Introduction
Hepatocellular carcinoma (HCC) is the fifth most
common type of cancer and the third leading cause of cancer-related
mortality worldwide (1,2). Of the risk factors attributed to liver
carcinogenesis, chronic hepatitis B virus (HBV) infection has been
demonstrated as a major factor (3).
The double-stranded DNA genome of HBV contains four overlapping
open-reading frames that encode the surface protein, the core
protein, a polymerase and the HBV X protein (HBx) (4). HBx is a multifunctional protein that
does not bind directly to DNA, but exerts transcriptional
activation through its interaction with nuclear transcription
factors and the modulation of cytoplasmic signal transduction
pathways, including NF-κB signaling (5). HBx has been demonstrated to accelerate
the progress of HCC in numerous processes, including apoptosis,
proliferation, inflammation, angiogenesis, immune responses,
multi-drug resistance, invasion and metastasis (6). Thus, there is increasing evidence
demonstrating the crucial role of HBx in the development and
progression of HCC.
The circadian clock is the inner rhythm of
organisms, which has been formed over the course of long-term
evolution and it regulates daily rhythmic variations in various
physiological processes, including sleep and activity, appetite,
hormone levels, metabolism and gene expression (7,8). The
pacemakers are located in the suprachiasmatic nucleus (SCN) of the
hypothalamus and produce self-sustaining circadian rhythms that are
synchronized by external cues (9).
Circadian rhythms similar to those operating in the SCN have been
found in the majority of mammalian cells and peripheral tissues.
These peripheral circadian rhythms can be driven or synchronized by
the central pacemaker in the SCN through neuronal and humoral
factors (10). In humans, the
molecular clocks that these intrinsic rhythmic changes are based
upon are the transcription-translation feedback loops of multiple
biological clock genes, including CLOCK, BMAL1,
Per (Per1, Per2 and Per3), Cry
(Cry1 and Cry2) and casein kinase 1ɛ
(CKIɛ) (11).
The CLOCK-BMAL1 heterodimer initiates
transcription by binding E-boxes, which are CACGTG nucleotide
sequences in promoters, and drives the rhythmic transcription of
the three Per and two Cry genes. As Per and
Cry are translated in the cytoplasm, they form
Per-Cry complexes and translocate into the nucleus to
inhibit further CLOCK-BMAL1 transcriptional activity
(12). In addition, CKIɛ has
also been found to be involved in the transcriptional loops by
controlling the stability of the PER and BMAL1
proteins via phosphorylation (13).
On the basis of epidemiological and experimental
studies, the potential association between disruption of the
circadian rhythm and tumor development has become a focus of
investigation (14,15). The disturbance of circadian clock
gene expression is common in colorectal (16), breast (17) and pancreatic cancers (18), and is generally associated with the
development and progression of these types of tumor. The
disturbance of circadian clock gene expression has also been found
in HCC (19), however, the
predominant factors that cause the circadian clock disorder have
not yet been elucidated. The present study aimed to reveal the role
of HBx in contributing to the disturbance of the expression of
circadian clock genes in HCC.
Materials and methods
Tumor specimens and cell lines
Tissues were obtained from 30 HCC patients who
underwent surgical resection at the Department of Hepatobiliary
Surgery, Affiliated Hospital of Guiyang Medical College (Guiyang,
China) between October 2010 and June 2011. All the tissue samples
were obtained from the patients prior to any medical treatment. All
patients tested positive for the HBV surface antigen, HBsAg, and
negative for antibodies to the hepatitis C virus (anti-HCV) and
human immunodeficiency virus (anti-HIV). The mean patient age was
44.9 years (range, 23–62 years), with 26 male and four female
patients. Clinical data, tumor characteristics, and American Joint
Committee on Cancer staging of these patients are demonstrated in
Table I (20). All specimens were obtained between
9:00 a.m. and 12:00 p.m. on the same day. The viable tumor and
adjacent healthy tissues were dissected immediately and snap-frozen
in liquid nitrogen. The human HCC BEL-7404 cell line was obtained
from the American Type Culture Collection (Manassas, VA, USA) and
cultured in Eagle’s Minimum Essential Medium (Sigma-Aldrich, St.
