Introduction
Telmisartan is an antihypertensive drug that exerts
its effect by antagonizing the angiotensin I (AT1)
receptor blocker (ARB). Beyond this mechanism, Shupp et al
(1) revealed partial peroxisome
proliferator-activated receptor (PPAR) agonism in 3T3-L1
preadipocytes. PPAR belongs to the steroid hormone receptor
superfamily (2) and can be divided
into three types: PPARα, PPARβ/δ and PPARγ. Alternative splicing of
PPARγ leads to four isoforms: PPARγ1–4. Translating the
mRNA of PPARγ1,3,4 results in an identical protein
(3). By contrast, the
PPARγ2 protein differs from PPARγ1 due to the
additional 30 amino acids at its N-terminal, and it predominantly
exists in adipocytes, but is also highly expressed in colon
epithelium, pancreatic β cells, endothelial tissue and macrophages.
In addition, PPARγ1 is present in human urological
cancer cells (i.e., renal cell, prostate, bladder and testicular
cancer) (4) and colon cancer cells
(5), where ligand-dependent
activation by antidiabetic drugs, such as thiazolidinediones, which
include pioglitazone, rosiglitazone, troglitazone and ciglitazone,
leads to apoptosis (6) and an
antiproliferative effect (7).
Arterial hypertension and colorectal cancer (CRC)
have a high prevalence in industrialized nations, with an estimated
proportion of 25% for hypertension and an age standardized
incidence (Europe) for CRC of 67.0% for males and 44.5% for females
in Germany (8). Synchronous
manifestation is common, however, at present, it is unclear whether
telmisartan partially activates PPARγ1 in human colon
cancer cells and whether its partial agonism is sufficient for
inhibiting proliferation and stimulating apoptosis in a significant
manner.
Materials and methods
Cell culture
Human colon cancer cells (HT-29, SW-480 and SW-620)
were obtained from the American Type Culture Collection (Rockville,
MD, USA). The HT-29 and SW-480 cells originated from
well-differentiated colorectal adenocarcinoma (9) and the SW-620 cells were derived from
colorectal lymph node metastasis. The cell lines were maintained in
RPMI-1640 culture medium (Life Technologies, Darmstadt, Germany),
containing 10% fetal calf serum and 1% penicillin and streptomycin
(all Biochrom AG Biotechnologie, Berlin, Germany), at 37°C in a 5%
CO2 humidified atmosphere. Each cell line was treated
with telmisartan (0.2–5.0 μM; Boehringer Ingelheim, Ingelheim,
Germany) or the full PPARγ agonist, pioglitazone (0.2–5.0 μM;
Zhejiang Huahai Pharmaceutical Co., Ltd., Zhejiang, China), as a
positive control for 24 h. Pioglitazone and telmisartan were each
dissolved in 0.05% dimethyl sulfoxide (DMSO; Carl Roth GmbH &
Co., KG, Karlsruhe, Germany). DMSO served as a negative
control.
MTT cytotoxicity assay
The measured activity of mitochondrial succinate
dehydrogenase quantitatively determines the degree of cytotoxicity.
The enzyme converts tetrazolium salt (MTT; Sigma-Aldrich Chemie
GmbH, Taufkirchen, Germany) into a blue dye, and the absorption is
measured. Higher levels of absorption indicate higher levels of
enzyme activity and increased cell viability. According to the
manufacturer’s instructions, the cells were detached using 3 ml
trypsin and then transferred to a 96-well microplate. Each well
contained 3×103 cells per 0.2 ml, and the cells were
incubated at 37°C and 95% relative atmospheric humidity for 24 h.
Following three days of incubation with 100 μl medium and 10 μl MTT
solution [50 mg/10 ml phosphate-buffered saline (PBS); PAA
Laboratories GmbH, Cölbe, Germany ], the cells were incubated for a
further four hours. Cell lysis was initiated by the addition of 100
μl SDS (10%; 5 g/50 ml double-distilled water) purchased from Carl
Roth GmbH & Co., KG. The cell suspension was incubated
overnight and the absorbance at 570 nm was measured on the
following day with a reference at 650 nm.
