Introduction
C-ros oncogene 1, receptor tyrosine kinase
(ROS1) gene rearrangements are reported in 1–2% of lung
adenocarcinomas and are sensitive to the small-molecule tyrosine
kinase inhibitor crizotinib (1,2).
Fluorescent in situ hybridization (FISH) is the gold
standard technique used for detecting ROS1-positive adenocarcinomas
and determining whether the cancer would respond well to crizotinib
treatment. However, FISH is an expensive and time-consuming assay,
requiring specialized microscopy equipment and technical expertise
(1). Currently there are no reports
of the use of immunohistochemistry (IHC) in the detection of
ROS1 rearrangements in order to guide treatment of advanced
non-small cell lung cancer (NSCLC). The present study reports the
case of a patient with advanced lung adenocarcinoma who was
ROS1-positive by IHC, but negative by FISH; the patient was
determined to have had a partial response to crizotinib. Written
informed consent was obtained from the patient’s family.
Case report
A 36-year-old male presented to the Department of
Oncology of Changzhou Tumor Hospital (Changzhou, China) with a
persistent cough, which he had suffered with for two years, 1-day
hemoptysis and shortness of breath. The patient was currently
smoking 100 packets of cigarettes per year and had an otherwise
unremarkable medical history.
A physical examination revealed decreased breath
sounds in both lung fields, however no other signs were revealed.
Positron emission tomography/computed tomography (CT) scans
revealed a mass measuring 6.3×6.2 cm in the right middle lung lobe,
and the maximum standardized uptake value (SUVmax) was
measured as 9.4, with atelectasis of the right lower lobe. There
were multiple ground-glass opacities (GGOs) in both lungs, the
largest of which was positioned in the right apicoposterior
segmental lung lobe, which measured ~2.0×2.5 cm, and the
SUVmax was 2.4. The supraclavicular lymph nodes were
grossly enlarged on both sides. There was no metastasis observed in
any other organ.
A fiber-optic bronchoscopy detected a stenotic
lesion at the opening of the right middle lobe bronchus. A
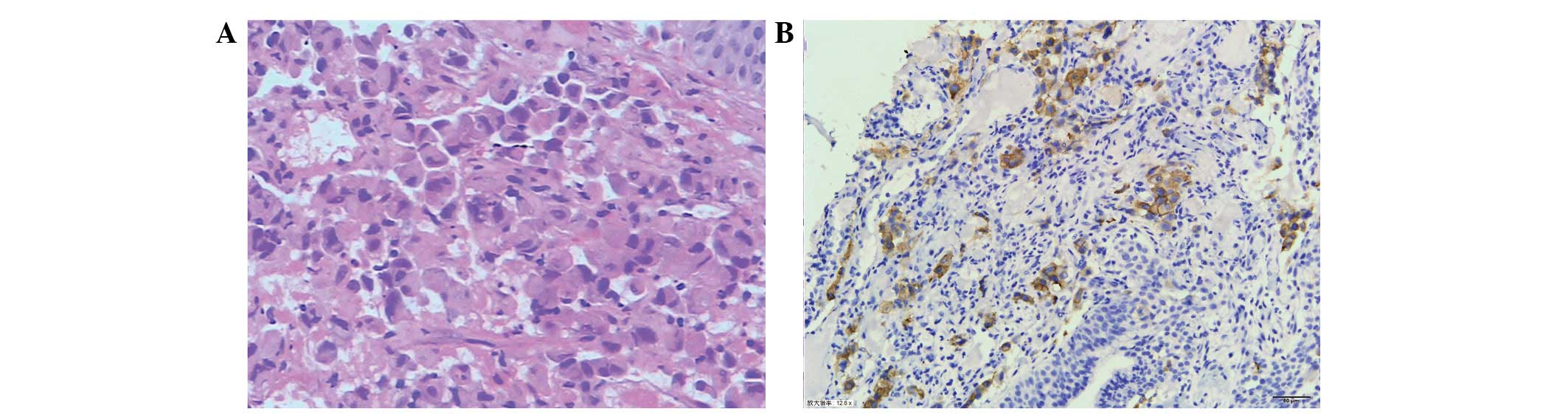
bronchoscopic biopsy confirmed the diagnosis of adenocarcinoma
(Fig. 1A). Genotype analysis, using
an amplification refractory mutations system, determined that the
patient had expression of wild-type epidermal growth factor
(EGFR), a commonly mutated oncogene. FISH and IHC showed
that the patient was negative for the echinoderm
microtubule-associated protein-like 4 (EML4)-anaplastic lymphoma
kinase (ALK) fusion protein. The patient’s clinical stage was
determined as T4(IpsiNod) N3M1a (contralateral lung)
(stage IV).
