Introduction
Granular cell tumors (GCTs), which were first
described by Abrikossoff in 1926, are uncommon mesenchymal
soft-tissue neoplasms (1,2). In 1931, Abrikossoff identified that,
although they were originally noted in the tongue, GCTs were also
associated with the breast (2).
Female patients are more likely than their male counterparts to be
affected. Patients of African descent have a higher incidence
compared with those who are not of African descent (3). GCTs of the breast (GCTB) account for
between 5 and 15% of all GCTs (4,5) and it
is extremely rare to find the tumors in lactating females. GCTBs
are difficult to distinguish from breast carcinoma by clinical,
radiological or other observational techniques. Thus, pathological
and immunohistochemical examinations are necessary (2). Although the association between GCT,
estrogen and prolactin has not been proven, increased GCT incidence
in the presence of hyperestrogenic and hyperprolactinemic states
has been reported (3,6–10). The
purpose of the present study was to review the management of GCTB
and to present and discuss cases with GCT in the presence of
hyperestrogenism or hyperprolactinemia. To the best of our
knowledge, this report is the first Chinese case of a GCTB during
lactation in the English literature. Written informed consent was
obtained from the patient.
Case report
A 29-year-old female presented to the Women’s
Hospital (Hangzhou, Zhejiang, China) with a mass on the right
breast, which had first been noticed four years earlier. The mass
had increased in size in the latter half of the gestation and
lactation periods. The patient had no medical history of
