Introduction
Trastuzumab, an anti-human epidermal growth factor
receptor 2 (HER2) monoclonal antibody and lapatinib, an epidermal
growth factor receptor (EGFR) and HER2 tyrosine kinase inhibitor
were the only two anti-HER2 agents that had been approved in Japan
as of September 2013. Administration of anti-HER2 agents in
combination with chemotherapy is the first-line treatment for
patients presenting with HER2-type breast cancer (1–4).
However, in the case outlined in the present study, the patient was
elderly and had a history of hepatitis B (HB); therefore,
discarding the use of chemotherapy was considered and the patient
was treated with trastuzumab monotherapy. However, after the
patient was assessed as achieving clinical partial response,
cutaneous metastases developed, which resulted in disease
progression. However, clinical complete response (cCR) was
sustained in the long-term subsequent to switching to treatment via
lapatinib monotherapy. In the present study, the clinical course of
a breast cancer patient is reported. Written informed consent was
obtained from the patient.
Case report
In December 2009, a 72-year-old female was admitted
to the Tokyo Women’s Medical University (Tokyo, Japan) presenting
with a mass in the left axillary lymph node. The patient had been
diagnosed with HB (cause unknown) at the age of 37 years. During
medical treatment at the age of 52 years, the following antigen
loss was observed: Hepatitis B surface (HBs) antigen negative (−),
HB virus (HBV) DNA real-time (−), HBs antibody positive (+) and
Hepatitis B core antibody (+). The patient’s older sister had been
diagnosed with breast cancer, a younger sister suffered from
pancreatic cancer and a younger brother had esophageal cancer. In
November 2009, the patient presented with a mass in the left axilla
and visited Fukujuji Hospital (Tokyo, Japan). A mass biopsy was
performed and the patient was diagnosed with metastatic
adenocarcinoma of the axillary lymph node.
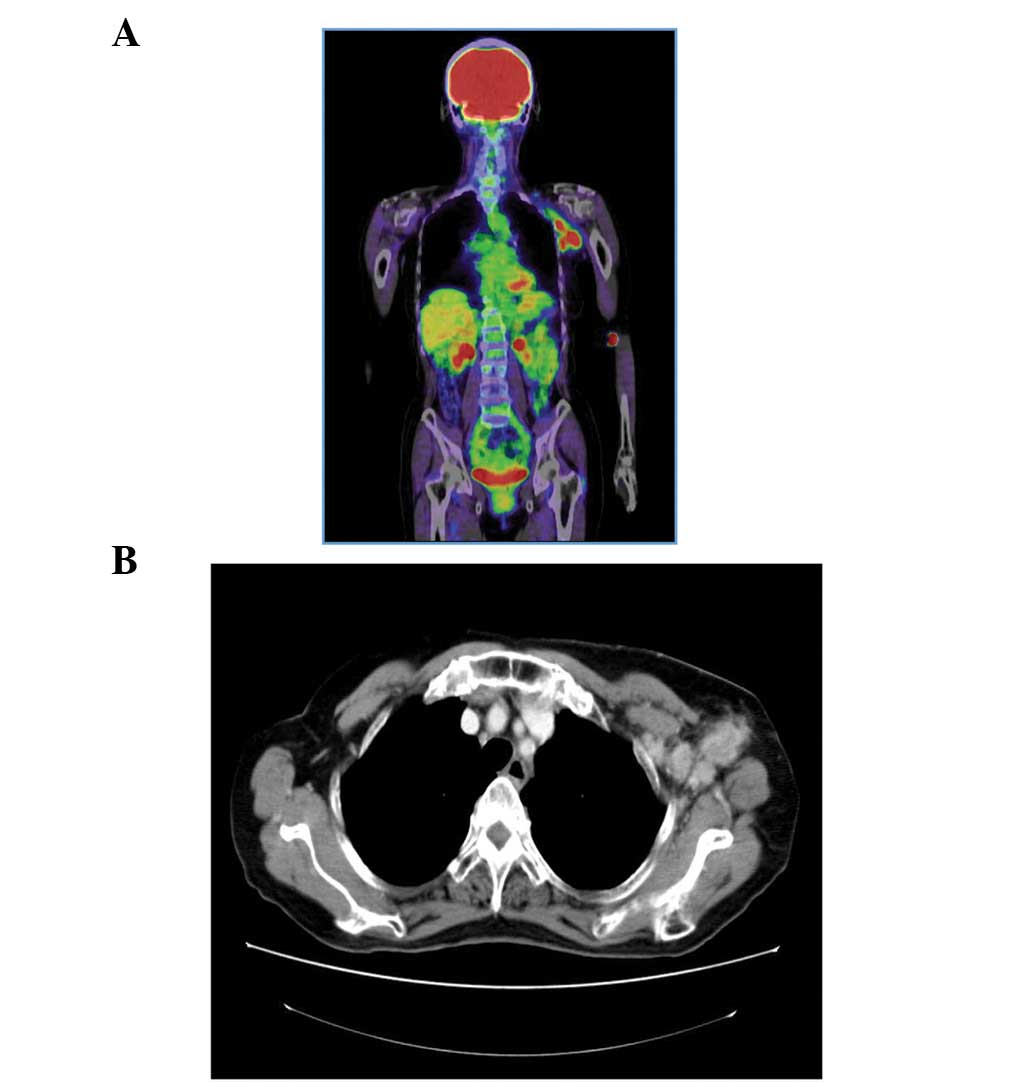
The primary tumor was not detected during
fludeoxyglucose-position electron tomography (Fig. 1A). In addition, no primary tumor was
observed by chest computed tomography (CT; Fig. 1B), abdominal-pelvic CT or via a
gynecological screening. Therefore, the patient was referred to
Tokyo Women’s Medical University hospital to undergo a breast
cancer screening.
