Introduction
Proteins play a central role in cell structure and
function. The serum contains a mixture of proteins that differ in
origin and function, and the amount of protein in the vascular
compartment depends on the balance between the rate of synthesis
and the rate of catabolism or loss (1). It is a well-established and
evidence-based fact that serum protein levels may undergo changes
during the process of breast cancer (2). Proteins are also present in other
tissue fluids, in addition to serum (1).
Breast cancer presents a major healthcare burden to
females as it is the most common type of cancer occurring in
females worldwide (3) and is the
most common type of cancer in Jordan. Breast cancer is responsible
for 20% of the total cancer cases with a 22% mortality. The
age-standardized incidence rate of breast cancer increased from
29/100,000 in 1996 to 50/100,000 in 2008, and therefore, early
diagnosis is of great importance in reducing mortality (4). However, the biological and clinical
progression of breast cancer is not easily predicted, as there are
a number of types of this disease that behave differently between
patients (5). It is well known that
proteins are the elemental constituents of all living cells and
they are included in numerous substances, including enzymes,
hormones and antibodies. Changes in the concentrations of serum
protein have been associated with cancer disease processes and can
be indicative of health problems that may provide important
diagnostic information (1). The
amount of protein in the serum depends on the balance between the
rate of its synthesis, and that of its catabolism or loss. A total
plasma protein test measures the total amount of protein in the
plasma, as well as the amounts of albumin, globulin and fibrinogen
(6), and it also shows the actual
functioning of an organism (7).
Saliva is an important biological fluid for the
detection of physiological and pathological conditions of the human
body (8,9). Whole saliva is a mixed fluid that is
predominantly derived from three pairs of major salivary glands,
submandibular (70%), parotid (25%) and sublingual (5%) (10), and the remainder is from the minor
salivary glands that are located at various oral mucosal sites.
Increasing attention has focused on diagnosis by saliva-based
analysis of biologically active compounds, as saliva has been shown
to represent the clinically relevant compounds (11,12).
Saliva analysis is able to prevent, predict and diagnose numerous
health problems and diseases (13),
and the methodology for saliva collection is a simple, non-invasive
method. Human saliva contains clinically relevant proteins and ~30%
of blood proteins are also present in the saliva, emphasizing its
use in clinical applications. Previously, attention has bene given
to salivary analysis, not only for detecting abundant proteins, but
also for the detection of other components, including pollutants,
hormones, enzymes, amino acid, proteins, statherin, histatin, mucin
and cystatins (14), and their
association with bacterial and viral infections, as well as being
an indicator for systemic diseases (15–17).
The major protein species in the saliva have extensively undergone
post-translational modifications, including phosphorylation,
sulfation and glycosylation (18,19).
The aim of the present study was to determine the total protein
levels in the saliva and serum from female patients with breast
cancer in comparison to the levels in the healthy control
group.
Materials and methods
Subjects
The present study was conducted at different public
hospitals in Amman (Jordan) and at the Medical Allied Sciences
Department, Zarka University College (Amman, Jordan) between
October 2012 and November 2013. The study included two groups of
females, aged 50–70 years, and an evaluation of a full medical
history was completed for any pre-existing systemic diseases. The
first group consisted of 40 female patients, diagnosed with breast
cancer by clinical and histological analysis, and the second group
was 40 healthy females subjects that constituted the control group.
The total protein concentrations obtained from these two groups
were measured in each saliva and serum sample.
Specimen collection
Venous blood samples were obtained from all patients
in plain tubes, and following coagulation the sera were separated
and stored at −20°C until analysis. Simultaneously, the saliva was
also collected.
The collection of the saliva involved obtaining 2 ml
of unstimulated whole saliva under a resting condition ≥1 h after
eating and drinking. The patients and control subjects were asked
to wash their mouths several times with de-ionized water.
Subsequently, the saliva that was accumulated in the floor of the
mouth, under the tongue, was drawn by plastic pipettes (20). The saliva samples were centrifuged
at 699 × g for 10 min to eliminate any debris. The samples were
frozen immediately and stored at −20°C until analysis.
