Introduction
Post-transplant lymphoproliferative disorder (PTLD)
is a rare, but critical complication that occurs following solid
organ and hematopoietic stem cell transplantation (1). PTLD encompasses a heterogeneous group
of disorders, ranging from benign self-limited lesions to
aggressive widely disseminated disease. Generally, PTLD is
considered to be an iatrogenic complication due to the intensive
immunosuppressive treatment administered following transplantation
(2). The overall incidence of PTLD
in adult kidney transplantation surgery was between 1 and 3%
worldwide (3). However, early-onset
PTLD (<1 year from transplantation to presentation of PTLD) is
closely associated with the Epstein-Barr virus (EBV) infection and
exhibits a predilection for allograft localization (4), occasionally occuring at sites adjacent
to the allograft. The current study describes a rare case of PTLD
that presented as a tumor adjacent to the allograft within the
first year following renal transplantation. The treatment strategy
included surgical resection that was followed by a reduction in
immunosuppression and low-dose rituximab-based chemotherapy. This
study may lead to future improvements for the treatment of
post-transplant lymphoproliferative disorder. Written informed
consent was obtained from the patient.
Case report
In March 2012, a 42-year-old male exhibiting
end-stage renal disease secondary to hypertension received a kidney
transplant at the Second Xiangya Hospital of Central South
University (Changsha, China). The donor was a 27-year-old male who
had succumbed to cardiac failure caused by craniocerebral trauma.
The human leukocyte antigen (HLA) type of the donor and the
recipient were A11/A2-B58/B13-DR4/DR53, DR9/DR53 and
A2/A2-B13/B61-DR15/DR51, DR9/DR53, respectively. The recipient was
EBV seronegative, however, the donor’s EBV serologic status was
unknown, as EBV serologic status was not tested routinely at the
time of donation. Furthermore, the donor and recipient were
seronegative for cytomegalovirus, and the Hepatitis B and C
viruses. There were no complications during surgical follow-up. The