Upon clinical breast examination, no evidence of
skin retraction or nipple dimpling was observed. In addition, there
was no apparent evidence of a mass in the mammary gland. However,
the breast cancer screening revealed a well-defined 30-mm diameter
non-movable mass in the left axillary lymph node. A mammography did
not reveal any abnormalities on either side and during breast
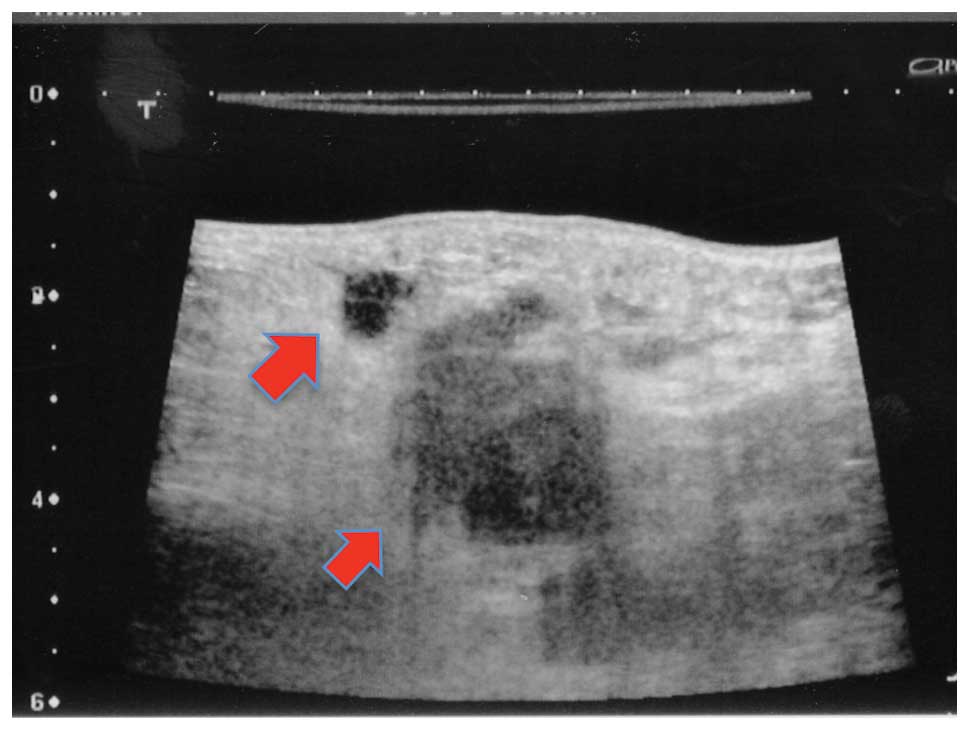
ultrasonography, no marked tumor lesions were detected in the
mammary gland. A suspected metastases (maximum diameter, 35 mm) was
observed in a lymph node in the left axilla (Fig. 2) and a minimum of 20 irregular
masses of varying sizes were observed in this area. No evidence of
metastases was detected in the lymph nodes proximal to the sternum
and clavicle fossae.
When analyzing the tumor markers (Table I) a marginal increase from the
standard value in serum HER2 levels was detected, however there
were no increases observed in the other markers.
 | Table ITumor markers examined during the
patient’s initial visit. |
Table I
Tumor markers examined during the
patient’s initial visit.