malignancy. A physical examination of the breast revealed a firm,
painless and vague nodularity in the upper-outer quadrant, near the
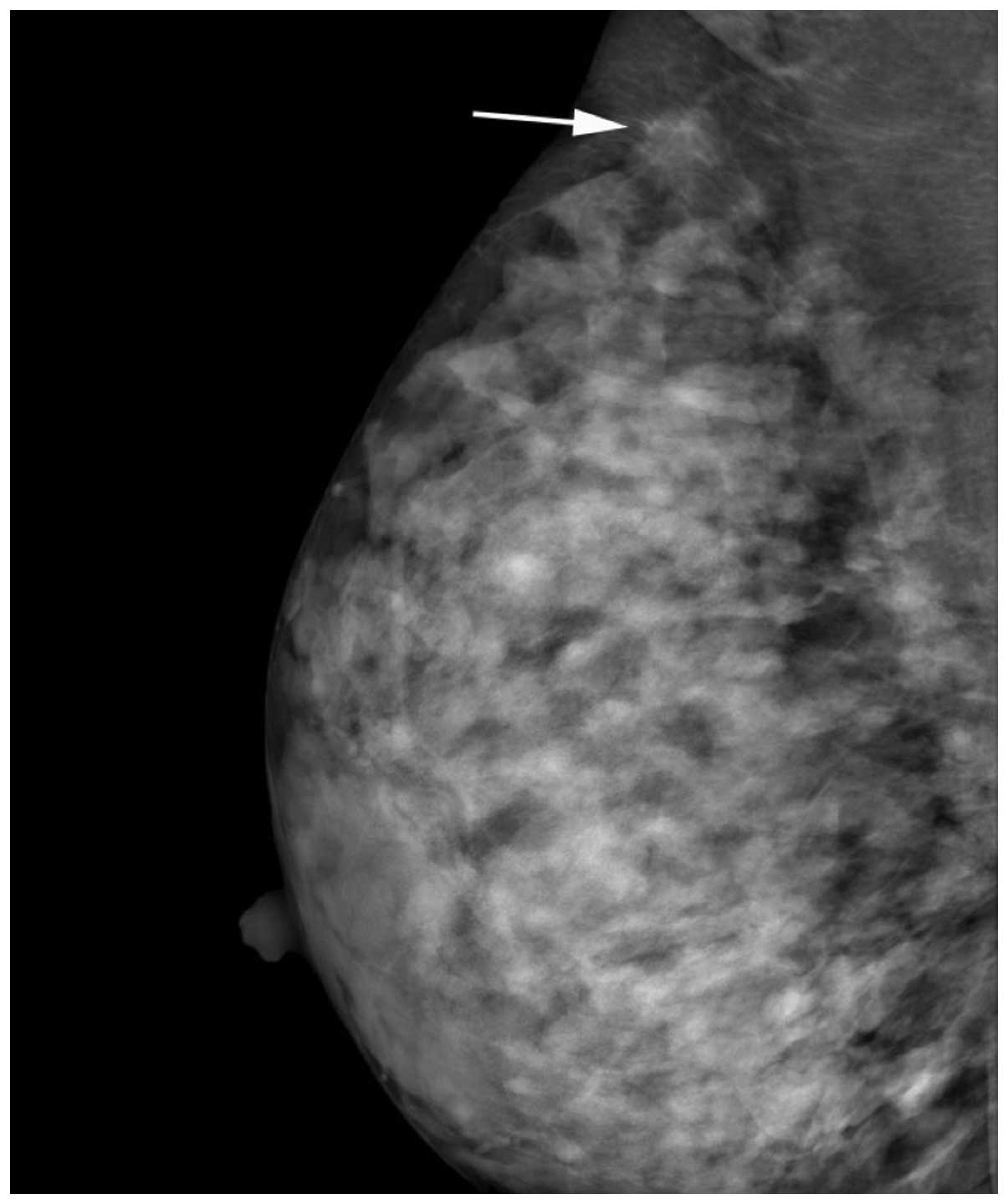
axillary tail. Mammography revealed an isodense right-sided mass
with ill-defined borders [Breast Imaging Reporting and Data System
(BI-RADS) 4C; Fig. 1]. Ultrasound
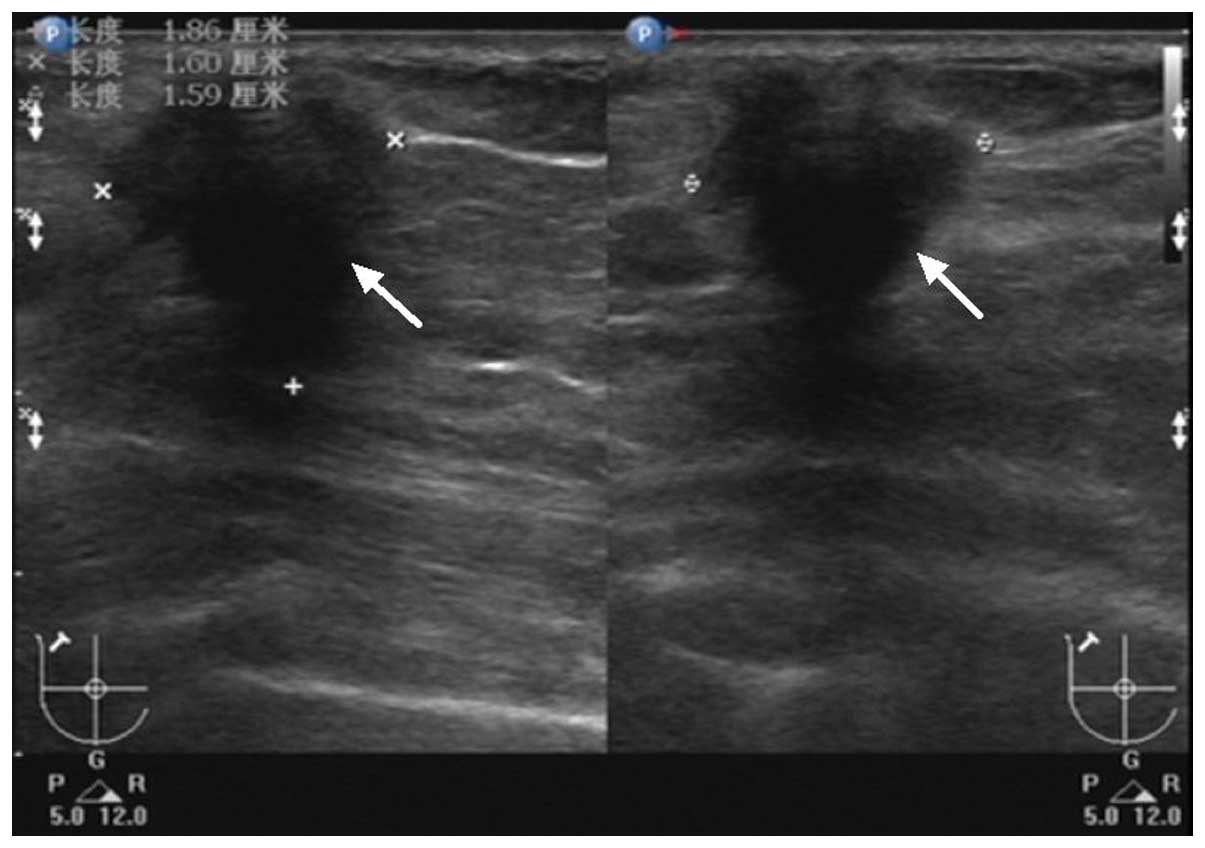
examination demonstrated a 1.9×1.6×1.6-cm hypoechoic, hypovascular
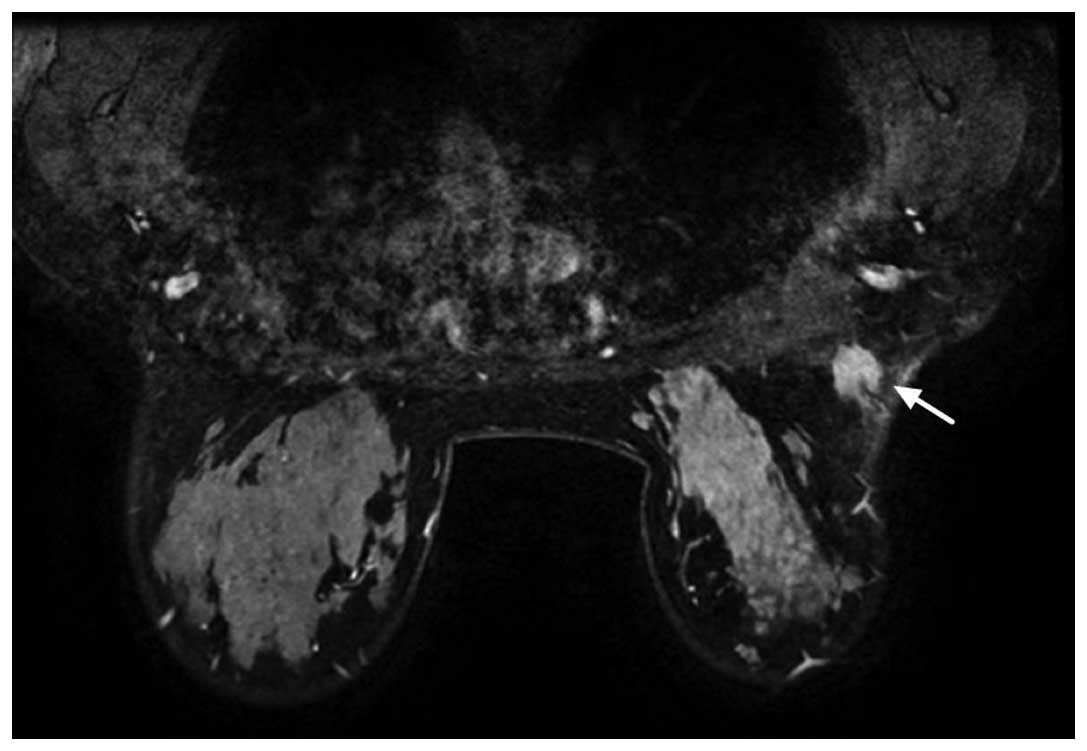
and poorly-defined mass (BI-RADS 5; Fig. 2). Dynamic magnetic resonance (MR)
mammography revealed homogeneous enhancement on a
post-gadolinium-enhanced T1-weighted MR imaging (MRI) sequence and
a high signal rim on a T2-weighted sequence (Fig. 3). The clinical and radiological
findings were suggestive of malignancy.