Cell count
Following 24 h of incubation with DMSO, pioglitazone
and telmisartan in the presence or absence of the PPARγ antagonist,
GW9662, the cell culture medium was carefully removed, followed by
multiple PBS-washing cycles. Cell counting was performed using a
Neubauer cell count chamber (Brand GmbH + Co., KG, Wertheim,
Germany).
Caspase 3–7 assay
The Caspase-Glo® 3/7 assay (AnaSpec Inc.,
Seraing, Belgium) is a homogeneous, luminescent assay that measures
caspase-3 and -7 activity. The assay provides a luminogenic
caspase-3/7 substrate, which contains the Asp-Glu-Val-Asp
tetrapeptide sequence, in a reagent optimized for caspase activity,
luciferase activity and cell lysis. Adding a single
Caspase-Glo® 3/7 reagent in an ‘add-mix-measure’ format
results in cell lysis, followed by caspase cleavage of the
substrate and generation of a ‘glow-type’ luminescent signal,
produced by luciferase. Luminescence is proportional to the amount
of caspase activity present.
Quantitative polymerase chain reacton
(qPCR)
Primers were purchased from TIB Molbiol
Syntheselabor GmbH (Berlin, Germany) with the following sequences:
PPARγ Sense, 5′-CAAGCCCTTCACTACTGTTG-3′ and antisense,
5′-CTTTATCTCCACAGACACG-3′; and AT1 receptor sense,
5′-ACAGCTTGGTGGTGATAGTC-3′ and antisense,
5′-CAATGCTGAGACACGTGAG-3′. The qPCR was performed using the Roche
LightCycler® carousel-based system (Roche Diagnostics
GmbH, Mannheim, Germany) under the following conditions: Activation
at 95°C for 10 min; 40 cycles of amplification at 95°C for 5 sec,
64°C for 10 sec and 72°C for 20 sec; and a brief melting curve