| Tumor marker | Serum level | Standard value |
|---|
| CEA (ng/ml) | 1.7 | ≤5.0 |
| CA15-3 (U/ml) | 10.3 | <25 |
| BCA225 (U/ml) | 47.0 | <160 |
| NCC-ST-439
(U/ml) | 4.2 | <7.0 |
| Serum HER2 protein
(ng/ml) | 18.8 | ≤15.2 |
| Serum p53 antibodies
(U/ml) | ≤0.4 | ≤1.3 |
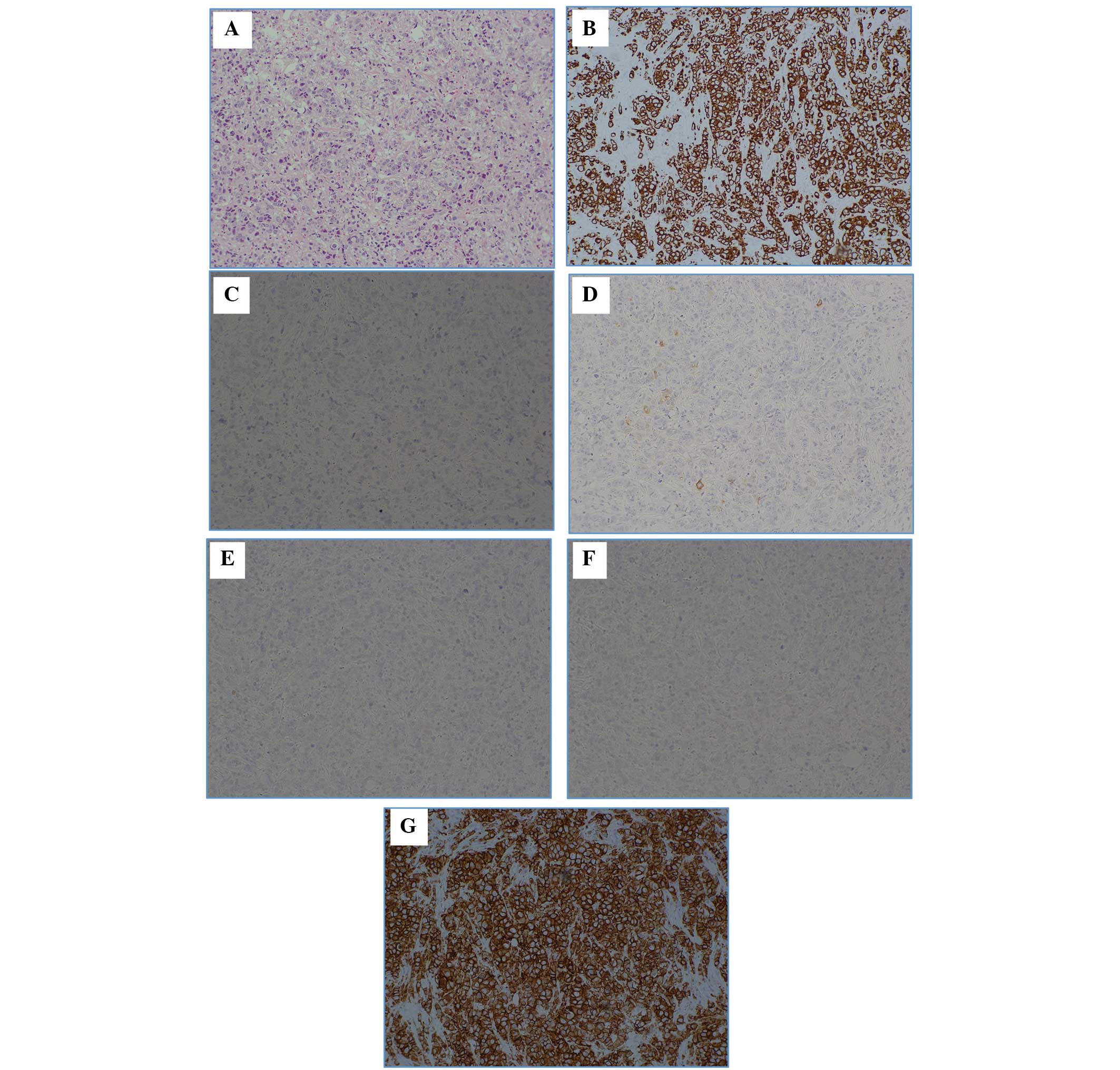
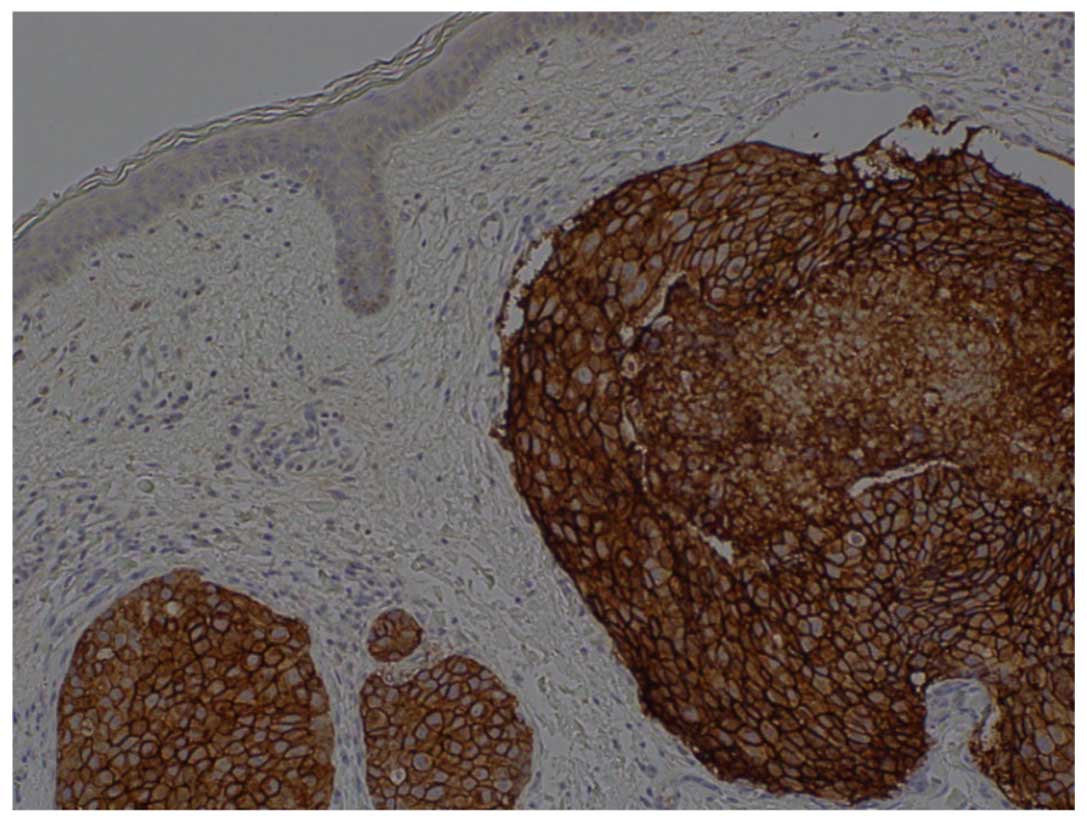
Pathological analyses were conducted on the left
axillary lymph node mass (Fig. 3).
In the lymph node, hematoxylin and eosin staining detected a
metastatic carcinoma and the immunostaining results were as
follows: Cytokeratin (CK)7 (+), CK20 (−), gross cystic disease
fluid protein 15 (partial +), estrogen receptor (ER; −) and
progesterone receptor (PgR; −). The lungs, mammary glands, ovary,
uterus, and mesothelium was hypothesized to have primary lesions
and the HER2 score was 3+.
However, no primary lesions were identified,
including in the mammary gland. The most frequent type of malignant