Louis, MO, USA), supplemented with 10% fetal bovine serum. The
BEL-7404 cells were transfected with pEGFP-HBx and pEGFP empty
vectors, and incubated in a selection media containing 800 mg/l
G418 (Gen-View Scientific Inc., Calimesa, CA, USA) for 14 days. The
BEL-7404 cells transfected with pEGFP empty vectors served as the
control. Stable colonies were isolated and identified by reverse
transcription-polymerase chain reaction (RT-PCR) and western blot
analysis.
 | Table IClinicopathological features of 30
patients with hepatocellular carcinoma. |
Table I
Clinicopathological features of 30
patients with hepatocellular carcinoma.
| Clinicopathological
parameter | Cases, n (%) |
|---|
| Mean age (range) | 45 years (23–62
years) |
| Gender |
| Male | 26 (86.7) |
| Female | 4 (13.3) |
| Cirrhosis |
| Presence | 17 (56.7) |
| Absence | 13 (43.3) |
| Tumor size |
| <3 cm | 11 (36.7) |
| ≥3 cm | 19 (63.3) |
| Vascular
invasion |
| Presence | 5 (16.7) |
| Absence | 25 (83.3) |
| Tumor number |
| 1 | 25 (83.3) |
| >1 | 5 (16.7) |
| Tumor
differentiation |
| Well | 5 (16.7) |
| Moderate | 19 (63.3) |
| Poor | 6 (20.0) |
| AJCC stage |
| I | 16 (53.3) |
| II | 8 (26.7) |
| III | 6 (20.0) |
RNA extraction and first-strand
complementary (c)DNA synthesis
Fresh frozen tissues (weight, 150–200 mg) were used
to isolate total RNA with 1 ml TRIzol® reagent
(Invitrogen Life Technologies, Carlsbad, CA, USA). The quantity and
quality of the extracted RNA was determined using a NanoDrop
Spectrophotometer (ND-2000; NanoDrop Technology, Wilmington, DE,
USA). The cDNA synthesis was performed at 42°C for 60 min in a
25-μl volume, which contained 2 μg RNA, 1.6 μM Oligo(dT)18, 0.6 μM
deoxyribonucleotides, 200 units Moloney murine leukemia virus
reverse transcriptase (Promega, Madison, WI, USA) and the reaction
buffer that was supplied.
qPCR
Following RT, the cDNA samples were diluted in
RNAse-free water (ratio, 1:5). The primer sequences used in the
current study are presented in Table
II. Each reaction used the SYBR® Green mix
(Invitrogen Life Technologies), a cDNA template, 10 μM forward
primer, 10 μM reverse primer and double-distilled H2O in
a total volume of 20 μl, and was performed on the ABI 7500
Real-Time PCR System (Applied Biosystems Life Technologies, Foster
City, CA, USA). The reaction conditions were as follows:
Denaturation at 94°C for 60 sec, 40 cycles of annealing at 55°C for
60 sec and extension at 72°C for 60 sec. The program was set to
automatically record the average fluorescence value of the last 10%
of time in the final cycle, which was equal to the quantity of
amplification at the end of each cycle. Following completion of the
reactions, the baseline and threshold were adjusted on the ABI 7500
Real-Time PCR System software, where the cycle threshold (Ct) value
of each reaction well was read. The data were analyzed according to
the comparative Ct method and normalized according to the GAPDH
expression in each sample. The primers were designed according to
the cDNA sequences in the GenBank database (National Center for
Biotechnology Information, Bethesda, MD, USA) using Primer Express
software (Applied Biosystems Life Technologies) and are listed in
Table II. The qPCR was performed
in triplicate.
 | Table IIPolymerase chain reaction primers and
conditions. |
Table II
Polymerase chain reaction primers and
conditions.