Estimation of total protein in the saliva
and serum
The salivary and serum total protein estimations
were conducted using the Biuret method (21). The method for the total protein is
founded on the method proposed by the American Association for
Clinical Chemistry and National Committee for Clinical Laboratory
Standards (22). The principle of
the method depends on the enzymatic reaction sequence used in the
assay of the total protein. The total protein was determined using
a Roche Cobas automated clinical chemistry analyzer (Roche
Diagnostics GmbH, Mannheim, Germany Systems).
Statistical analysis
The collected data were analyzed by Excel (2010),
using the Statistical Package for Social Sciences (SPSS Ver. 19;
SPSS, Inc., Chicago, IL, USA) for the inferential statistics. For
the purpose of data presentation and interpretation, the patient
cases were presented as figures to indicate the changes in total
protein in healthy individuals compared to breast cancer patients.
The t-test was used to analyze significant differences between
healthy individuals and breast cancer patients. P<0.05 was
considered to indicate a statistically significant difference. Data
are presented as mean ± standard deviation.
Results
Total proteins in the serum and
saliva
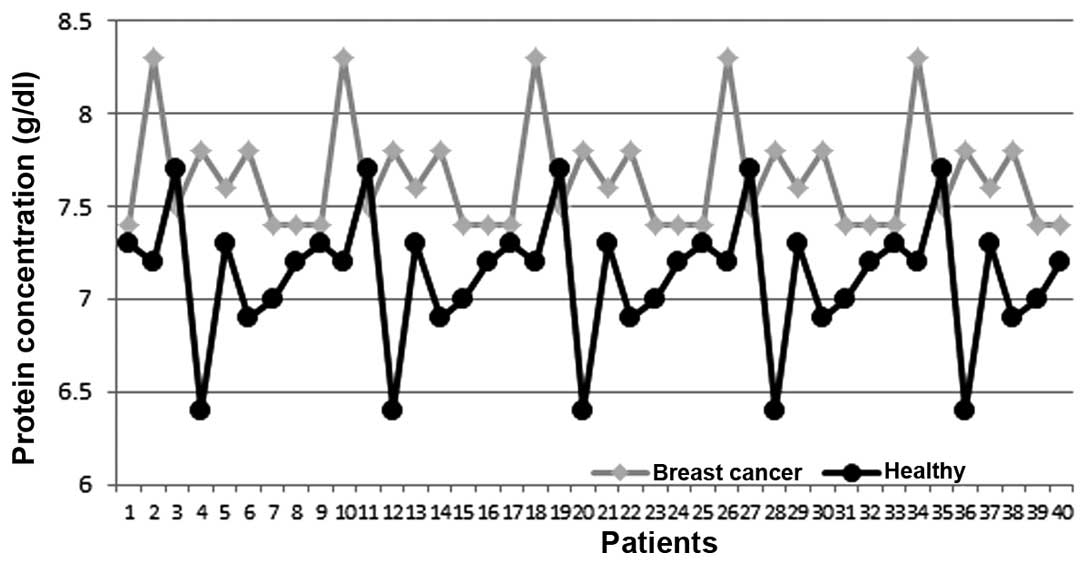
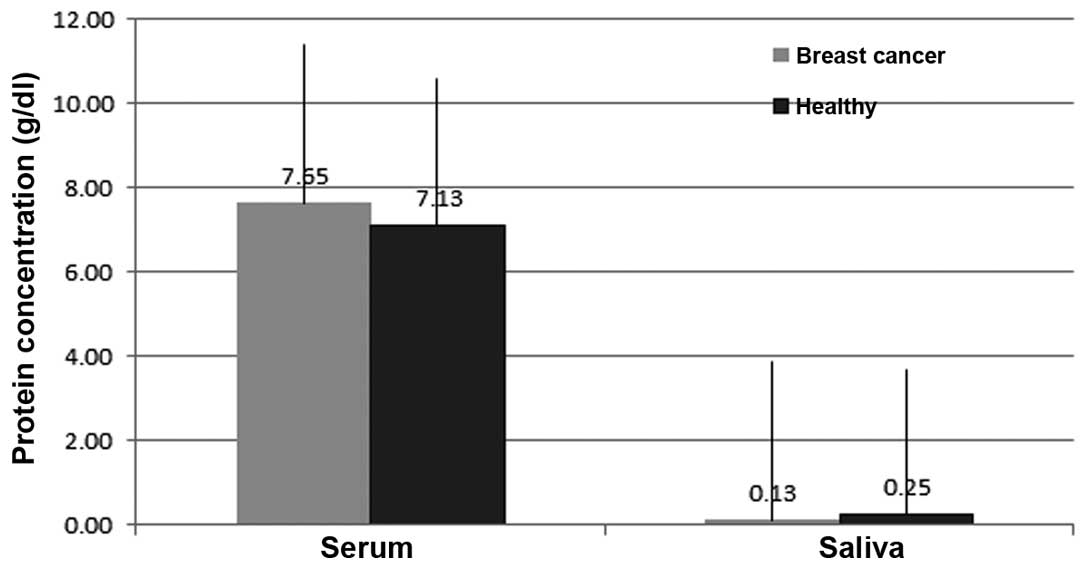
Table I shows the
total protein concentrations in the serum and saliva of female
patients with breast cancer and the control group. The results show
that the levels in the serum of the control group compared to those
levels in breast cancer patients were 6.14±1.84 and 7.63±SD 0.41
g/dl, respectively (Figs. 1 and
2). The mean values of the protein
concentration in the serum of breast cancer patients were
significantly higher than those in healthy individuals with a
statistical significance (P<0.05), whereas the mean levels of
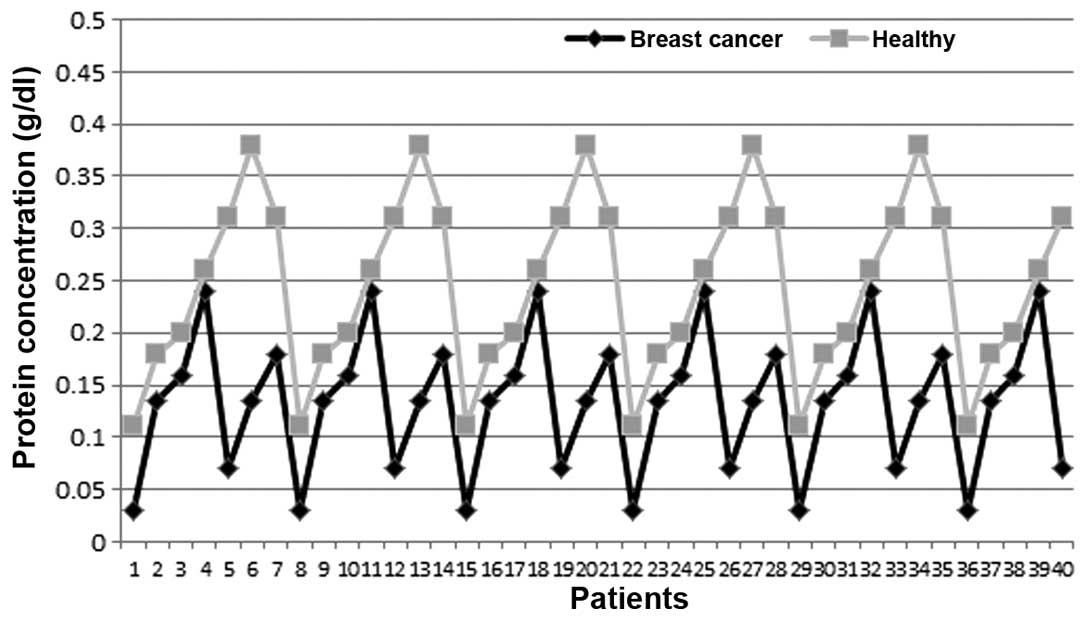
the saliva protein concentrations in the breast cancer group were
lower than the mean level for the healthy individuals, with
0.25±0.09 and 0.14±0.07 g/dl in the healthy controls and the
patients with breast cancer, respectively (Figs. 2 and 3). The differences were statistically
significant (P<0.05).
 | Table ITotal protein in the serum and saliva
from females with breast cancer and the control group. |
Table I
Total protein in the serum and saliva
from females with breast cancer and the control group.