tumor exhibiting only axillary lymph node enlargement is breast
cancer, which accounts for >50% cases, worldwide (5–8). The
primary occult breast cancer lesion is not identified in 0.3–1.0%
of resectable breast cancer cases, worldwide (5). The patient in the current case study
was treated for occult breast cancer as a result of the
immunohistochemical staining results and the presentation of
axillary lymph node metastases alone
(T0N2M0; Stage IIIA) (9). Primary systemic therapy was
considered, as the patient was Stage IIIA. However, as the patient
had a history of HBV infection, there was a risk of de novo
HB development due to administration of anticancer agents. This, in
addition to the age of the patient, resulted in the patient being
initially treated with trastuzumab monotherapy.
Trastuzumab was administered as a monotherapy from
February 2010. Mammary ultrasonography was performed 10 months
following the initial trastuzumab administration, and the irregular
masses in the left axillary lymph node reduced in size and number.
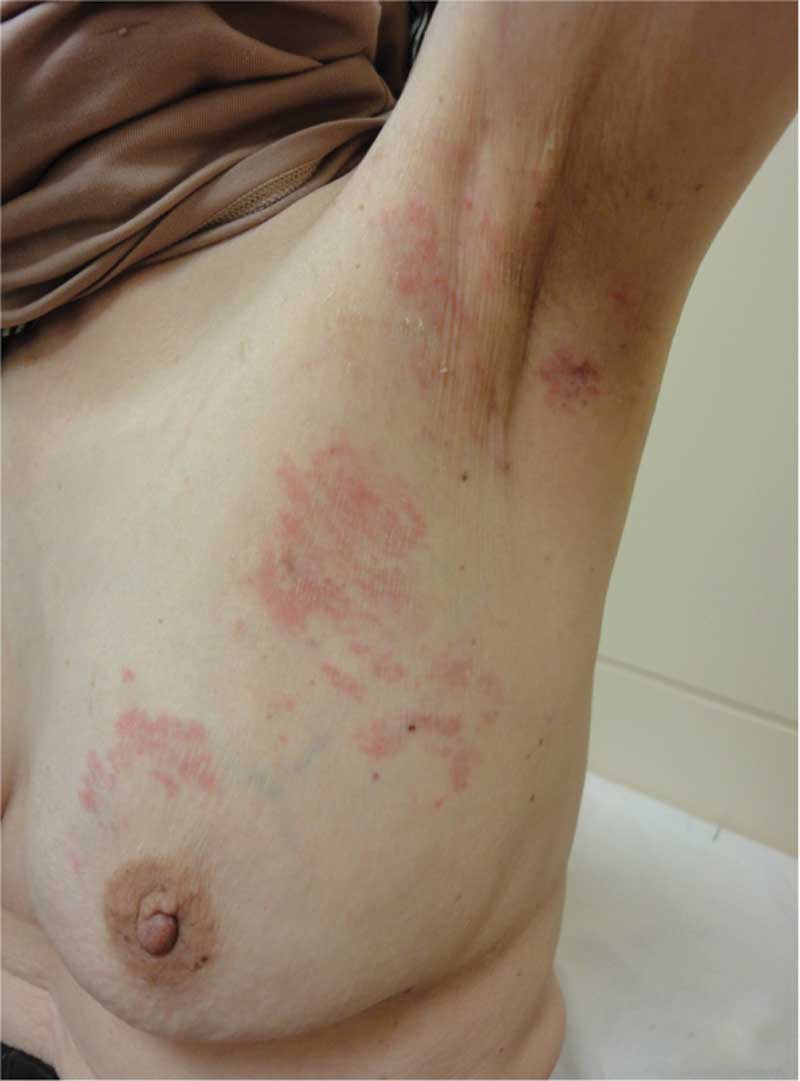
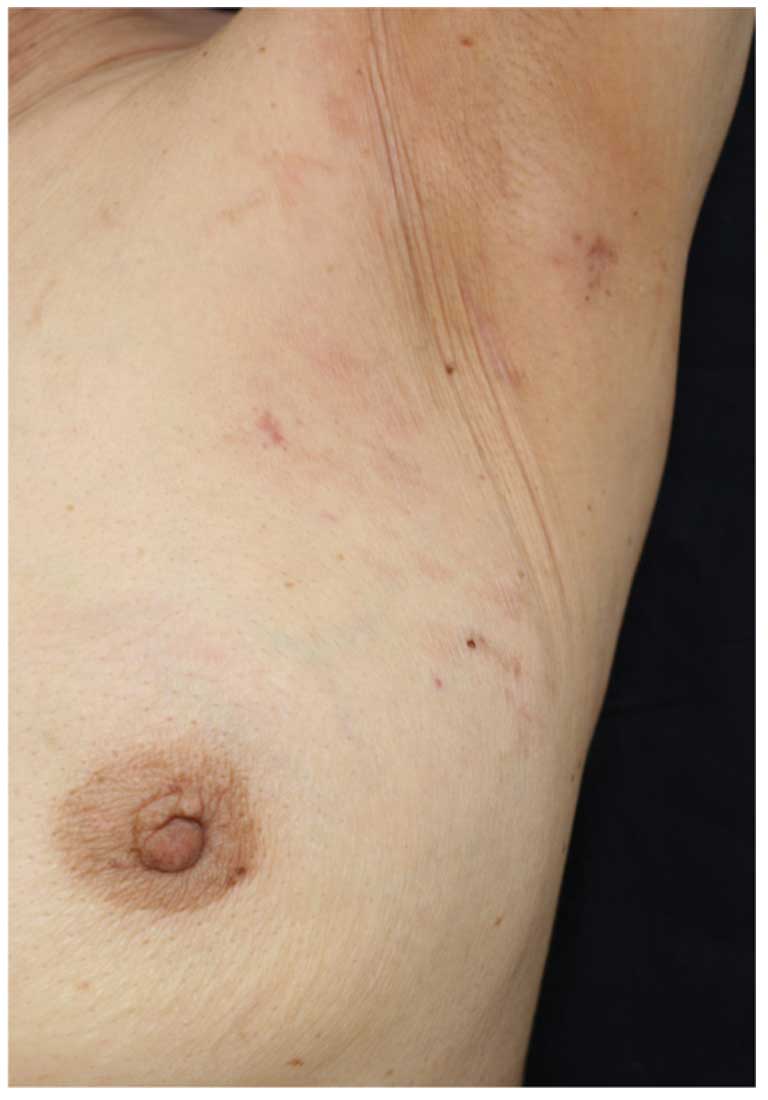
However, a skin rash (erythema) was observed encompassing the left
breast and extending into the axilla (Fig. 4). The pathological findings from the
skin biopsy of the affected area, as well as the immunostaining
findings, were comparible with those of the malignant lymph nodes
(Fig. 5). Therefore, the patient
was diagnosed with occult breast cancer with cutaneous metastases.
In February 2011, administration of the anti-HER2 agent was
switched from trastuzumab to lapatinib. The erythema completely
disappeared following two months of lapatinib administration
(Fig. 6).
At present, 34 months following initiation of
lapatinib treatment, no new lesions or severe side-effects have
been observed. Fig. 7 illustrates
the progress during the treatment period.