| Gene | Primer | Temperature
(°C) | Product size
(bp) |
|---|
| Per1 |
5′-AGGCAACGGCAAGGACTC-3′
5′-GGCTGTAGGCAATGGAACTG-3′ | 60.2 | 101 |
| Per2 |
5′-CTACAGCAGCACCATCGTC-3′
5′-CCACTCGCAGCATCTTCC-3′ | 58.9 | 78 |
| Per3 |
5′-TGGTGGTGGTGAATGTAAGAC-3′
5′-GGCTGTGCTCATCGTTCC-3′ | 57.2 | 104 |
| Cry1 |
5′-CAACCTCCATTCATCTTTCC-3′
5′-CTCATAGCCGACACCTTC-3′ | 58.9 | 151 |
| Cry2 |
5′-TGGGCTTCTGGGACTGAG-3′
5′-GGTAGGTGTGCTGTCTTAGG-3′ | 57.2 | 136 |
| CLOCK |
5′-GCAGCAGCAGCAGCAGAG-3′
5′-CAGCAGAGAGAATGAGTTGAGTTG-3′ | 61.9 | 149 |
| BmalI |
5′-TGCCACCAATCCATACACAGAAG-3′
5′-TTCCCTCGGTCACATCCTACG-3′ | 60.9 | 123 |
| CKIɛ |
5′-TCAGCGAGAAGAAGATGTC-3′
5′-GAAGAGGTTGCGGAAGAG-3′ | 58.9 | 149 |
| β-actin |
5′-CCCATTTATGAGGGCTACGCG-3′
5′-CGATGAAGGAGGGCTGGAAGA-3′ | 61.9 | 313 |
| GAPDH |
5′-GCGCTGAGTACGTCGTGGAG-3′
5′-GCTGATGATCTTGAGGCTGTTG-3′ | 59.8 | 173 |
| HBx |
5′-CGTCCTTTGTCTACGTCCCG-3′
5′-AAGTTGCATGGTGCTGGTGA-3′ | 59.4 | 408 |
Protein preparation and western blot
analyses
Cells were collected and lysed in lysis buffer
containing 50 mmol/l Tris-HCl (pH 8.5), 150 mmol/l NaCl, 0.2 g/l
NaN3, 0.1 g/l SDS, 100 μg/ml phenylmethylsulfonyl
fluoride, 1 μg/ml aprotinin, 10 ml/l NP-40, and 5 g/l sodium
deoxycholate. Cells were centrifuged at 20,800 × g for 15 min to
remove the cellular debris. The protein concentrations were
determined by the Bradford method. A total of 30–50 μg of protein
was separated by sodium dodecyl sulfate (SDS)-polyacrylamide gel
electrophoresis using 12% SDS polyacrylamide gels, transferred to
polyvinylidene fluoride membranes, blocked in 5% non-fat milk in
Tris-buffered saline containing 0.1% Tween-20 for 2 h, incubated
with primary mouse monoclonal antibodies raised against baculovirus
expressed recombinant Hep B xAg (obtained from Santa Cruz
Biotechnology, Inc. (sc-57760, Dallas, TX, USA) for HBx (1:400),
and β-actin raised against gizzard actin (1:2,000; sc-47778) for 1
h at 37°C and incubated overnight at 4°C, followed by a 1-h
incubation with the appropriate horse-radish peroxidase conjugated
monoclonal secondary goat anti-mouse IgG antibody. The bands were
visualized using an enhanced chemiluminescence (ECL) detection
system (Thermo Fisher Scientific Inc., Rockford, IL, USA).
Statistical analysis
Data were expressed as means ± standard deviation
and were analyzed using SPSS version 13.0 (SPSS Inc., Chicago, IL,
USA). The gene expression levels in the liver cancer tissues were
compared with those of the adjacent normal tissues using the
Wilcoxon test. In addition, the differences between the
Bel-7404-HBx and control cells were analyzed using the Mann-Whitney
U test and P<0.05 was considered to indicate a statistically
significant difference.