patient’s post-transplant immunosuppressive regimen included:
Intravenous methylprednisolone (dose during surgery, 0.5 g; dose
for the first three days following surgery, 0.5 g/day); followed by
cyclosporine (CsA; initial dose, 6 mg/kg/day; trough concentration
was adjusted to 220–250 ng/ml 0–6 months following transplantation
and 180–220 ng/ml over the next 6–12 months); oral mycophenolate
mofetil (MMF; dose, 0.75 g per 12 h; gradually reduced to 0.5 g per
12 h for long-term maintenance immunosuppression); and prednisolone
(initial dose, 80 mg/day; gradually reduced to 10 mg/day over the
next 6 months for long-term maintenance immunosuppression). Good
renal allograft function was observed immediately following
surgery. On the twelfth postoperative day, the patient exhibited a
serum creatinine level of 133 μmol/l (normal range, 44–133 μmol/l)
and was discharged. The allograft function remained normal during
the out-patient follow-up, however, seven months
post-transplantation, the patient developed a fever, oliguria and
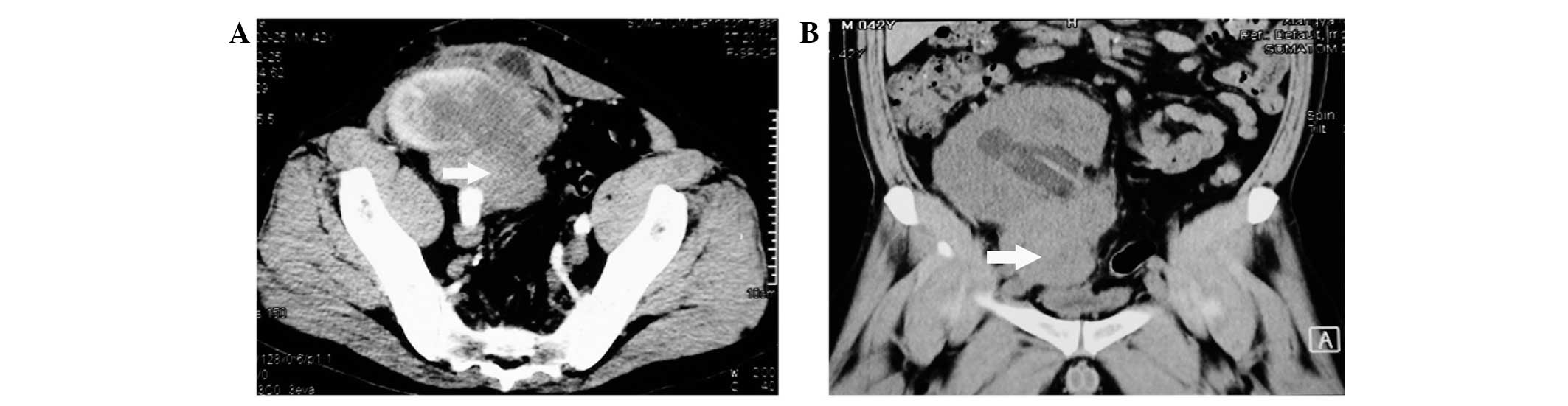
an elevated serum creatinine level (410.7 μmol/l). Sonography and
computed tomography revealed a solid mass adjacent to the renal
allograft (Fig. 1A) and computed
tomography with coronal multiplanar reformation revealed a solid
mass (size, 6×4×8 cm) in the lower pole of the allograft and
hydronephrosis (Fig. 1B).
Percutaneous nephrostomy tubes were inserted, resulting in a
decline in the serum creatinine level. Subsequently, surgical
resection was performed and a tumor with a poorly defined margin,
located adjacent and in close proximity to the lower pole of the
allograft, was removed. Postoperatively, serum creatinine returned
to within the normal range following treatment of the urinary
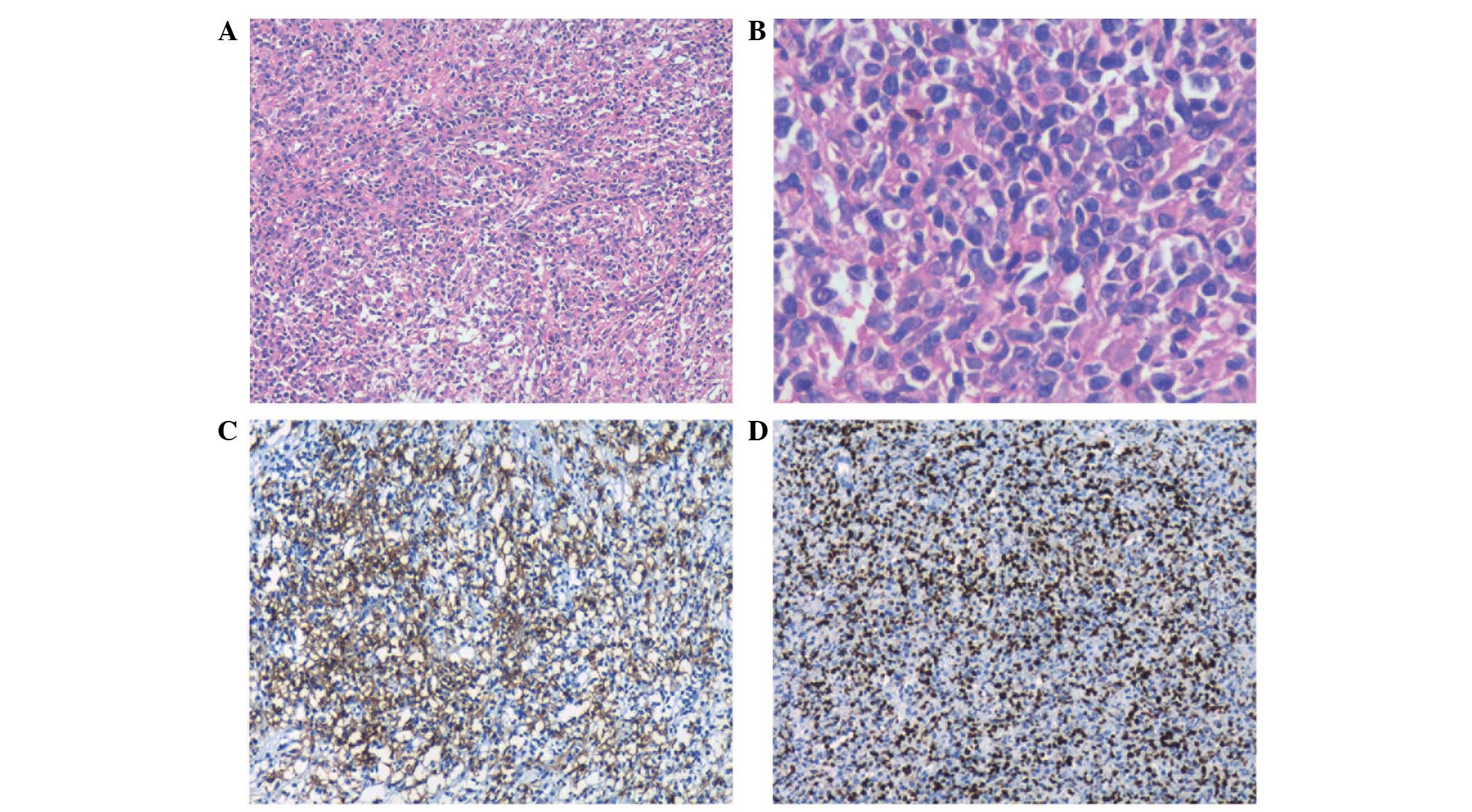
obstruction. Intra- and postoperative histopathological assessments
determined a diagnosis of polymorphic PTLD with positive stains for
cluster of differentiation (CD)20, CD79a, CD3 and EBV-encoded RNA
(Fig. 2). The proliferation index
of Ki-67 was 40%. Upon diagnosis, the blood EBV DNA level was