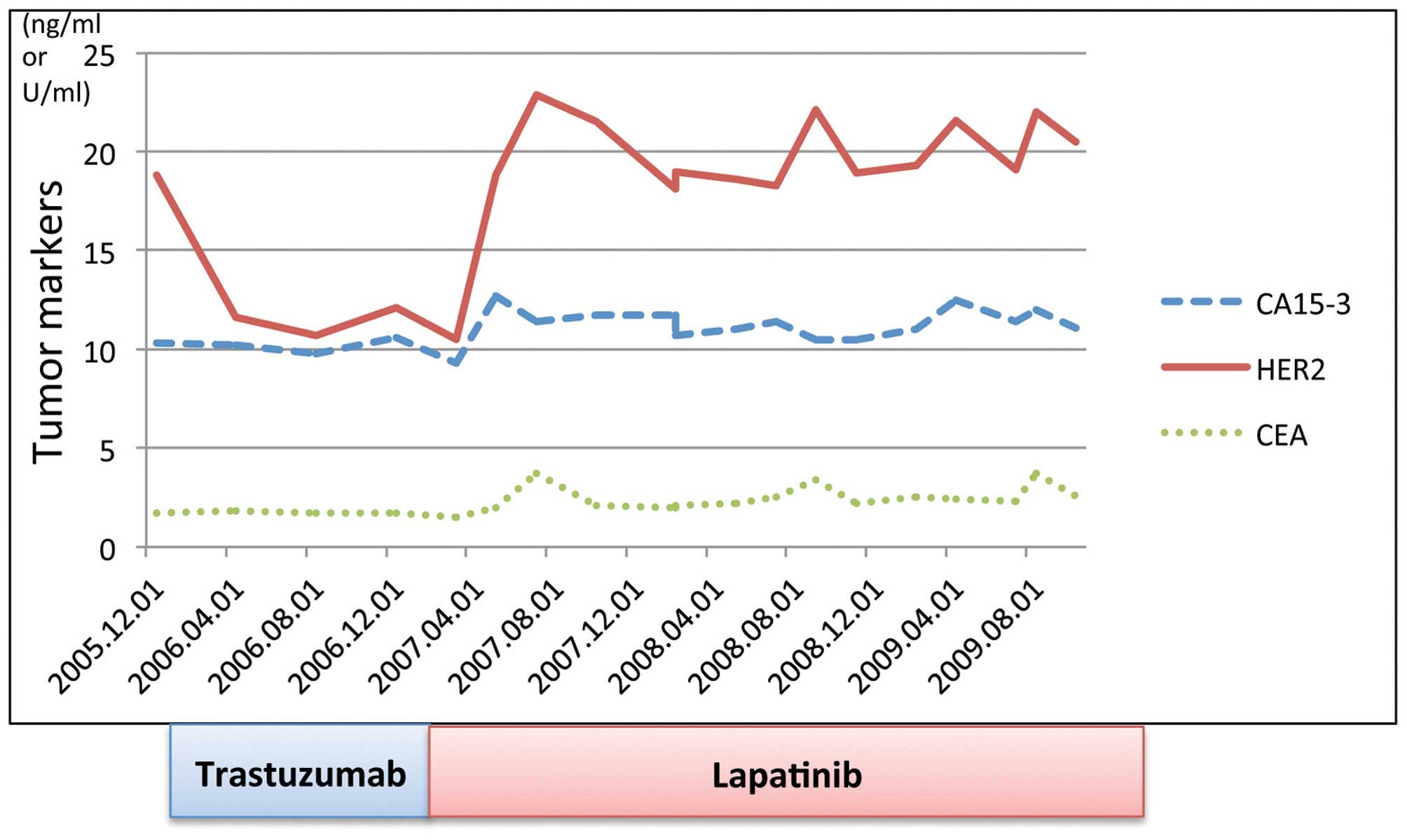 | Figure 7Treatment progress. Transition and
progression of tumor marker levels. February 12, 2010: Initiation
of trastuzumab administration. The HER2 serum concentration
immediately declined to below the reference value following
initiation of therapy. December 2010: A patch of eczema appeared in
the area between the upper left breast and axilla, which was
identified as skin metastases; the HER2 serum concentration was
normal when this occurred. February 19, 2011: Initiated lapatinib
administration. Skin metastases disappeared two months after
switching to lapatinib; however, the HER2 serum concentration was
18.8 ng/ml, which exceeded the reference value (15.2 ng/ml).