An ultrasound-guided core biopsy was performed and
confirmed that the mass was a GCT, due to the cytological features
observed and the immunohistochemical profile of the mass, thus, a
wide local excision was performed. The intraoperative frozen
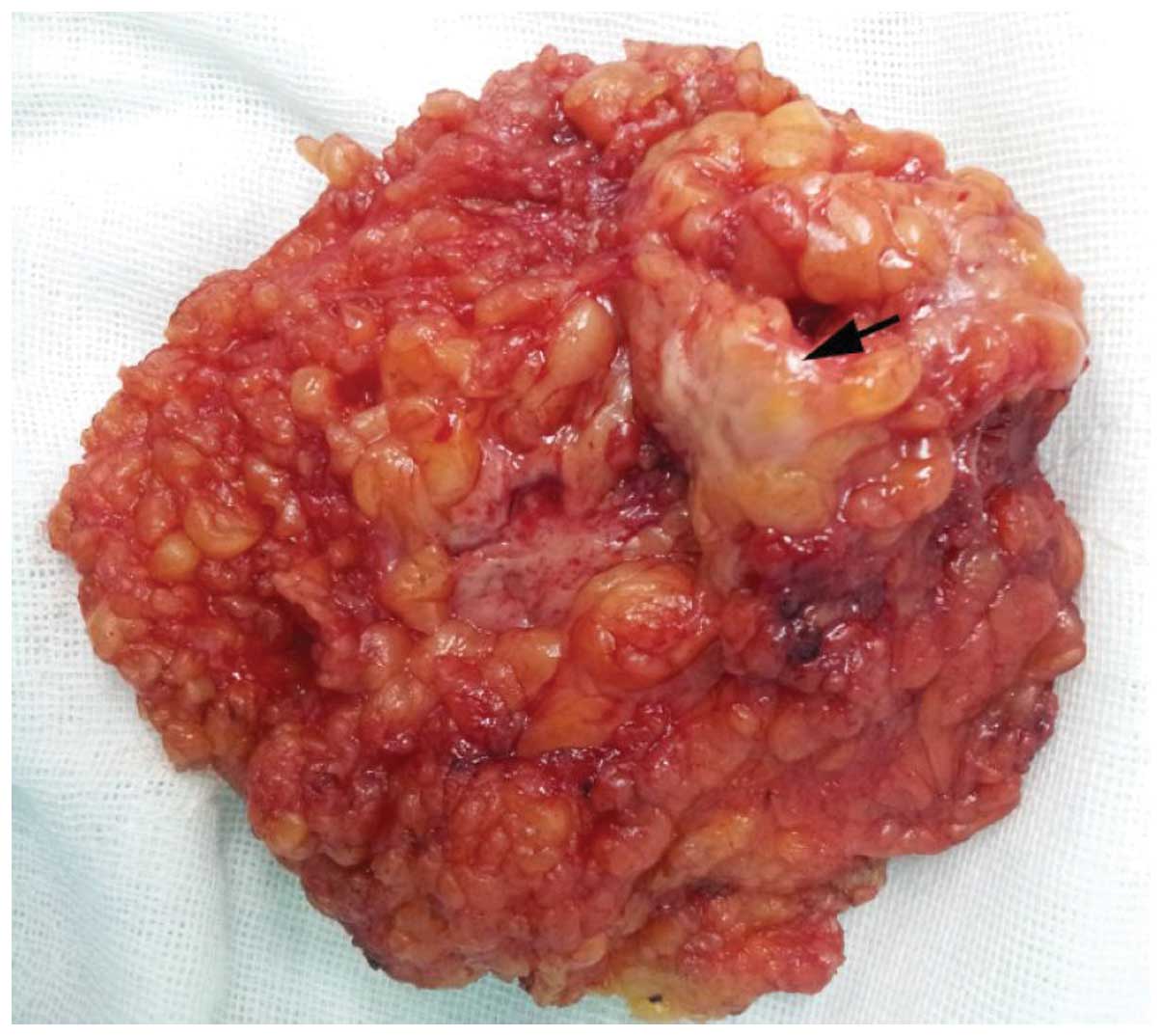
section demonstrated that the mass was a GCT. On gross examination,
the surgically excised specimen was comprised of fatty tissue