1.3×104 copies/ml, and the lactate dehydrogenase level
(normal range, 135–215 U/l) and bone marrow biopsy were normal.
Therefore, MMF therapy was discontinued and CsA was replaced with
Rapamune (Wyeth Pharmaceuticals, Dallas, TX, USA) (trough
concentration adjusted to 6–8 ng/ml) to reduce the level of
immunosuppression. Furthermore, four cycles of adjuvant low-dose
chemotherapy were administered, including rituximab (300
mg/m2), cyclophosphamide (500 mg/m2),
vincristine (1.2 mg/m2) and prednisolone (50
mg/m2). The patient’s blood was negative for EBV DNA
following the first cycle of chemotherapy. During the 16-month
follow-up after resection, the patient remained in remission,
neither EBV viremia nor PTLD recurred and renal allograft function
was preserved. Outpatient follow-up is ongoing to determine the
long-term outcome of the treatment strategy.
Discussion
PTLD is the second most commonly occurring
malignancy in solid organ transplant recipients, worldwide.
Approximately 20% of kidney transplant patients developed PTLD
within the first year following surgery (5). Early-onset PTLD occurs more commonly
in EBV seronegative recipients compared with EBV seropositive
recipients and is characterized by an EBV in situ
hybridization-positive, CD20-positive phenotype and allograft
involvement (6). An
immunosuppressed state and EBV infection are considered to be the
two most important risk factors in PTLD development (2). In the majority of EBV-associated cases
of PTLD, immunosuppression depresses the EBV-specific cellular
immune response, which may promote uncontrolled EBV-infected
lymphocyte proliferation, resulting in PTLD (7). The rare case described in the current
report presented as a tumor adjacent to the lower pole of the renal
allograft and developed into a urinary obstruction. To the best of
our knowledge, few similar cases have been reported to date
(8–12). In the present case, the risk factors
for the development of PTLD included EBV seronegativity prior to
transplantation, EBV infection post-transplantation, mismatching at
the HLA-B locus and a high dose of CsA (13,14).