Thereafter, no evident new lesions were observed, and the HER2
serum concentration was maintained between 18.1 and 22.9 ng/ml,
marginally greater than the reference value. CA15-3, cancer antigen
15-3; HER2, human epidermal growth factor receptor 2, CEA,
carcinoembryonic antigen. |
Discussion
Accounting for the extent of the axillary lymph node
metastases, the standard treatment for the patient in the present
case study would usually have been neoadjuvant chemotherapy via
administration of anticancer agents, such as anthracycline and
taxane (plus the anti-HER2 agent, trastuzumab), followed by surgery
and radiation therapy, followed up with administration of
trastuzumab as an adjuvant therapeutic agent. However, the decision
to initiate treatment using trastuzumab as a monotherapy was
determined based on the following factors: i) The patient had a
history of HBV infection and there was a risk of developing de
novo HB as a result of administering the anticancer agents
(10) and ii) the patient was
elderly (age, 72 years). Although cCR in the axillary lymph node
had almost been reached 10 months subsequent to the initiation of
trastuzumab administration, cutaneous metastases appeared in the
skin in the area between the upper left breast and axilla. Distant
metastases were not observed in any of the other areas. However,
the extent of skin metastases had spread to the medial side of the
arm beyond the axillary side. The severity reached a level such
that skin grafting was required via radical surgery. Therefore, the
anti-HER2 agent, trastuzumab was replaced with lapatinib
monotherapy. The skin metastases disappeared after two months of
lapatinib administration and the patient was considered to be in
cCR. Currently, 34 months subsequent to lapatinib administration,
no new lesions or severe side-effects have been observed.
When the tumor markers were examined in the present
case study, the serum HER2 levels exhibited the only variation.
HER2 serum concentration is a measurement of the serum
extracellular domain (ECD) level. HER2/neu proteins located in the
ECD are cleaved and separated by shedding, and remain as ECD in the
serum. The upper limit of normal serum HER2 levels is defined as
15.2 ng/ml (11).
In the present case study, the HER2 serum
concentration level at the time of the initial visit was 18.8
ng/ml. This level was immediately reduced to the reference value
subsequent to trastuzumab treatment initiation. However, the HER2
serum concentration was at the normal value when the skin
metastases presented. Two months after switching to treatment with
lapatinib, when skin metastases had disappeared, the HER2 serum
concentration was 18.8 ng/ml, which exceeded the reference value
(15.2 ng/ml). However, since then, no apparent new lesions have
been observed, and the HER2 serum concentration has been maintained
between 18.1 and 22.9 ng/ml, which is marginally greater than the
reference value. Scaltriti et al (12) reported that the HER2 protein
expression levels were reduced following trastuzumab administration
in mice; by contrast, the HER2 protein expression levels were
increased subsequent to lapatinib administration.
In the present case, there were no observations that
indicated cancer recurrence. This may have been due to the
lapatinib administration, whereby occult breast cancer cells,
including circulating tumor cells and other cells observed in
normal tissue, such as in the myocardial cell, induced the HER2
proteins.
Trastuzumab and lapatinib were the only two