analysis at 95°C and 65°C for 15 sec, followed by an increase of
0.1°C/sec to 95°C.
Statistical analyses
Statistical significance was determined using the
independent Student t-test, SPSS Version 19.0.1 (IBM, Armonk, NY,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
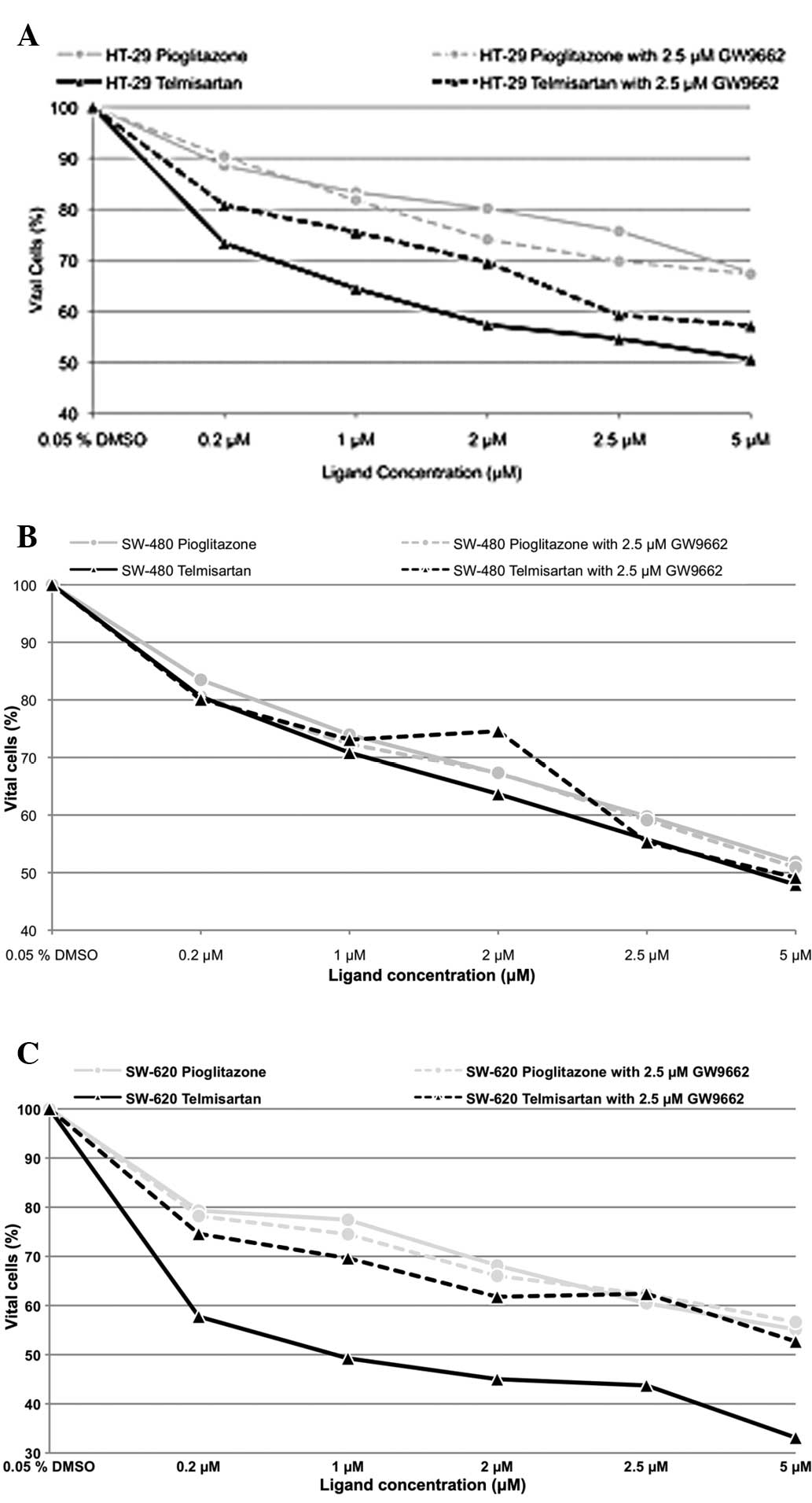
Telmisartan affects cell viability of
human colon cancer cells
The incubation of the human colon cancer cell lines
with pioglitazone and telmisartan exhibited a dose-dependent effect
on cell viability (Fig. 1), while
incubation of the HT-29 cells with 0.2 μM telmisartan resulted in
98.65% cell viability (P>0.05). Additional blocking of PPARγ
with 2.5 μM GW9662 resulted in 96.60% (P>0.05) cell viability
(telmisartan vs. telmisartan combined with GW9662; P>0.05). In
the SW-480 and SW-620 cells, incubation with 0.2 μM telmisartan
alone resulted in a cell viability of 98.13 and 96.33% (P>0.05),
respectively. In the presence of 2.5 μM GW9662, the cell viability
was reduced to 88.53 and 86.77% (P<0.001). A concentration of
0.2 μM pioglitazone alone resulted in 97.31, 99.63 and 96.74% cell
viability for the HT-29, SW-480 and SW-620 cells, respectively
(P>0.05). Additional blocking of PPAR with 2.5 μM GW9662
resulted in 98.10 (P>0.05), 95.23 and 91.70% (P<0.001) cell
viability for the HT-29, SW-480 and SW-620 cells, respectively. No
significant differences were identified with regard to the effect
on cell viability between telmisartan and pioglitazone regardless
of the use of GW9662 in all three human colon cell lines.