| Group | | Mean±SD | P<0.05 |
|---|
| TP serum | Normal | 6.14±1.84 | 0.013 |
| Breast cancer | 7.63±0.41 | |
| TP saliva | Normal | 0.25±0.09 | 0.043 |
| Breast cancer | 0.14±0.07 | |
Discussion
Breast cancer is estimated to be the most commonly
diagnosed neoplasm in females according to the latest statistics
from the Jordan National Cancer Registry (23). In 2008, 864 females were diagnosed
with breast cancer, which constitutes 18.8% of the overall new
cancer cases. Breast cancer came first among the types of cancer in
females, accounting for 36.7% of total female cancers, and it has
been shown to be the foremost cause of cancer mortalities among
females in Jordan. The median age at diagnosis is ≥51 years
(24).
In the present study, the measurements of the serum
total protein revealed that there was an increase in the total
protein concentration in the serum of female patients with breast
cancer. This result is in accordance with several previous studies
that have explored the evaluation of serum total protein in
patients with a brain tumor (1),
and it also concurs with a study that found significant increases
in several protein profile levels in the sera of lung cancer
patients, using protein microarray and immunoassay techniques
(25). This increase in total serum
protein concentration can be due to the fact that total serum
protein is composed of albumin and other proteins, collectively
termed as globulins, and it is known that the serum albumin
concentration may change under oxidative stress, such as the stress
associated with cancer (26). In
addition, as the plasma circulates through the tissues, it collects
proteins that are released from their original locations due to
certain physiological events, including tissue remodeling, trauma
and cell death, which lead to an increase in total serum protein.
The ability of differentiating between the proteins that truly
reside in the plasma with those that are present following their
release due to physiological events, remains to be resolved.
However, the latter is inconsistently found, and is usually only
present at an extremely low concentration. By contrast, the present
results differ with the results of a study conducted by Jsiem et
al (27) in the evaluation of
serum and/or tissue proteins in patients with bladder cancer and in
patients with gynecological malignancies (28). However, with regards to the levels
of the total saliva protein of patients with breast cancer, the
present study revealed a decrease in the total saliva protein
compared to the control group. This result is in accordance with
the results from the study by Ozturk et al (29) who assessed female patients with
breast cancer who underwent chemotherapy treatment.
The original sources of the total proteins present
in the saliva may be affected by the patient conditions and type of
treatment. Among these sources are leakage of plasma into the
saliva and the outflow of gingival crevicular fluid (30). There are two known transport methods
for the molecules that are not part of the normal salivary
secretions from the serum to the saliva. These are the
transcellular route by passive diffusion and active transport, and
the paracellular route (ultrafiltration) through tight junctions
(31). However, the exact mechanism
of plasma-protein leakage and whether there is selective transport
of specific plasma proteins into the salivary system, remains
unknown.
Another reason for the decrease of the total protein
in the saliva may be due to the transport system in which the
transport depends on the polarity and the charge of the molecule.
Thus far, the majority of the identified biomarkers are not part of
the intrinsic components of saliva, but are small molecular-weight
inflammatory markers derived from the serum that are transported
into the saliva. Additionally, the decrease in the total protein in
the saliva may be due to the quantity and constitution of secreted
human saliva, which depends on particular factors, including flow
rate, circadian rhythm, type and size of the salivary gland,
duration and type of stimulus, diet, drugs, age, gender, blood type
and physiological status (8).
Saliva is a ‘real-time’ fluid due to the exocrine
salivary glands that generate protein profiles, which are
representative of an individual’s health and well-being status at
the time of collection (32). It is
known that blood is contained within a closed-loop circuit, whereas
the saliva is continually produced and excreted in an open-ended
circuit. Thus, as a circulating media, saliva may be a more useful
biological fluid than blood for representing the protein profile as
it is easier to test than blood, and is altered in the presence of
malignant diseases (33). As the
subjects in the present study were exposed to chemotherapy
treatment, this may be the decisive factor for the decrease in the
total protein in the saliva. The changes in the salivary protein
can now be observed quantitatively in different physiological or
pathological stages (34). However,
in order for a valid analysis, there are necessary precautions that
can be followed to avoid proteolysis, deglycosylation and
dephosphorylation of salivary proteins by microbial and host
enzymes in the saliva. In the present study, precautionary measures
were considered. Breast cancer is one of the conditions that
initiate the acute phase response by increasing the levels of
specific hepatic proteins, such as positive acute-phase proteins
(35). At the same time, there are
groups of proteins in the body that have been reported to decrease
in concentration due to the enhancement in their catabolism, rather
than in their synthesis. These proteins are known as negative-phase
proteins, including albumin and prealbumin (36). Therefore, the detection of
hypoproteinemia can be the result of the net balance between the
protein synthesis of positive acute-phase proteins, and the
catabolism of negative acute-phase proteins. Protein modifications,
including glycosylation, phosphorylation and proteolysis, occur in
a dynamic environment that is determined by the continual supply of
newly synthesized proteins and the removal of proteins by
swallowing. Evaluating the whole saliva proteome in a continuous
turnover environment is necessary for understanding the
physiological and pathological processes that are relevant to oral
health, and may be critical for the identification of important
biomarkers (19).