Results
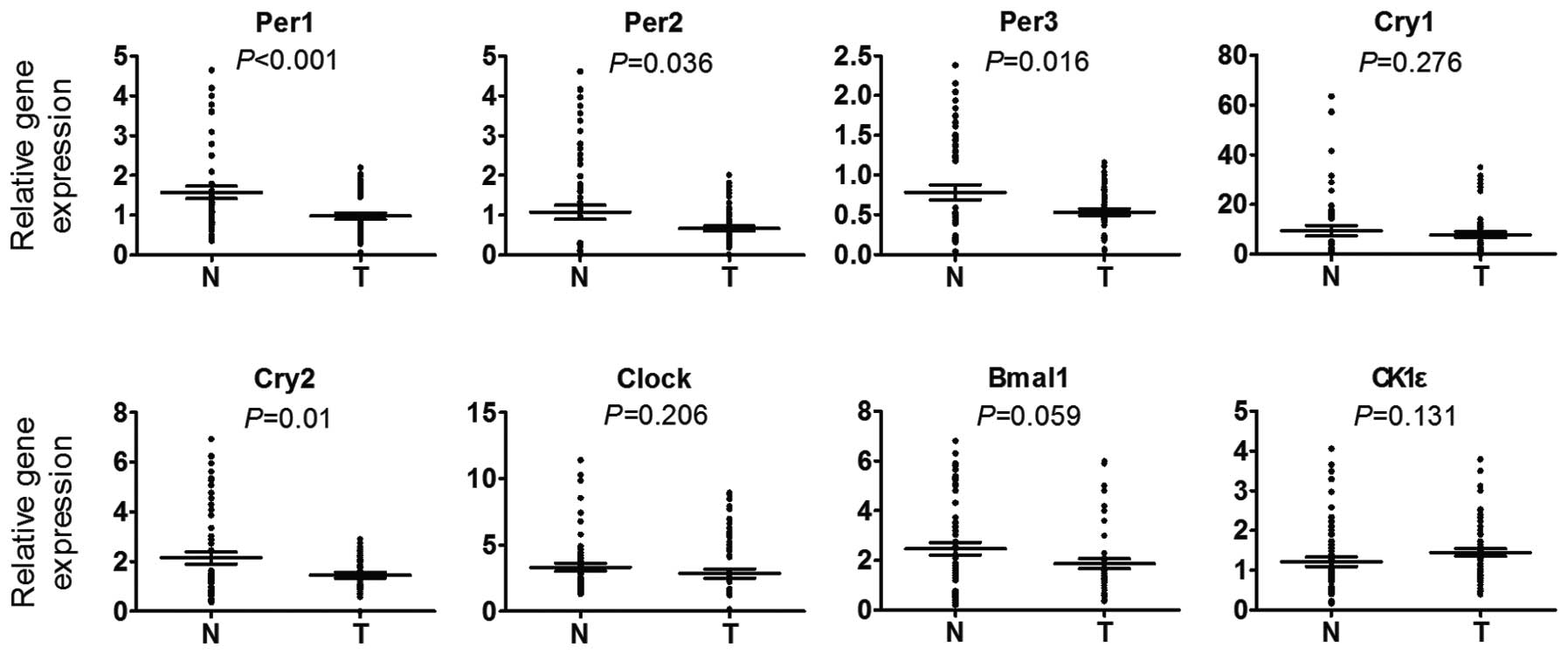
Disturbance of the expression of
circadian clock genes is commonly identified in HCC
The mRNA levels of circadian clock genes were first
determined in 30 HCC and the paired peritumorous tissues using
RT-qPCR. It was found that the mRNA levels of Per1,
Per2, Per3 and Cry2 in HCC tissues were
markedly decreased in comparison with those in the paired
peritumoral tissues, while no significant difference was observed
in the mRNA levels of CLOCK, BMAL1, Cry1 and
CK1ɛ compared with the peritumoral tissues (Fig. 1). In these cases, the majority
(70–90%) of the HCC cancerous tissues exhibited downregulation of
the circadian clock gene, whereas the remaining cases were either
downregulated in the non-cancerous tissues or were cases in which
no differential expression of the circadian clock genes was
detected. In particular, Per1 and Per3 downregulation
was found in 27 (90%) out of the 30 cases analyzed and only three
cases (10%) exhibited Per1 and Per2 upregulation in
the HCC tissues. It was also found that the majority of the HCC
tissues exhibited downregulation of at least four circadian clock
genes. Therefore, it could be inferred that the disturbance of
circadian clock gene expression is a common event in HCC and
results in the disruption of normal circadian rhythm in cancerous
cells.