One cycle of chemotherapy was administered,
containing pemetrexed (500 mg/m2, d1) and cisplatin (75
mg / m2, d1). One month later, the patient’s disease had
progressed; the shortness of breath had worsened, and the pulmonary
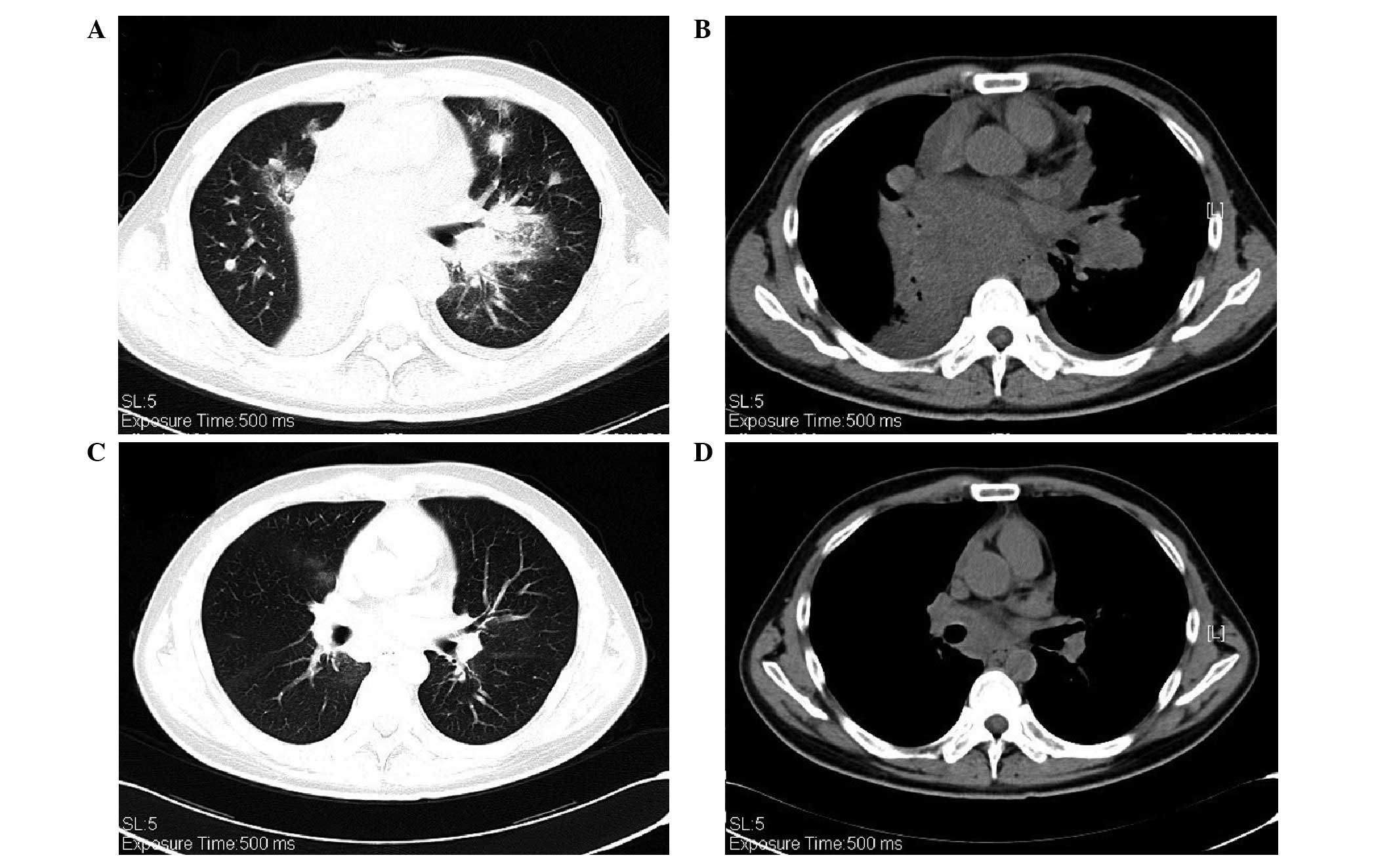
stenosis (PS) had deteriorated from 1 to 3. The CT scans showed
that the targeted lesion in the right middle lobe had shrunk,;
however, the nodules in the left lung had become enlarged, and
there was a new lesion measuring ~1.0×1.3 cm at the hilus of the
left lung. The presence of GGOs in both lungs had increased and
enlarged (Fig. 2A and B). The ROS1
protein was detected using IHC, with a staining intensity of
>70% 2+ or 3+ (Fig. 1B)
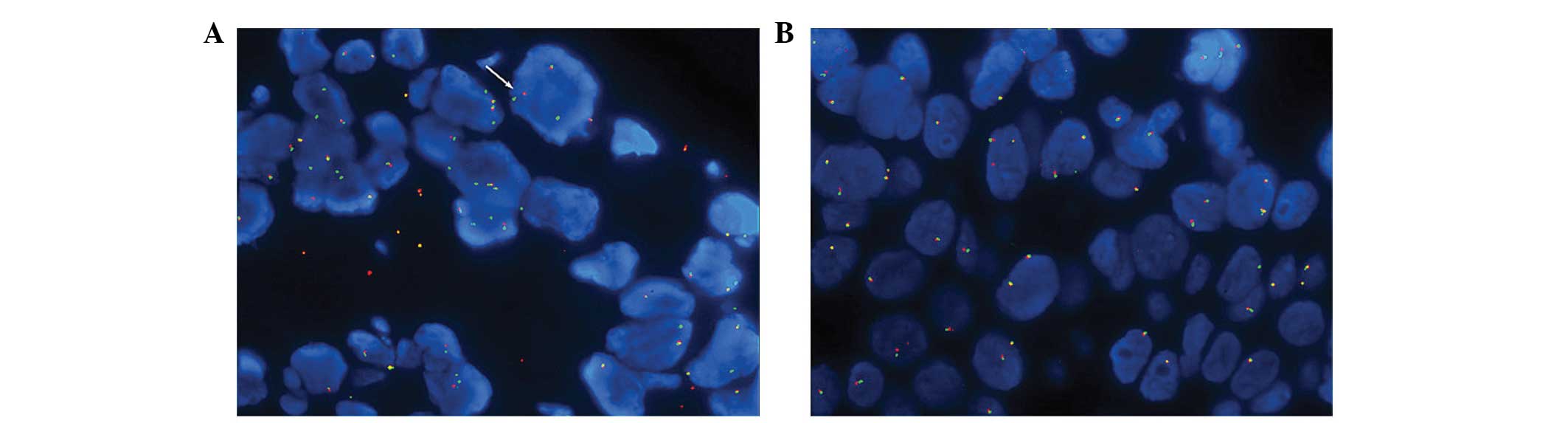
(3). Furthermore, a break-apart
FISH procedure was used to test for ROS1 gene
rearrangements; however, the result was negative when the cutoff
value was set at 15% (Fig. 3A and
B) (4).
The patient had severe PS and was being treated with
no other targeted therapies, therefore crizotinib treatment was
orally administered at the standard dose of 250 mg twice daily. One
week later, the patient’s symptoms had improved and the PS was
measured at 1, a decrease as compared with the previous
measurement. Following four weeks of crizotinib treatment, the CT
scans detected an improvement; the targeted lesions in the right
lung and left lung hilus had shrunk and the GGOs had almost
disappeared (Fig. 2C and D).
According to the Response Evaluation Criteria in Solid Tumors
criteria, version 1.1 (5), the
patient’s cancer had elicited a partial response to the crizotinib
treatment.