fragments measuring 2.5×2.0×2.0 cm, and contained a firm,
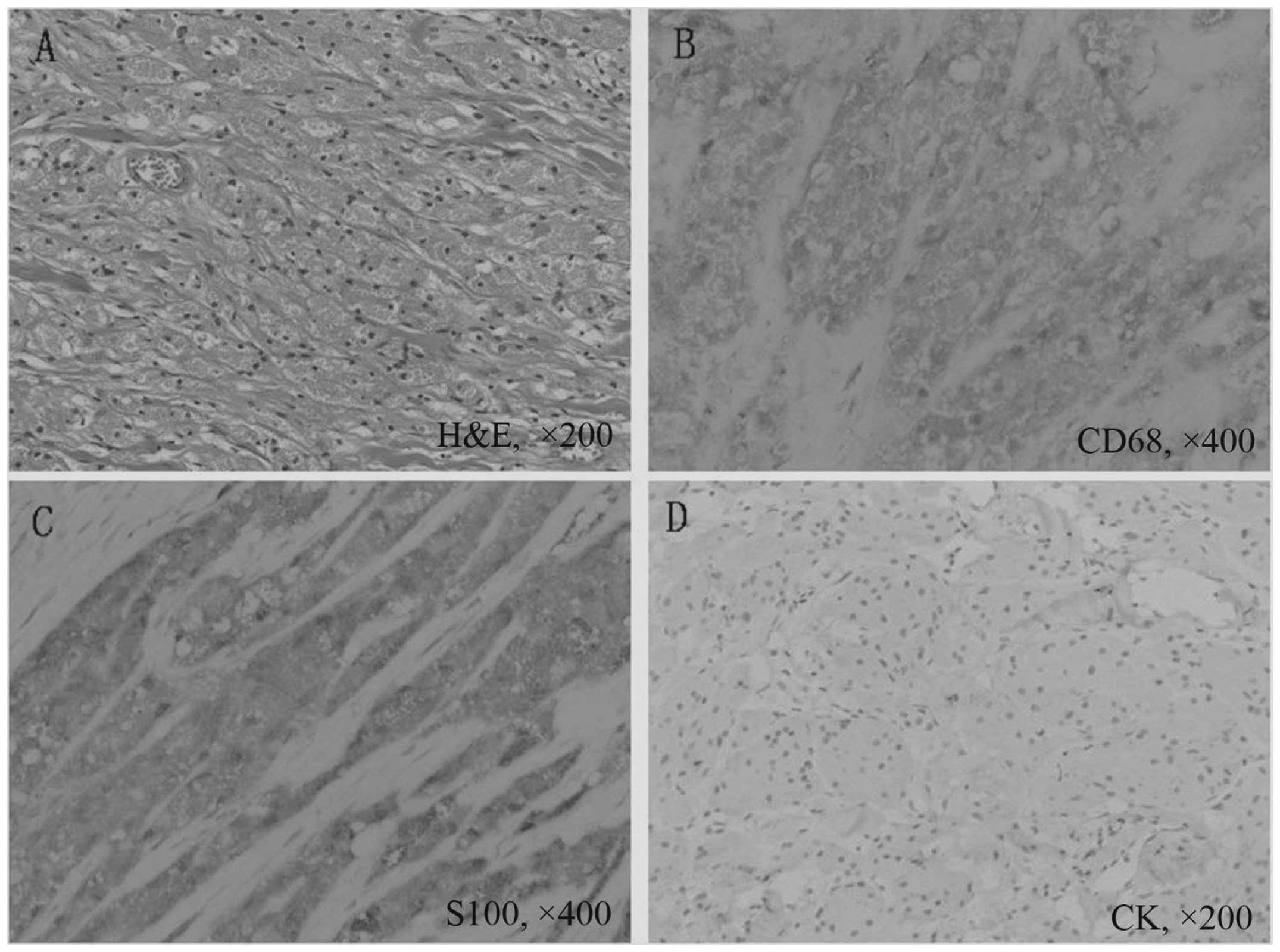
well-limited and yellow-white nodule (Fig. 4). Microscopically, the specimen was
composed of nests or sheets of cells that contained cytoplasmic
eosinophilic granules. The cells were generally uniform, large and
polygonal. The nuclei were round to oval in shape, and the nucleoli
were prominent (Fig. 5A).