HBx causes disturbance of circadian clock
gene expression in HCC cells
HBx is a multifunctional protein that plays a vital
role in the development and progression of HCC. To identify the
influence of HBx on the expression of circadian genes, a stable
HBx-transfected Bel-7404 cell line was established, termed
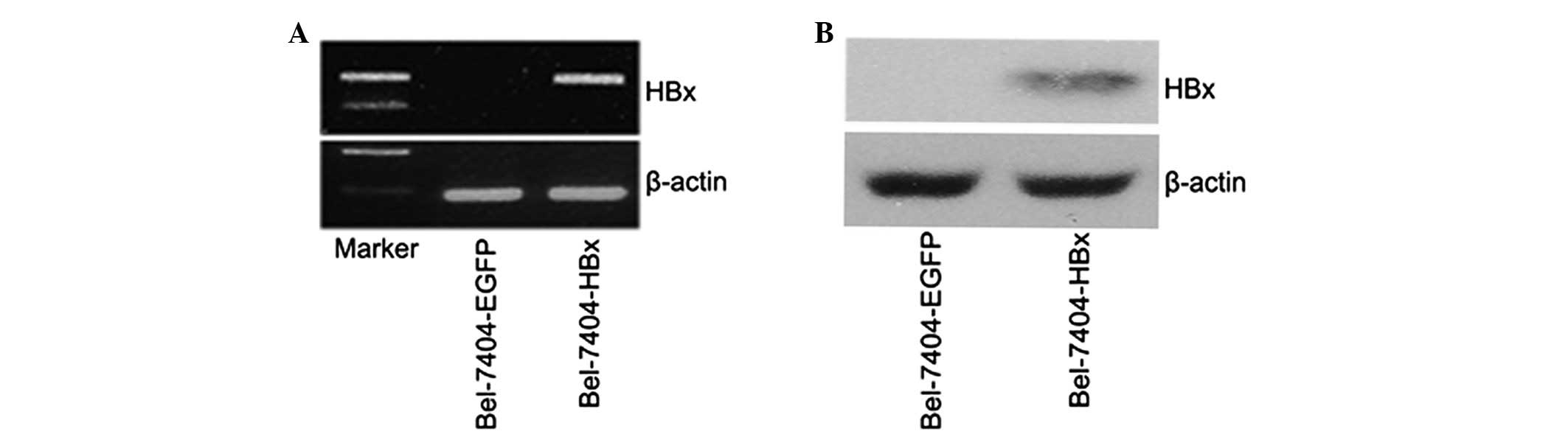
Bel-7404-HBx cells. Subsequently, HBx mRNA and protein expression
was identified in the HBx-transfected cells. As shown in Fig. 2A, HBx mRNA was highly expressed in
the Bel-7404-HBx cells, with no HBx mRNA detected in the control
cells. In addition, western blot analysis detected the 17-kDa HBx
protein in the lysates of the Bel-7404-HBx cells (Fig. 2B). Therefore, HBx was successfully
transfected and expressed in the Bel-7404-HBx cells. The expression
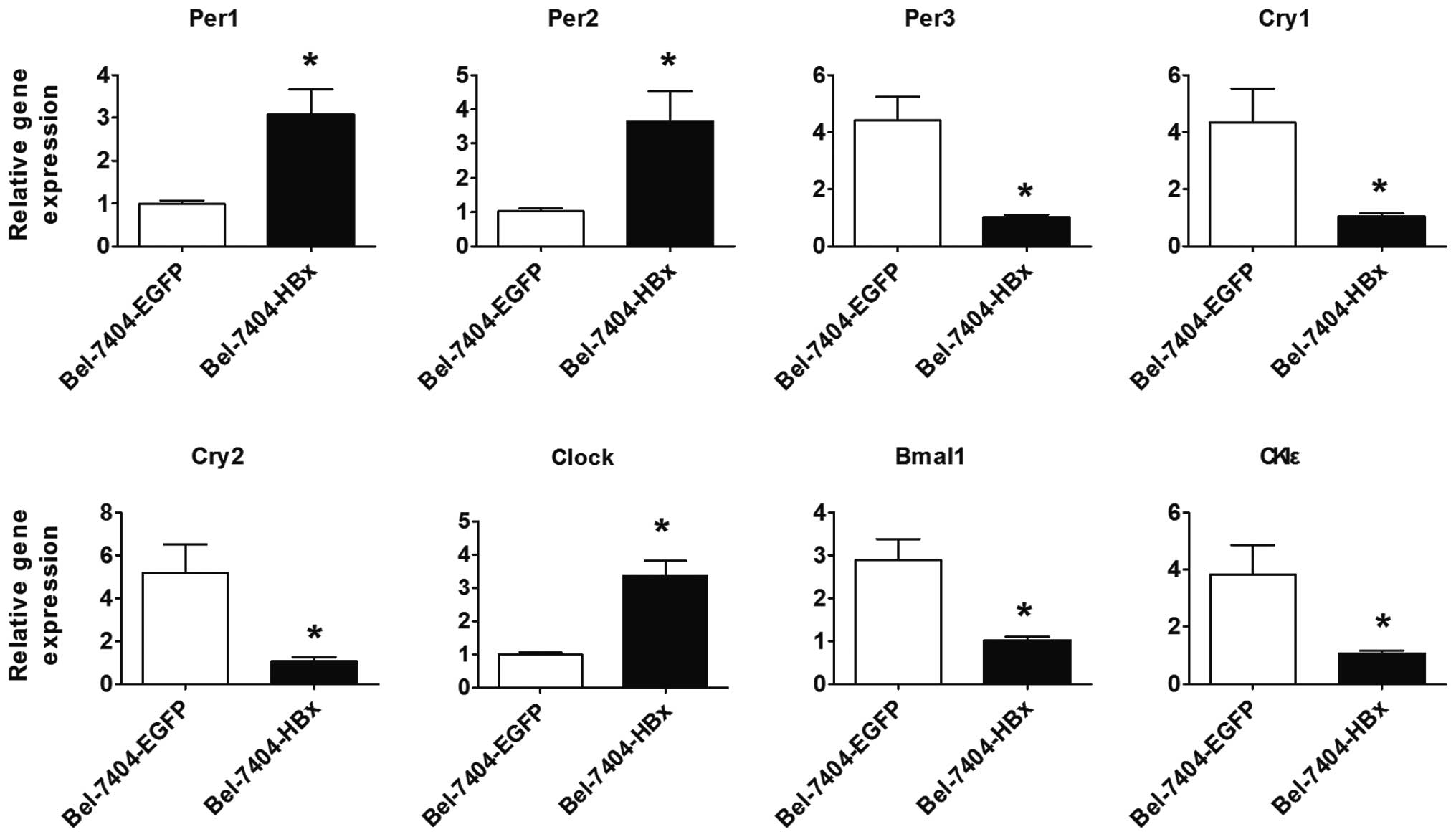
of circadian clock genes in these cells was assessed. Compared with
the control cells, the expression of CLOCK, Per1 and
Per2 mRNA was significantly increased in the Bel-7404-HBx
cells, while the expression of BMAL1, Per3,
Cry1, Cry2 and CKIɛ mRNA was significantly
decreased (Fig. 3; P<0.05).
Therefore, HBx may disturb circadian clock gene expression in HCC
cells.
Discussion
An increasing number of studies have demonstrated
that breast, colon and prostate cancers are more common among
individuals whose circadian rhythms are constantly disturbed as a
result of shift work, jet lag or increased exposure to light at
night (16,21,22).
Further studies have revealed that the disturbance of circadian
clock gene expression is particularly common in various
malignancies (15,23,24),
and that circadian clocks guide a number of cancer-related genes,
including genes that regulate cell division, DNA repair and
apoptosis (25,26). Therefore, the disruption of
circadian clock genes and their downstream clock-controlled genes
may enhance cancer development, and this viewpoint has already been
demonstrated in murine cancer models (27,28).
The association between circadian clock genes and