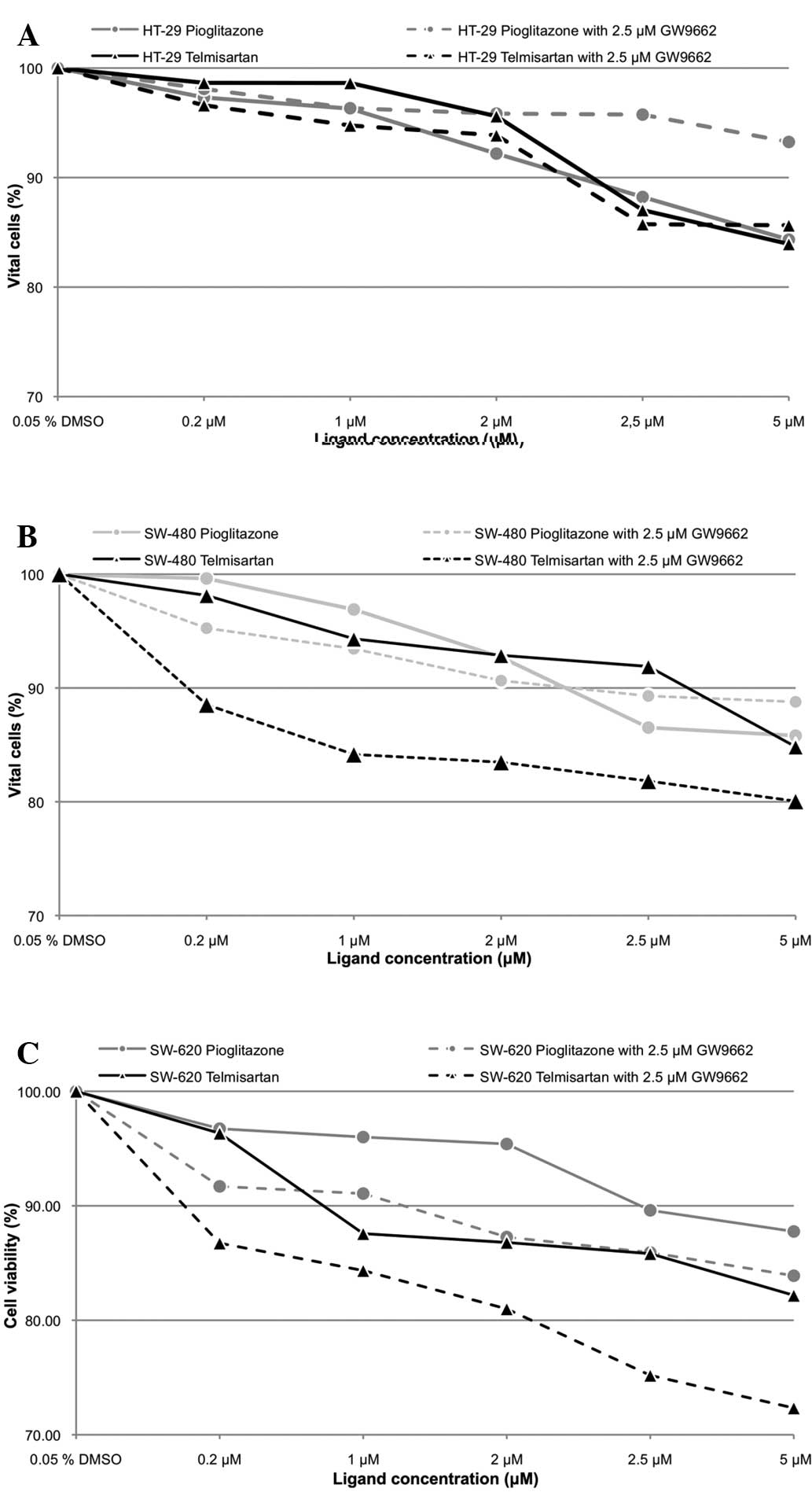
Antiproliferative effect of telmisartan
in human colon cancer cells
Telmisartan and pioglitazone inhibited cell
proliferation in a dose-dependent manner (Fig. 2). Telmisartan (0.2 μM) significantly
reduced cell survival in the HT-29, SW-480 and SW-620 cells (73.33,
80.56 and 57.75%, respectively; P<0.001). Additional PPARγ
blockage with 2.5 μM GW9662 led to a significant increase in cell
survival in the HT-29 and SW-620 cells (80.78 and 74.59%,
respectively; P<0.001), however, this increase was not observed
in the SW-480 cells (80.08%; P>0.05). The incubation of the
HT-29, SW-480 and SW-620 cells with 0.2 μM pioglitazone resulted in
a significantly reduced cell count (88.51, 83.49 and 79.30%;
P<0.001). However, no significant differences in cell count were
observed by adding 2.5 μM GW9662 (90.27% for HT-29, 80.56% for
SW-480 and 78.21% for SW-620; P>0.05). The difference in the
antiproliferative effect between pioglitazone and telmisartan was
not significant in the HT-29 cells, regardless of the use of
GW9662. By contrast, the difference in the antiproliferative effect
was significant in the SW-480 cells (P<0.001; P<0.05 with
GW9662), as well as in the SW-620 cells in the absence of GW9662
(P<0.05; P>0.05 with GW9662).