Immunohistochemical staining was strongly positive for cluster of
differentiation (CD)68 (Fig. 5B)
and S100 expression (Fig. 5C) and
negative for cytokeratin expression (Fig. 5D). The GCT diagnosis was confirmed.
The patient received no further treatment, and 15 months after
surgery was in good health, with no tumor recurrence.
Discussion
GCTBs account for between 5 and 15% of all GCTs
(1,4,5). To
the best of our knowledge, <400 cases of GCTBs have been
reported in the literature. The tumors are most common in
middle-aged premenopausal females and in African-American females
(11), and occur largely in the
upper-inner quadrant of the breast. However, in the present case,
the mass was located in the upper-outer quadrant. GCTBs often
present as a firm and painless mass, which may occur in the deep
parenchyma, with fixation to the pectoral muscle, or in the
subcutis, causing skin retraction, thus mimicking a malignancy
(12).
Mammographically, GCTBs are known to exhibit a
variable appearance, which ranges from well-circumscribed
benign-appearing nodules to highly suspicious spiculated masses
associated with skin retraction and thickening (13). Mammograms also often reveal GCTBs as
stellate lesions without calcifications. The ultrasound appearance
of a GCTB is usually a hypoechoic, ill-defined mass with posterior
shadowing and a high boundary echo (14). A variety of MRI findings were
illustrated in the cases reviewed in the present study, so no
specific features of GCTB have been outlined. The patient in the
present case exhibited all of the clinical and imaging features
that have been classically associated with breast carcinoma, such
as a firm and vague nodularity, an irregular spiculated mass on
mammography and spiculated margins on sonography along with
posterior shadowing. Irshad et al (13) reported spiculations as a common
imaging feature that mimic carcinoma when present, and the present
case was in agreement with this, since all the images exhibited a
spiculated mass.
Although the clinical and radiological findings are
mostly misleading, pathological investigations are essential in the
diagnosis of GCTB. According to the European Society of Breast
Cancer Specialists, pre-operative histological confirmation with a
core biopsy can avoid mastectomy and axillary dissection (15).
GCTBs are usually firm and ill-defined masses with
coloration ranging from white to tan (4,16,17).
The majority of GCTBs are well-circumscribed lesions, but a
significant proportion of them may be poorly circumscribed.
Additionally, a lack of circumscription is now considered a common
feature of GCTBs (18).
Microscopically, the cytological features of GCTs include uniform
cells, voluminous cytoplasm with fragile membranes, abundant
granular eosinophilic cytoplasm and absent bare or bipolar nuclei
(2). GCTs are also generally
uniform, large, bland and polygonal, and are arranged in nests and
sheets. Granular change is caused by the cytoplasmic accumulation
of lysosomes. GCTs do not exhibit mitoses, pleomorphism, nuclear
multiplicity or atypia (19,20).
Immunohistochemical staining is useful in differentiating GCTBs
from mammary carcinoma. The tumor cells in GCTs are strongly
immunoreactive to S100 and CD68. CD68 expression is an
immunohistochemically distinctive feature of GCTs and is associated
with an abundance of phagolysosomes (20). S100 is a sensitive marker for GCTB,
but it is not specific, as certain breast malignancies are also
S100-positive (21). CD68 and S100
stain negative for cytokeratins, epithelial membrane antigen and
mucin (22). CD68 and S100 aid in
the differentiation between a GCT and apocrine carcinoma.