No consensus on the optimal management of PTLD has
been determined, however, a reduction in immunosuppression (RI) has
been demonstrated to be an effective initial treatment modality for
PTLD. In a recent analysis of 148 solid organ transplant-associated
PTLD cases, Reshef et al (15) reported that the overall response
rate for a RI alone was 45% and the three year overall survival
rate was 55%. Recent guidelines recommend commencing the reduction
of immunosuppression therapy as soon as possible in all PTLD
patients (16) and in specific
cases of localized PTLD, surgical excision of isolated lesions or
debulking of the tumor may be an effective component of first-line
treatment. Reshef et al (15) identified that patients who underwent
surgery and adjuvant RI exhibited a favorable outcome, with 27%
patients relapsing at a median of five months. Rituximab is a
monoclonal antibody against the B lymphocyte-specific CD20 antigen
(17). Recent data indicates that
immediate commencement of rituximab-based therapy followed by
anthracycline-based chemotherapy (cyclophosphamide, doxorubicin,
vincristine and prednisolone) may result in durable
progressive-free survival in PTLD patients (18) and may reduce the risk of renal graft
impairment following the reduction of immunosuppression (19). In the present case, surgical
excision was performed for the diagnosis and resolution of the
urinary obstruction. RI, followed by rituximab-based therapy
combined with low-dose chemotherapy, for four months, the standard
dose is as follows: rituximab (375 mg/m2, day 1),
cyclophosphamide (400 mg/m2, days 1–5), vincristine (1.4
mg/m2, day 1) and prednisolone (100 mg/m2,
days 1–5) and was prescribed due to the aggressive nature of the
disease. The therapeutic strategy administered to the patient in
the present study resulted in complete remission with few
manageable side-effects, including nausea/vomiting and leukopenia.
Consistent with the present case, two previous studies reported
that surgical intervention in combination with other therapies
achieved durable remission in specific patients exhibiting
localized PTLD (20,21). The maintenance of immunosuppression
remains a challenge in renal recipients who develop PTLD. The use
of calcineurin inhibitors has been associated with an increased
incidence of PTLD (22), however,
treatment with rapamycin and its analogs has demonstrated
immunosuppression and antiproliferative action. Previous studies
demonstrated that rapamycin immunosuppressant therapy produced
favorable effects in kidney transplant patients exhibiting PTLD
(23,24). Therefore, serolimus was introduced
in the present case for maintenance immunosuppression. However, the
use of rapamycin in PTLD patients remains controversial (25), thus, further studies are required to
reach a consensus for the optimal management of PTLD.
In conclusion, the current report presents a rare
case of PTLD that manifested as an obstructive uropathy within the
first year following kidney transplantation. Surgical excision
followed by a reduction in immunosuppression and low-dose
rituximab-based chemotherapy may present as an effective and safe
strategy for specific cases of localized and resectable PTLD.
References
|
1
|
Everly MJ, Bloom RD, Tsai DE and Trofe J:
Posttransplant lymphoproliferative disorder. Ann Pharmacother.
41:1850–1858. 2007.
|
|
2
|
Dierickx D, Tousseyn T, De Wolf-Peeters C,
Pirenne J and Verhoef G: Management of posttransplant
lymphoproliferative disorders following solid organ transplant: an
update. Leuk Lymphoma. 52:950–961. 2011.
|
|
3
|
Caillard S, Lelong C, Pessione F and
Moulin B; French PTLD Working Group. Post-transplant
lymphoproliferative disorders occurring after renal transplantation
in adults: report of 230 cases from the French Registry. Am J
Transplant. 6:2735–2742. 2006.
|
|
4
|
Khedmat H and Taheri S: Early onset post
transplantation lymphoproliferative disorders: analysis of
international data from 5 studies. Ann Transplant. 14:74–77.
2009.
|
|
5
|
Morton M, Coupes B, Roberts SA, et al:
Epidemiology of posttransplantation lymphoproliferative disorder in
adult renal transplant recipients. Transplantation. 95:470–478.
2013.
|
|
6
|
Ghobrial IM, Habermann TM, Macon WR, et
al: Differences between early and late posttransplant
lymphoproliferative disorders in solid organ transplant patients:
are they two different diseases? Transplantation. 79:244–247.
2005.
|
|
7
|
Tsao L and Hsi ED: The clinicopathologic
spectrum of posttransplantation lymphoproliferative disorders. Arch
Pathol Lab Med. 131:1209–1218. 2007.
|
|
8
|
Kew CE II, Lopez-Ben R, Smith JK, et al:
Posttransplant lymphoproliferative disorder localized near the
allograft in renal transplantation. Transplantation. 69:809–814.