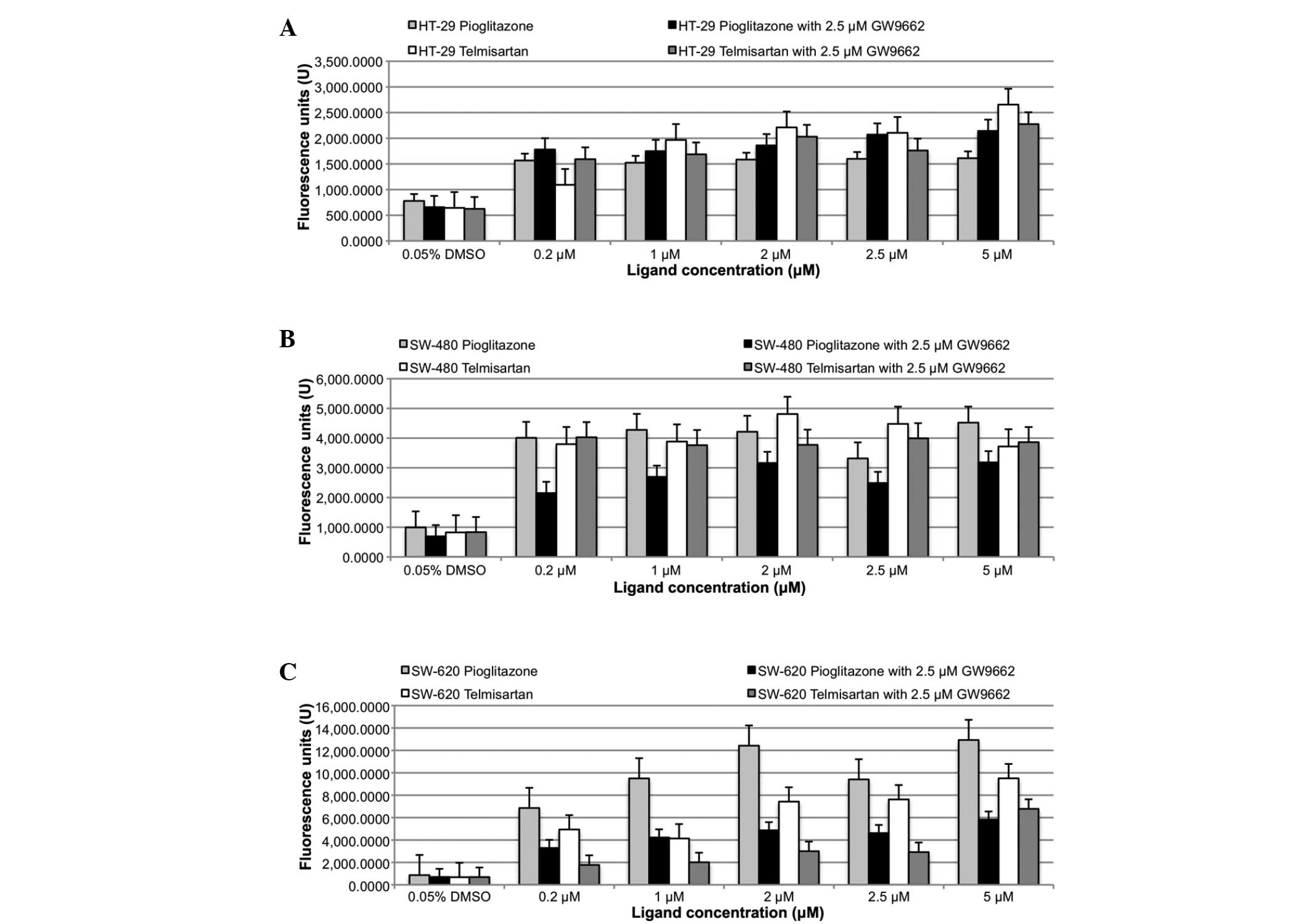
Telmisartan induces apoptosis
The induction of apoptosis was determined by the
caspase 3/7 assay (Fig. 3).
Significant caspase 3/7 activation was measured with 0.2 μM
pioglitazone and telmisartan (P<0.05) in all three colon cancer
cell lines (HT-29, SW-480 and SW-620). However, no significant
difference was observed between the apoptotic effects of
telmisartan and pioglitazone. Similarly, the addition of 2.5 μM of
the PPARγ blocker, GW9662, also resulted in significant caspase 3/7
activation (P<0.05) in all three colon cancer cell lines. No
significant differences were identified in caspase 3/7 activation
between telmisartan alone versus telmisartan in combination with
GW9662, and the same was true for pioglitazone.