HCC has not been fully elucidated. The present study has found that
the mRNA levels of Per1, Per2, Per3 and
Cry2 in HCC cancerous tissues were significantly lowered
compared with the paired peritumoral tissues, while no significant
difference was observed in the expression levels of CLOCK,
BMAL1, Cry1 and CK1ɛ. This finding was
consistent with the study by Lin et al (19), which revealed that the expression of
Per1, Per2, Per3 and Cry2 was lower in
HCC tissue samples. The expression of these genes was also found to
be lower in colorectal and pancreatic cancer (18,29).
However, it was demonstrated that there was a significant
correlation between the low levels of Per1, Per2,
Per3, and Cry2 and poor patient survival rates,
implying that these genes may be closely associated with the
carcinogenesis and development of abdominal cancer (18,29).
The Per genes have been studied extensively
and it has been proposed that these genes act as tumor suppressors,
with prior reports indicating that decreased Per gene
expression is associated with tumorigenesis and disease progression
(30,31). The molecular link between Per
genes and cell cycle control incorporates their inhibitory effect
on cyclin D1, c-Myc and Wee1, leading to either a repressed
G1-S transition or an enhanced G2-M
transition (32,33). Per1 and Per2 are
involved in the DNA damage response signaling pathways, perhaps as
cofactors with checkpoint kinase 2 (Chk2) for the activation of
ataxia telangiectasia mutated (ATM). In murine models, Per2
mutations result in temporal changes in mRNA expression for genes
associated with cell cycle regulation and tumor suppression,
including c-Myc, cyclins and mouse double minute 2 homolog,
resulting in an impaired DNA damage response and accelerated tumor
growth (34,35). Per3 interacts with ATM and
Chk2. In addition, silencing of Per3 expression terminates
Chk2 activation upon induction of DNA damage as Per3
overexpression alone activates Chk2, resulting in decreased
cellular proliferation and apoptosis (36).