Discussion
Alongside mutations in the EGFR gene and
EML4-ALK rearrangements, ROS1 rearrangements have
been shown to define a new molecular subgroup in NSCLC (6). ROS1 shares a 49% amino acid sequence
homology with EML4-ALK in the kinase domains, and several EML4-ALK
inhibitors have demonstrated an inhibitory effect against ROS1
in vitro (7). Updated
efficacy and safety data have been presented for the use of
crizotinib in patients with advanced ROS1 rearrangements in
NSCLC (8). An ongoing phase I
clinical trial, investigating the effects of crizotinib
(NCT00585195), enrolled patients with advanced
ROS1-rearranged NSCLC, as determined by FISH. The patients
receive a standard oral dose of crizotinib: 250 mg twice daily. As
observed in EML4-ALK-positive NSCLC, treatment with
crizotinib has so far shown promising antitumor activity, with an
overall response rate of 56% (2).
FISH is an expensive and difficult assay to support,
requiring specialized microscopy equipment and technical expertise.
By contrast, IHC is relatively cheap in comparison and can be
performed in numerous pathology laboratories (1,9). A
previous study showed that if IHC thresholds of 2+ and 3+ are
considered to indicate positive expression, ROS1 IHC is 100%
sensitive and 92% specific for ROS1 rearrangements with FISH
(1).
To the best of our knowledge, the present case
report is the first to show positive ROS1-testing by IHC, but
negative by FISH where the patient responded well to crizotinib
treatment. These results provide evidence that IHC may be a useful
screening method for the detection of ROS1 rearrangements,
thus allowing for targeted precision therapy.
Acknowledgements
The present study was supported by the Key Lab
System Project of Guangdong Science and Technology Department -
Guangdong Key Lab of Lung Cancer Translational Medicine (China).
This work was also supported by a grant awarded to Professor
Yi-Long Wu, supplied by the National Natural Science Foundation of
China (grant no. 81272618).
References
|
1
|
Sholl LM, Sun H, Butaney M, et al: ROS1
immunohistochemistry for detection of ROS1-rearranged lung
adenocarcinomas. Am J Surg Pathol. 37:1441–1449. 2013.
|
|
2
|
Ou SI, Bang Y, Camidge DR, et al: Efficacy
and safety of crizotinib in patients with advanced
ROS1-rearranged non-small cell lung cancer (NSCLC). J Clin
Oncol. 31:80322013.
|
|
3
|
Kang W, Tong JH, Chan AW, et al:
Yes-associated protein 1 exhibits oncogenic property in gastric
cancer and its nuclear accumulation associates with poor prognosis.
Clin Cancer Res. 17:2130–2139. 2011.
|
|
4
|
Davies KD, Le AT, Theodoro MF, et al:
Identifying and targeting ROS1 gene fusions in non-small cell lung
cancer. Clin Cancer Res. 18:4570–4579. 2012.
|
|
5
|
Eisenhauer EA, Therasse P, Bogaerts J, et
al: New response evaluation criteria in solid tumours: revised
RECIST guideline (version 1.1). Eur J Cancer. 45:228–247. 2009.
|
|
6
|
McLeer Florin A, Mescam-Mancini L,
Moro-Sibilot D, et al: Detection of ROS1 translocations in
triple-negative lung adenocarcinomas. J Clin Oncol.
31:80992013.
|
|
7
|
Ou SH, Tan J, Yen Y and Soo RA: ROS1 as a
‘druggable’ receptor tyrosine kinase: lessons learned from
inhibiting the ALK pathway. Expert Rev Anticancer Ther. 12:447–456.
2012.
|
|
8
|
Shaw AT, Camidge DR, Engelman JA, et al:
Clinical activity of crizotinib in advanced non-small cell lung
cancer (NSCLC) harboring ROS1 gene rearrangement. J Clin Oncol.
30:75082012.
|
|
9
|
Cai W, Li X, Su C, et al: ROS1 fusions in
Chinese patients with non-small-cell lung cancer. Ann Oncol.
24:1822–1827. 2013.
|