Telmisartan downregulates PPARγ and
upregulates cystatin A (CSTA)
PPARγ was found to downregulate its relative mRNA
expression following ligand activation (Fig. 4). Relative PPARγ mRNA expression was
significantly downregulated in the HT-29, SW-480 and SW-620 cells
with 0.2 μM telmisartan (Δ0.53, Δ0.53 and Δ0.36; P<0.05). The
addition of 2.5 μM GW9662 also resulted in a significant
downregulation of relative PPARγ mRNA expression (HT-29, Δ0.34;
SW-480, Δ0.67; and SW-620, Δ0.31; P<0.05). However, no
significant differences were observed between telmisartan alone and
telmisartan combined with GW9662. The incubation of the HT-29,
SW-480 and SW-620 cells with 0.2 μM pioglitazone also resulted in a
significant downregulation of relative PPARγ mRNA expression
(Δ0.27, Δ0.32 and Δ0.27, respectively; P<0.05). PPARγ blockage
did not neutralize the effect of pioglitazone on PPARγ mRNA
expression in all three colon cancer cell lines (HT-29, Δ0.42;
SW-480, Δ0.48; and SW-620, 0.27; P<0.05). AT1
receptor mRNA was downregulated significantly through telmisartan
in all three colon cancer cell lines (P<0.05), as predicted.
Notably, pioglitazone had the same effect in all three colon cancer
cell lines (P<0.05). CSTA, a well-known PPARγ1 target
gene, which is upregulated upon PPARγ activation, was used as a
positive control. Relative CSTA mRNA expression was not
significantly affected by 0.2 μM telmisartan or telmisartan
combined with 2.5 μM GW9662 in the HT-29 and SW-620 cells. While
significant upregulation was observed in the SW-480 cells (Δ0.5;
P<0.05), the addition of 2.5 μM GW9662 (P>0.05) did not cause
this result. Relative CSTA mRNA expression in the HT-29 cells was
not significantly affected by 0.2 μM pioglitazone, alone or in
combination with GW9662. By contrast, relative CSTA mRNA expression
in the SW-620 cells was significantly upregulated (Δ0.50 alone and
Δ0.20 with GW9662; P<0.05).
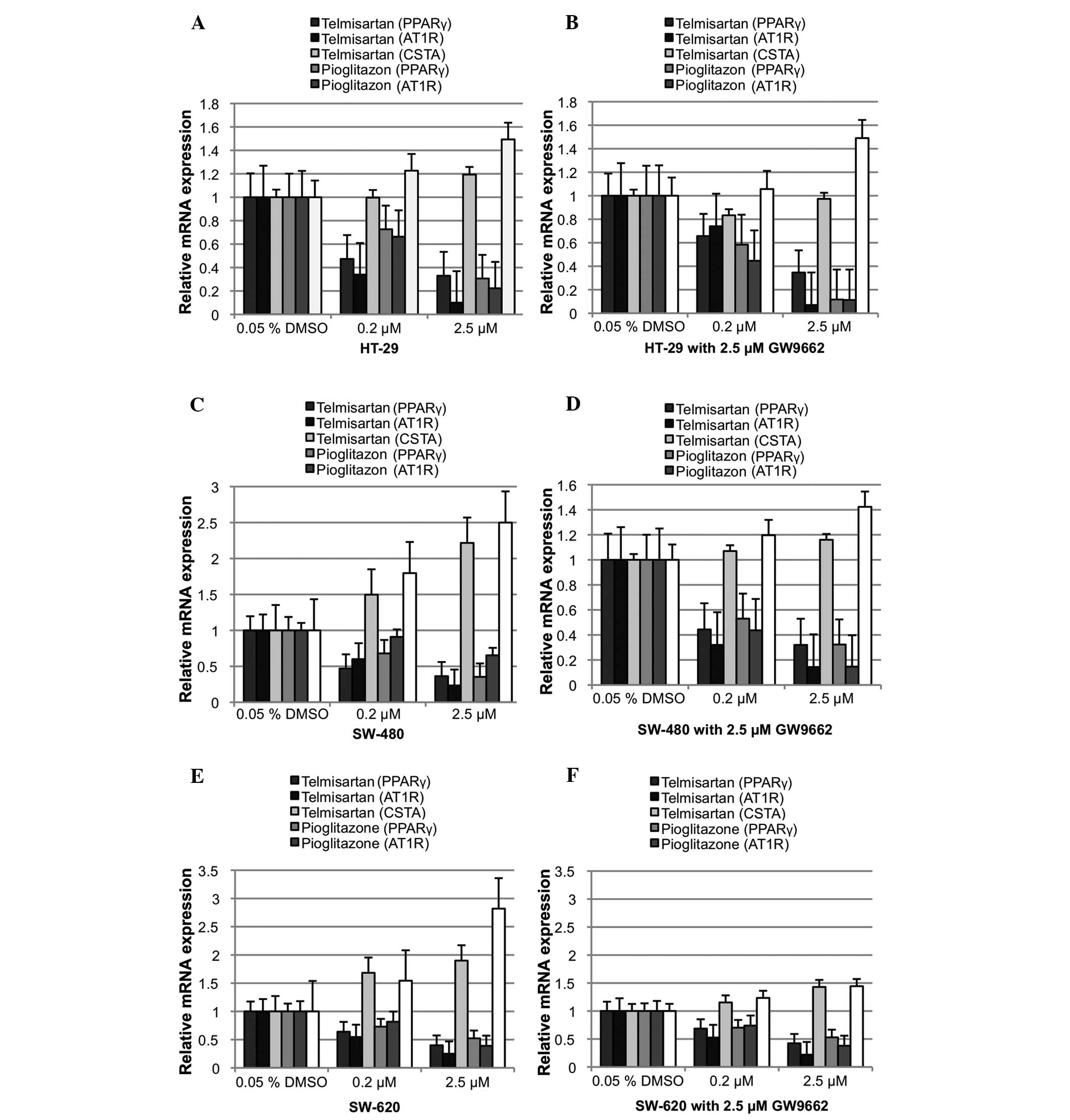 | Figure 4Relative mRNA expression of PPARγ, the
PPARγ target gene, CSTA and AT1R following 24 h of incubation with
DMSO, pioglitazone and telmisartan. (A) HT-29 without and (B) with