Although circadian clock genes were found to be the
most prevalent, abnormally expressed genes in the tumor cells the
underlying mechanisms of circadian rhythm disorders remain unclear.
The present study reveals that HBx may alter the expression of the
circadian clock genes at the level of mRNA in HCC cell lines. The
expression of CLOCK, Per1, and Per2 mRNA was
upregulated, while the expression of BMAL1, Per3,
Cry1, Cry2 and CKIɛ mRNA was downregulated.
This observation may be an alternative cause of HBx-induced HCC
carcinogenesis and development. Therefore, a comparison between the
alteration trends of HCC tissues and HBx-expressing HCC cells was
made. It was found that only Per3 and Cry2 were
downregulated in the two cell lines; therefore, HBx disturbs the
expression of circadian clock genes, however, it may not be the
major regulator in HCC.
Further investigation is required to fully elucidate
the influence of HBx on the circadian clock, and its association
with carcinogenesis and development of HCC. In addition, more
studies are necessary to obtain an improved understanding of the
association between circadian rhythm disruption and HCC, which may
broaden current knowledge on the occurrence and development of HCC
(37). This association may provide
novel approaches and methods for the treatment of HCC.
Acknowledgements
The present study was supported by a grant from The
National Natural Science Foundation of China (grant no.
81160311).
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010.
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011.
|
|
3
|
Xu C, Zhou W, Wang Y and Qiao L: Hepatitis
B virus-induced hepatocellular carcinoma. Cancer Lett. 345:216–222.
2014.
|
|
4
|
Guerrieri F, Belloni L, Pediconi N and
Levrero M: Molecular mechanisms of HBV-associated
hepatocarcinogenesis. Semin Liver Dis. 33:147–156. 2013.
|
|
5
|
Liu LP, Liang HF, Chen XP, et al: The role
of NF-kappaB in Hepatitis b virus X protein-mediated upregulation
of VEGF and MMPs. Cancer Invest. 28:443–451. 2010.
|
|
6
|
Xia L, Huang W, Tian D, et al: Upregulated
FoxM1 expression induced by hepatitis B virus X protein promotes
tumor metastasis and indicates poor prognosis in hepatitis B
virus-related hepatocellular carcinoma. J Hepatol. 57:600–612.
2012.
|
|
7
|
Barclay JL, Tsang AH and Oster H:
Interaction of central and peripheral clocks in physiological
regulation. Prog Brain Res. 199:163–181. 2012.
|
|
8
|
Eckel-Mahan K and Sassone-Corsi P:
Metabolism and the circadian clock converge. Physiol Rev.
93:107–135. 2013.
|
|
9
|
Li JD, Hu WP and Zhou QY: The circadian
output signals from the suprachiasmatic nuclei. Prog Brain Res.
199:119–127. 2012.
|
|
10
|
Dibner C, Schibler U and Albrecht U: The
mammalian circadian timing system: organization and coordination of
central and peripheral clocks. Annu Rev Physiol. 72:517–549.
2010.
|
|
11
|
Mazzoccoli G, Pazienza V and Vinciguerra
M: Clock genes and clock-controlled genes in the regulation of
metabolic rhythms. Chronobiol Int. 29:227–251. 2012.
|
|
12
|
Isojima Y, Okumura N and Nagai K:
Molecular mechanism of mammalian circadian clock. J Biochem.
134:777–784. 2003.
|
|
13
|
Eide EJ and Virshup DM: Casein kinase I:
another cog in the circadian clockworks. Chronobiol Int.
18:389–398. 2001.
|
|
14
|
Greene MW: Circadian rhythms and tumor
growth. Cancer Lett. 318:115–123. 2012.
|
|
15
|
Savvidis C and Koutsilieris M: Circadian
rhythm disruption in cancer biology. Mol Med. 18:1249–1260.
2012.
|
|
16
|
Brudnowska J and Pepłońska B: Night shift
work and cancer risk: a literature review. Med Pr. 62:323–338.