Acknowledgements
The author would like to thank Al-Balqa Applied
University, Zarqa University College (Amman, Jordan) and all the
people and institutions that have extended their help with the
present study.
References
|
1
|
Hamad AWR, Ibrahim MA, Al-Mohtasib SI and
Al-Kobasi KN: Comparative study on saliva proteins in patients and
healthy individuals of brain tumours. Trends Med Res. 4:16–23.
2009.
|
|
2
|
Gast MC, Van Gils CH, Wessels LF, Harris
N, Bonfrer JM, Rutgers EJ, Schellens JH and Beijnen JH: Serum
protein profiling for diagnosis of breast cancer using SELDI-TOF
MS. Oncol Rep. 22:205–213. 2009.
|
|
3
|
Parkin DM, Muir CS, Whelan SL, et al:
Cancer Incidence in Five Continents. 6IARC Scientific Publications.
(120)Lyon: IARC; 1992
|
|
4
|
Colditz GA and Frazier AL: Models of
breast cancer show that risk is set by events of early life:
prevention efforts must shift focus. Cancer Epidemiol Biomarkers
Prev. 4:567–571. 1995.
|
|
5
|
MacMahon B: Epidemiology and the causes of
breast cancer. Int J Cancer. 118:2373–2378. 2006.
|
|
6
|
Yilmaz IA, Akçay T, Cakatay U, et al:
Relation between bladder cancer and protein oxidation. Int Urol
Nephrol. 35:345–350. 2003.
|
|
7
|
Santamaria-Kisiel L, Rintala-Dempsey AC
and Shaw GS: Calcium-dependent and -independent interactions of the
S100 protein family. Biochem J. 396:201–214. 2006.
|
|
8
|
Schipper RG, Silletti E and Vingerhoeds
MH: Salivary as research material: biochemical, physicochemical and
practical aspects. Arch Oral Biol. 52:1114–1135. 2007.
|
|
9
|
Lawrence HP: Salivary markers of systemic
disease: non-invasive diagnosis of disease and monitoring of
general health. J Can Dent Assoc. 68:170–174. 2002.
|
|
10
|
Rosen FS and Bailey BJ: Anatomy and
Physiology of the Salivary Glands, Grand Rounds Presentation. 5th
edition. UTMB; 2001
|
|
11
|
Lee YH and Wong DT: Saliva: an emerging
biofluid for early detection of diseases. Am J Dent. 22:241–248.
2009.
|
|
12
|
Choi M: Saliva diagnostics integrate
dentistry into general and preventive health care. Int J
Prosthodont. 23:1892010.
|
|
13
|
Kunsman K: Oral fluid testing arrives.
Occup Health Saf. 69:28–30. 2000.
|
|
14
|
Oppenheim FG, Salih E, Siquerira WL, Zhang
W and Helmerhorst EJ: Salivary proteome and its genetic
polymorphisms. Ann NY Acad. 1098:22–50. 2007.
|
|
15
|
Kazmi SH, Naglik JR, Sweet SP, et al:
Comparison of human immunodeficiency virus type 1-specific
inhibitory activities in saliva, and other human mucosal fluids.