the PPARγ antagonist, GW9662. (C) SW-480 without and (D) with the
PPARγ antagonist, GW9662. (E) SW-620 without and (F) with the PPARγ
antagonist, GW9662. PPARγ, peroxizome proliferator-activated
receptor γ; DMSO, dimethyl sulfoxide; CSTA, cystatin A; AT1R,
angitensin I receptor. |
Discussion
The thiazolidinedione, pioglitazone, is an oral
antidiabetic drug that enhances insulin sensitivity through PPARγ
activation. However, it also activates PPARγ in colon cancer cells
in vitro, inducing the suppression, differentiation,
apoptosis and reversal of malignant changes (7,10).
Telmisartan is an ARB and a common antihypertensive drug with
partial PPARγ-agonism (1).
Therefore, it is of note whether telmisartan partially activates
PPARγ1 in colon cancer cells, subsequently inducing a
reduction in cell viability, inhibiting cell proliferation and
inducing apoptosis.
Following the oral intake of 20–120 mg telmisartan
by hypertensive patients, the therapeutic serum concentration has
been measured to range between 0.035 and 1.036 mM (11). Therefore, in the present study,
various colon cancer cells were incubated with telmisartan and
pioglitazone in comparable concentrations ranging between 0.2 and
5.0 μM. This resulted in a dose-dependent reduction in cell
viability, the inhibition of cell proliferation and the induction
of apoptosis in all three colon cancer cell lines. As predicted,
the effect of the full agonist, pioglitazone, was diminished by
adding the synthetic PPARγ antagonist, GW9662. PPARγ blockage with
GW9662 in the presence of telmisartan as a partial PPARγ agonist
did not lead to the diminished inhibition of the cell proliferation
and cell viability of pioglitazone. However, the reduction in cell
viability and the antiproliferative effect in the SW-480 and SW-620
cells were further increased compared with use of telmisartan
alone. Furthermore, telmisartan exhibited an apoptotic effect
equivalent to the full PPARγ agonist, pioglitazone. Contrary to the
prediction of a decreased apoptotic effect following the addition
of the PPARγ GW9662, a significant induction of caspase 3/7 was
observed, indicating PPARγ-independent apoptosis induction.