2000.
|
|
9
|
Palmer BF, Sagalowsky AI, McQuitty DA,
Dawidson I, Vazquez MA and Lu CY: Lymphoproliferative disease
presenting as obstructive uropathy after renal transplantation. J
Urol. 153:392–394. 1995.
|
|
10
|
Khedmat H and Taheri S: Characteristics
and prognosis of post-transplant lymphoproliferative disorders
within renal allograft: Report from the PTLD. Int Survey Ann
Transplant. 15:80–86. 2010.
|
|
11
|
Cosio FG, Nuovo M, Delgado L, et al: EBV
kidney allograft infection: possible relationship with a peri-graft
localization of PTLD. Am J Transplant. 4:116–123. 2004.
|
|
12
|
Caillard S, Porcher R, Provot F, et al:
Post-transplantation lymphoproliferative disorder after kidney
transplantation: report of a nationwide French registry and the
development of a new prognostic score. J Clin Oncol. 31:1302–1309.
2013.
|
|
13
|
Quinlan SC, Pfeiffer RM, Morton LM and
Engels EA: Risk factors for early-onset and late-onset
post-transplant lymphoproliferative disorder in kidney recipients
in the United States. Am J Hematol. 86:206–209. 2011.
|
|
14
|
Bakker NA, van Imhoff GW, Verschuuren EA,
et al: HLA antigens and post renal transplant lymphoproliferative
disease: HLA-B matching is critical. Transplantation. 80:595–599.
2005.
|
|
15
|
Reshef R, Vardhanabhuti S, Luskin MR, et
al: Reduction of immunosuppression as initial therapy for
posttransplantation lymphoproliferative disorder. Am J Transplant.
11:336–347. 2011.
|
|
16
|
Parker A, Bowles K, Bradley JA, et al:
Haemato-oncology Task Force of the British Committee for Standards
in Haematology and British Transplantation Society: Management of
post-transplant lymphoproliferative disorder in adult solid organ
transplant recipients - BCSH and BTS Guidelines. Br J Haematol.
149:693–705. 2010.
|
|
17
|
Svoboda J, Kotloff R and Tsai DE:
Management of patients with post-transplant lymphoproliferative
disorder: the role of rituximab. Transpl Int. 19:259–269. 2006.
|
|
18
|
Trappe R, Oertel S, Leblond V, et al;
German PTLD Study Group; European PTLD Network. Sequential
treatment with rituximab followed by CHOP chemotherapy in adult
B-cell post-transplant lymphoproliferative disorder (PTLD): the
prospective international multicentre phase 2 PTLD-1 trial. Lancet
Oncol. 13:196–206. 2012.
|
|
19
|
Trappe R, Hinrichs C, Appel U, et al:
Treatment of PTLD with rituximab and CHOP reduces the risk of renal
graft impairment after reduction of immunosuppression. Am J
Transplant. 9:2331–2337. 2009.
|
|
20
|
Foroncewicz B, Mucha K, Usiekniewicz J, et
al: Posttransplant lymphoproliferative disorder of the lung in a
renal transplant recipient treated successfully with surgery.
Transplant Proc. 38:173–176. 2006.
|
|
21
|
Moudouni SM, Tligui M, Doublet JD, Haab F,
Gattegno B and Thibault P: Lymphoproliferative disorder presenting
as a tumor of the renal allograft. Int Urol Nephrol. 38:779–782.
2006.
|
|
22
|
André N, Roquelaure B and Conrath J:
Molecular effects of cyclosporine and oncogenesis: a new model. Med
Hypotheses. 63:647–652. 2004.
|
|
23
|
Pascual J: Post-transplant
lymphoproliferative disorder - the potential of proliferation
signal inhibitors. Nephrol Dial Transplant. 22(Suppl 1): i27–i35.
2007.
|
|
24
|
Alexandru S, Gonzalez E, Grande C, et al:
Monotherapy rapamycin in renal transplant recipients with lymphoma
successfully treated with rituximab. Transplant Proc. 41:2435–2437.
2009.
|
|
25
|
DiNardo CD and Tsai DE: Treatment advances
in posttransplant lymphoproliferative disease. Curr Opin Hematol.
17:368–374. 2010.
|