The factors affecting the development of GCTB have
not been clearly confirmed, and the association between GCTB and
hormones remains inconclusive. Certain cases have reported that
GCTs occur during pregnancy and in hyperestrogenic and
hyperprolactinemic states (3,4,7–10,23,24).
In the present study, the size of the tumor increased rapidly in
the latter half of the gestation and lactation periods, and the
levels of estrogen and prolactin were high. Review of the
literature revealed five other cases of GCTs in which a history of
increased bodily estrogen was noted at the time of diagnosis.
Kommoss et al (4) reported a
GCTB in a 20-year-old, pregnant female of African descent. Ipakchi
et al (3) noted a patient
with recurrent GCT in subsequent pregnancies during the later
stages of pregnancy. Yang et al (23) reported the case of a 5-month
pregnant, 21-year-old patient who was found to have a malignant
mediastinal GCT. Mahoney et al (7) reported a thyroid GCT in a
nine-year-old patient receiving high-dose estrogen therapy. Benisch
et al (8) reported the case
of a GCT of the trachea in a first-trimester, 25-year-old patient.
The review of the literature revealed three additional cases of GCT
in which a history of increased prolactin was noted at the time of
diagnosis. Lee et al (9)
reported a GCT of the neurohypophysis in a 36-year-old female whose
laboratory examination revealed hyperprolactinemia. Higuchi et
al (10) noted a hypophyseal
GCT case, which presented as visual failure and hyperprolactinaemia
(serum prolactin level, 274 ng/ml; normal, <10 ng/ml). Popovic
et al (24) reported a case
of GCT of the sellar region with hyperprolactinemia. However, GCT
cells are also negative for estrogen and progesterone. The hormonal
effects on GCT have not been completely investigated.
Wide local excision is the recognized treatment for
GCTB. The local excision of lymph nodes or a sentinel lymph node
biopsy is not indicated, except in the case of malignant GCT
(1).
GCTB is a rare tumor that mimics breast malignancy
clinically and radiologically. Pathological correlation usually
clarifies the diagnosis, but further examination via
immunohistochemical staining is necessary. Although an association
between GCT, hyperestrogenism and hyperprolactinemia has been
postulated, the small number of studies investigating the
hypothesis precludes any definitive association.
Acknowledgements
The authors would like to thank the teachers at the
Department of Surgery and the support provided by the Departments
of Radiology and Pathology at the Women’s Hospital, School of
Medicine, Zhejiang University.
References
|
1
|
Qureshi NA, Tahir M and Carmichael AR:
Granular cell tumour of the soft tissues: a case report and
literature review. Int Semin Surg Oncol. 3:212006.
|
|
2
|
Pieterse AS, Mahar A and Orell S: Granular
cell tumour: a pitfall in FNA cytology of breast lesions.
Pathology. 36:58–62. 2004.
|
|
3
|
Ipakchi R, Zager WH, de Baca ME, Bloedon
E, McCue PA and Zwillenberg D: Granular cell tumor of the trachea
in pregnancy: a case report and review of literature. Laryngoscope.
114:143–147. 2004.
|
|
4
|
Kommoss F, Mercer L, Schmidt RA and
Talerman A: Granular cell tumor of the breast mimicking carcinoma
in pregnancy. Obstet Gynecol. 73:898–900. 1989.
|
|
5
|
Al-Ahmadie H, Hasselgren PO, Yassin R and
Mutema G: Colocalized granular cell tumor and infiltrating ductal
carcinoma of the breast. Arch Pathol Lab Med. 126:731–733.