Following ligand-dependent activation, the PPARγ mRNA level was
characteristically downregulated as a negative feedback mechanism,
whilst upregulating the mRNA of its target genes, including CSTA.
Incubation with pioglitazone or telmisartan only significantly
downregulated relative PPARγ mRNA expression in all three colon
cancer cell lines, while upregulating CSTA in an evidently
dose-dependent manner. However, the additional blockage with 2.5 μM
GW9662 resulted in a significant downregulation in relative PPARγ
mRNA expression.
In conclusion, the present study showed that
telmisartan reduced cell viability and inhibited cell proliferation
in selected colon cancer cell lines in a dose-dependent manner.
Furthermore, telmisartan showed a greater effect than pioglitazone
in the SW-480 and SW-620 cells, particularly in the presence of the
PPARγ blocker, GW9662. The apoptotic effect of telmisartan was
close to the full PPARγ agonist, pioglitazone, in all cell lines
and was not inhibited by GW9662. PPARγ and CSTA mRNA expression
appeared to be affected by the presence of GW9662. Therefore, the
observed reduction in cell viability, the inhibition of cell
proliferation and the induction of apoptosis by telmisartan cannot
be completely explained by ligand-dependent PPARγ1
activation.
Telmisartan may be an alternative drug for patients
presenting with arterial hypertension and colon cancer in their
medical history. Understanding its mechanism of action may lead to
similar pleiotropic drugs with increased potency.
Acknowledgements
The authors would like to thank Mr. Marco Arndt and
Mrs. Sonja Dullat for providing excellent technical assistance.
References
|
1
|
Schupp M, Janke J, Clasen R, Unger T and
Kintscher U: Angiotensin type 1 receptor blockers induce peroxisome
proliferator-activated receptor-gamma activity. Circulation.
109:2054–2057. 2004.
|
|
2
|
Mangelsdorf DJ, Thummel C, Beato M, et al:
The nuclear receptor superfamily: the second decade. Cell.
83:835–839. 1995.
|
|
3
|
Fajas L, Fruchart JC and Auwerx J:
PPARgamma3 mRNA: a distinct PPARgamma mRNA subtype transcribed from
an independent promoter. FEBS Lett. 438:55–60. 1998.
|
|
4
|
Matsuyama M, Funao K, Kuratsukuri K, et
al: Telmisartan inhibits human urological cancer cell growth
through early apoptosis. Exp Ther Med. 1:301–306. 2010.
|
|
5
|
Fajas L, Auboeuf D, Raspé E, et al: The
organization, promoter analysis, and expression of the human
PPARgamma gene. J Biol Chem. 272:18779–18789. 1997.
|
|
6
|
Bull AW: The role of peroxisome
proliferator-activated receptor gamma in colon cancer and
inflammatory bowel disease. Arch Pathol Lab Med. 127:1121–1123.
2003.
|
|
7
|
Sarraf P, Mueller E, Jones D, et al:
Differentiation and reversal of malignant changes in colon cancer
through PPARgamma. Nat Med. 4:1046–1052. 1998.
|
|
8
|
Bertz J, Dahm S, Haberland J, Kraywinkel
K, Kurth B-M and Wolf U: Spread of Cancer in Germany. Development
in the prevalence between 1990 and 2010. Robert Koch Institute;
Berlin: 2010, (In German).
|
|
9
|
Semple TU, Quinn LA, Woods LK and Moore
GE: Tumor and lymphoid cell lines from a patient with carcinoma of
the colon for a cytotoxicity model. Cancer Res. 38:1345–1355.
1978.
|
|
10
|
Kato M, Kusumi T, Tsuchida S, Tanaka M,
Sasaki M and Kudo H: Induction of differentiation and peroxisome
proliferator-activated receptor gamma expression in colon cancer
cell lines by troglitazone. J Cancer Res Clin Oncol. 130:73–79.
2004.
|
|
11
|
Stangier J, Su CA and Roth W:
Pharmacokinetics of orally and intravenously administered
telmisartan in healthy young and elderly volunteers and in
hypertensive patients. J Int Med Res. 28:149–167. 2000.
|