Clin Vaccine Immunol. 13:1111–1118. 2006.
|
|
16
|
Pink R, Simek J, Vondrakova J, et al:
Saliva as a diagnostic medium. Biomed Pap Med Fac Univ Palacky
Olomouc Czrch Repub. 153:103–110. 2009.
|
|
17
|
Hofman LF: Human saliva as a diagnostic
specimen. J Nutr. 131:1621S–1625S. 2001.
|
|
18
|
Helmerhorst EJ and Oppenheim FG: Saliva: a
dynamic protecome. J Dent Res. 86:680–693. 2007.
|
|
19
|
Castagnola M, Cabras T, Vitali A, Sanna MT
and Messana I: Biotechnological implications of the salivary
protecome. Trends Biotechnol. 29:409–418. 2011.
|
|
20
|
Nishanian P, Aziz N, Chung J, Detels R and
Fahey JL: Oral fluids as an alternative to serum for measurement of
markers of immune activation. Clin Diagn Lab Immunol. 5:507–512.
1998.
|
|
21
|
Doumas BT, Bayse DD, Carter RJ, Peters T
Jr and Schaffer R: A candidate reference method for determination
of total protein in serum. I Development and validation. Clin Chem.
27:1642–1650. 1981.
|
|
22
|
National Committee for Clinical Laboratory
Standards. Specification for Standardized Protein Solution (Bovine
Serum Albumin). National Committee Standards for Clinical
Laboratory. 2nd edition. Villanova; Pennsylvania, PA: pp. 19–85.
1979
|
|
23
|
Khoury SA and Mas’ad DF: Profile of cancer
family clustering in Jordan. East Mediterr Health J. 14:1101–1109.
2008.
|
|
24
|
Arkoob K, Al-Nsour M, Al-Nemry O and
Al-Hajawi B: Epidemiology of breast cancer in women in Jordan:
patient characteristics and survival analysis. East Mediteer Health
J. 16:1032–1038. 2010.
|
|
25
|
Gao WM, Kuick R, Orchekowski RP, et al:
Distinctive serum protein profiles involving abundant proteins in
lung cancer patients based upon antibody microarray analysis. BMC
Cancer. 5:1102005.
|
|
26
|
Halliwell B: Antioxidants: the basics -
what they are and how to evaluate them. Adv Pharmacol. 38:3–20.
1997.
|
|
27
|
Jsiem RH, Hasan RH and Husain MK: A
biochemical study for evaluation and analysis of serum protein of
patients with different kidney tumors. J Baghdad for Sci.
9:311–321. 2012.
|
|
28
|
Al-Ardee R: Evaluation of sialic acid
levels and lectin activity as tumour markers for gynaecologic
cancers (unpublished PhD thesis). University of Kufa; 2001
|
|
29
|
Oztürk LK, Emekli-Alturfan E, Kaşikci E,
et al: Salivary total sialic acid levels increase in breast cancer
patients: a preliminary study. Med Chem. 7:443–447. 2011.
|
|
30
|
Yan W, Apweiler R, Balgley BM, et al:
Systematic comparison of the human saliva and plasma proteomes.
Proteomics Clin Appl. 3:116–134. 2009.
|
|
31
|
Haeckel R and Hänecke P: Application of
saliva for drug monitoring. An in vivo model for
transmembrane transport. Eur J Clin Chem Clin Biochem. 34:171–191.
1996.
|
|
32
|
Bigler LR, Streckfus CF and Dubinsky WP:
Salivary biomarkers for the detection of malignant tumors that are
remote from the oral cavity. Clin Lab Med. 29:71–85. 2009.
|
|
33
|
Kuerer M, Thompson PA, Krishnamurthy S, et
al: High and differential expression of HER-2/neu extracellular
domain in bilateral ductal fluids from women with unilateral
invasive breast cancer. Clin Cancer Res. 9:601–605. 2003.
|
|
34
|
Siqueira WL and Dawes C: The salivary
proteomics: challenges and perspectives. Proteomics Clin Appl.
5:575–579. 2011.
|
|
35
|
Kaplen LA and Pesce AJ: Clinical
Chemistry: Theory, Analysis and Correlation. 4th edition. C.V.
Mosby Co; St. Louis, MO: 2003
|
|
36
|
Henry JB: Clinical Diagnosis and
Management by Laboratory Methods. 20th edition. W.B. Saunders Co;
Philadelphia, PA: 2001
|