2011.(In Polish).
|
|
17
|
Leonardi GC, Rapisarda V, Marconi A, et
al: Correlation of the risk of breast cancer and disruption of the
circadian rhythm (Review). Oncol Rep. 28:418–428. 2012.
|
|
18
|
Relles D, Sendecki J, Chipitsyna G, Hyslop
T, Yeo CJ and Arafat HA: Circadian gene expression and
clinicopathologic correlates in pancreatic cancer. J Gastrointest
Surg. 17:443–450. 2013.
|
|
19
|
Lin YM, Chang JH, Yeh KT, et al:
Disturbance of circadian gene expression in hepatocellular
carcinoma. Mol Carcinog. 47:925–933. 2008.
|
|
20
|
Edge SB, Byrd DR, Compton CC, et al: AJCC
Cancer Staging Manual. 7th edition. Springer; New York, NY:
2010
|
|
21
|
Menegaux F, Truong T, Anger A, et al:
Night work and breast cancer: a population-based case-control study
in France (the CECILE study). Int J Cancer. 132:924–931. 2013.
|
|
22
|
Lahti T, Merikanto I and Partonen T:
Circadian clock disruptions and the risk of cancer. Ann Med.
44:847–853. 2012.
|
|
23
|
Chen R, Yang K, Zhao NB, et al: Abnormal
expression of PER1 circadian-clock gene in oral squamous cell
carcinoma. Onco Targets Ther. 5:403–407. 2012.
|
|
24
|
Mazzoccoli G, Panza A, Valvano MR, et al:
Clock gene expression levels and relationship with clinical and
pathological features in colorectal cancer patients. Chronobiol
Int. 28:841–851. 2011.
|
|
25
|
Canaple L, Kakizawa T and Laudet V: The
days and nights of cancer cells. Cancer Res. 63:7545–7552.
2003.
|
|
26
|
Schibler U: The daily timing of gene
expression and physiology in mammals. Dialogues Clin Neurosci.
9:257–272. 2007.
|
|
27
|
Filipski E, Subramanian P, Carrière J,
Guettier C, Barbason H and Levi F: Circadian disruption accelerates
liver carcinogenesis in mice. Mutat Res. 680:95–105. 2009.
|
|
28
|
Logan RW, Zhang C, Murugan S, et al:
Chronic shift-lag alters the circadian clock of NK cells and
promotes lung cancer growth in rats. J Immunol. 188:2583–2591.
2012.
|
|
29
|
Karantanos T, Theodoropoulos G, Gazouli M,
et al: Expression of clock genes in patients with colorectal
cancer. Int J Biol Markers. 28:280–285. 2013.
|
|
30
|
Yang X, Wood PA, Ansell C and Hrushesky
WJ: Circadian time-dependent tumor suppressor function of period
genes. Integr Cancer Ther. 8:309–316. 2009.
|
|
31
|
Hwang-Verslues WW, Chang PH, Jeng YM, et
al: Loss of corepressor PER2 under hypoxia up-regulates
OCT1-mediated EMT gene expression and enhances tumor malignancy.
Proc Natl Acad Sci USA. 110:12331–12336. 2013.
|
|
32
|
Lee CC: Tumor suppression by the mammalian
Period genes. Cancer Causes Control. 17:525–530. 2006.
|
|
33
|
Chen-Goodspeed M and Lee CC: Tumor
suppression and circadian function. J Biol Rhythms. 22:291–298.
2007.
|
|
34
|
Fu L, Pelicano H, Liu J, Huang P and Lee
C: The circadian gene Period2 plays an important role in tumor
suppression and DNA damage response in vivo. Cell. 111:41–50.
2002.
|
|
35
|
Wood PA, Yang X and Hrushesky WJ: Clock
genes and cancer. Integr Cancer Ther. 8:303–308. 2009.
|
|
36
|
Im JS, Jung BH, Kim SE, Lee KH and Lee JK:
Per3, a circadian gene, is required for Chk2 activation in human
cells. FEBS Lett. 584:4731–4734. 2010.
|
|
37
|
Vinciguerra M, Mazzoccoli G, Piccoli C,
Tataranni T, Andriulli A and Pazienza V: Exploitation of host clock
gene machinery by hepatitis viruses B and C. World J Gastroenterol.
19:8902–8909. 2013.
|