2002.
|
|
6
|
Kintanar EB, Giordano TJ, Thompson NW and
Michael CW: Granular-cell tumor of trachea masquerading as
Hurthle-cell neoplasm on fine-needle aspirate: a case report. Diagn
Cytopathol. 22:379–382. 2000.
|
|
7
|
Mahoney CP, Patterson SD and Ryan J:
Granular cell tumor of the thyroid gland in a girl receiving
high-dose estrogen therapy. Pediatr Pathol Lab Med. 15:791–795.
1995.
|
|
8
|
Benisch BM, Abt AB and Abramson A:
Granular cell myoblastoma of trachea associated with pregnancy.
Chest. 63:832–833. 1973.
|
|
9
|
Lee CC, Liu CH, Wei CP and How SW:
Symptomatic granular cell tumor of the neurohypophysis. J Formos
Med Assoc. 103:58–62. 2004.
|
|
10
|
Higuchi M, Tsuji M and Ikeda H:
Symptomatic hypophyseal granular cell tumour: endocrinological and
clinicopathological analysis. Br J Neurosurg. 11:582–586. 1997.
|
|
11
|
Henry M and Perry A: Multiple cutaneous
granular cell tumors: case report of a 19-year-old African American
female. J Cutan Med Surg. 15:344–346. 2011.
|
|
12
|
Brown AC, Audisio RA and Regitnig P:
Granular cell tumour of the breast. Surg Oncol. 20:97–105.
2011.
|
|
13
|
Irshad A, Pope TL, Ackerman SJ and
Panzegrau B: Characterization of sonographic and mammographic
features of granular cell tumors of the breast and estimation of
their incidence. J Ultrasound Med. 27:467–475. 2008.
|
|
14
|
Ayub MF, Radhakrishna S and Chakravarthy
R: Images: granular cell tumour of the breast mimics a malignancy.
Indian J Surg Oncol. 3:47–49. 2012.
|
|
15
|
Bauerfeind I, Ditsch N, Sittek H and
Diebold J: Reduction mammaplasty in granular cell tumour of the
breast. Br J Plast Surg. 57:458–461. 2004.
|
|
16
|
Gibbons D, Leitch M, Coscia J, et al: Fine
needle aspiration cytology and histologic findings of granular cell
tumor of the breast: review of 19 cases with clinical/radiologic
correlation. Breast J. 6:27–30. 2000.
|
|
17
|
Quiroz-Rodriguez G, Robles-Vidal C,
Guzmán-Navarro L and Ortiz-Hidalgo C: Granular cell (Abrikossof)
tumor of the breast. Breast J. 12:4942006.
|
|
18
|
Adeniran A, Al-Ahmadie H, Mahoney MC and
Robinson-Smith TM: Granular cell tumor of the breast: a series of
17 cases and review of the literature. Breast J. 10:528–531.
2004.
|
|
19
|
Scaranelo AM, Bukhanov K, Crystal P,
Mulligan AM and O’Malley FP: Granular cell tumour of the breast:
MRI findings and review of the literature. Br J Radiol. 80:970–974.
2007.
|
|
20
|
Gavriilidis P, Michalopoulou I, Baliaka A
and Nikolaidou A: Granular cell breast tumour mimicking
infiltrating carcinoma. BMJ Case Rep. 2013:bcr2012008178. 2013.
|
|
21
|
El-Khalawany M, Mosbeh AS, Abd-Al Salam F
and Abou-Bakr A: Ulcerative granular cell tumor: a
clinicopathological and immunohistochemical study. J Skin Cancer.
2011:4976482011.
|
|
22
|
Filie AC, Lage JM and Azumi N:
Immunoreactivity of S100 protein, alpha-1-antitrypsin, and CD68 in
adult and congenital granular cell tumors. Mod Pathol. 9:888–892.
1996.
|
|
23
|
Yang SW, Hong SW, Cho MY and Kang SJ:
Malignant granular cell tumor at the retrotracheal space. Yonsei
Med J. 40:76–79. 1999.
|
|
24
|
Popovic V, Pekic S, Skender-Gazibara M,
Salehi F and Kovacs K: A large sellar granular cell tumor in a
21-year-old woman. Endocr Pathol. 18:91–94. 2007.
|