anti-HER2 agents that had been approved in Japan as of September
2013. Anti-HER2 agents may be administered in the following
treatment strategies: i) Anti-HER2 agent plus chemotherapy; ii)
anti-HER2 agent administered as a monotherapy (13–15);
and iii) anti-HER2 agent plus hormonal therapy (16,17).
The current recommendation for the first-line therapy is an
anti-HER2 agent plus chemotherapy. However, in the present case
study, the administration of an anti-HER2 agent as a monotherapy
was controversial due to the patient’s HER2 subtype, history of HB
and other such characteristics.
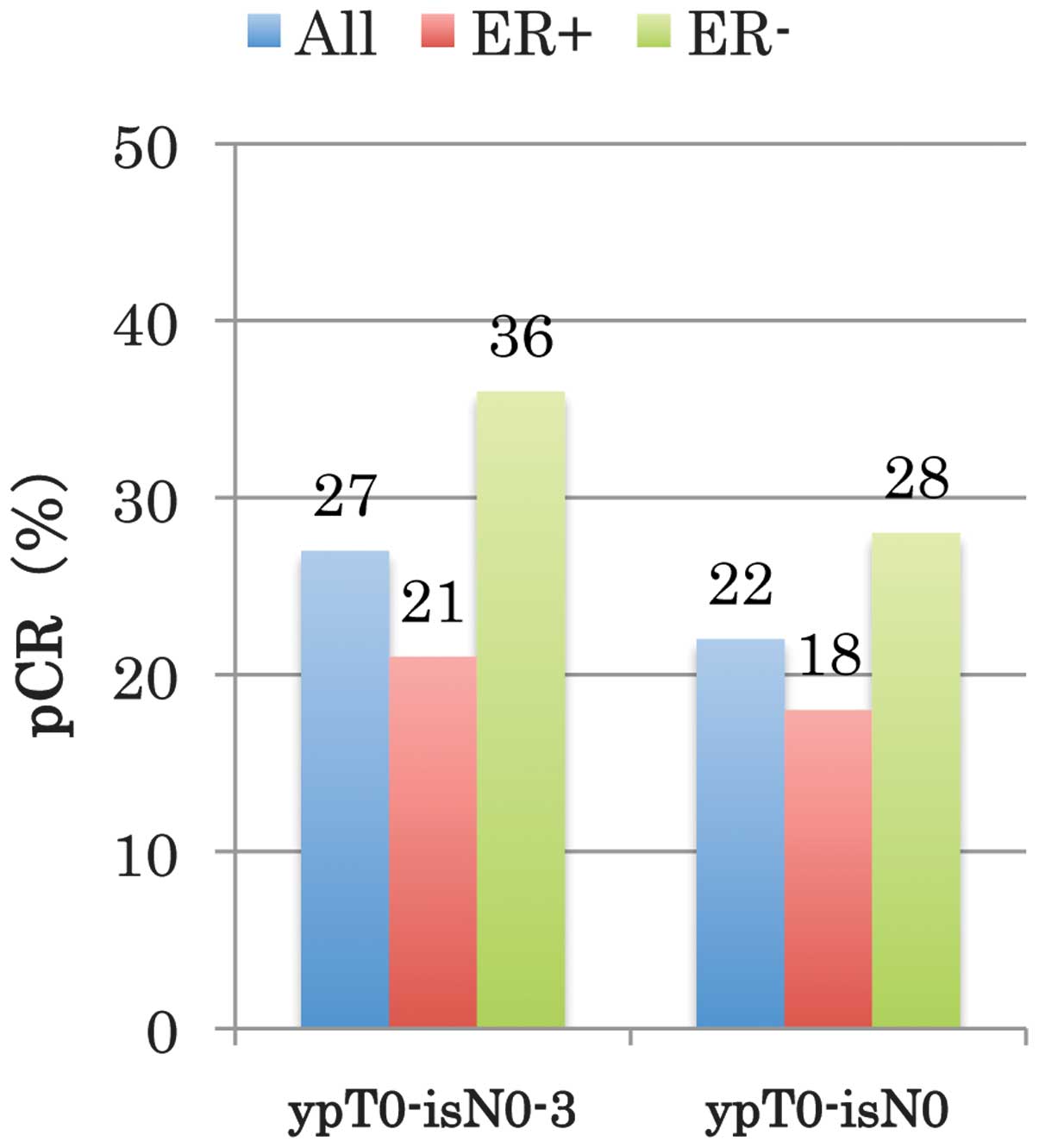
The TBCRC 006 trial (18), a single-arm phase II study, is a
clinical trial of neoadjuvant lapatinib and trastuzumab with
hormonal therapy and without chemotherapy in patients with
HER2-overexpressing breast cancer using only the two anti-HER2
agents [combined with hormonal therapy, in the case of the ER (+)
subjects]. The complete pathological response rate for ER (−)
(HER2-type) was high at 28% (Fig.
8) (18). In the present case,
the treatment was switched to lapatinib when the patient
demonstrated resistance to trastuzumab. Therefore, the results of
the abovementioned clinical trial cannot be directly applied.
However, the present results demonstrate that a type of breast
cancer exists upon which anti-HER2 monotherapy exerts an effect.
Pertuzumab, which is a HER dimerization inhibitor, has become
available in Japan (19,20). Identifying biomarkers of this type
of breast cancer, which exhibits a CR as a result of the
administration of anti-HER2 agents only, may significantly alter
the treatment strategies that are adopted for HER2-type breast
cancer.
In conclusion, in the present study, a case of a
patient presenting with occult breast cancer and cutaneous
metastases, who was successfully treated with lapatinib monotherapy
and who sustained cCR in the long-term is reported. After a total
of 34 months following the initiation of lapatinib monotherapy, no
novel lesions or severe side-effects have been identified.
The results of the current study may lead to the
development of numerous anti-HER2 agents. Future studies are
required to identify biomarkers of HER2-type breast cancer, which
may be used as monotherapy agents.
Acknowledgements
The present study was presented at the 73rd Annual
Congress of the Japan Surgical Association (Tokyo, November
2011).
References
|
1
|
Slamon DJ, Leyland-Jones B, Shak S, et al:
Use of chemotherapy plus a monoclonal antibody against HER2 for
metastatic breast cancer that overexpresses HER2. N Engl J Med.
344:783–792. 2001.
|
|
2
|
Marty M, Cognetti F, Maraninchi D, et al:
Randomized phase II trial of the efficacy and safety of trastuzumab
combined with docetaxel in patients with human epidermal growth
factor receptor 2-positive metastatic breast cancer administered as
first line treatment: the M77001 study group. J Clin Oncol.
23:4265–4274. 2005.
|
|
3
|
Burstein HJ, Harris LN, Marcom PK, et al:
Trastuzumab and vinorelbine as first-line therapy for
HER2-overexpressing metastatic breast cancer: multicenter phase II
trial with clinical outcomes, analysis of serum tumor markers as
predictive factors, and cardiac surveillance algorithm. J Clin
Oncol. 21:2889–2895. 2003.
|
|
4
|
Di Leo A, Gomez HL, Aziz Z, et al: Phase
III, double-blind, randomized study comparing lapatinib plus
paclitaxel with placebo plus paclitaxel as first-line treatment for
metastatic breast cancer. J Clin Oncol. 26:5544–5552. 2008.
|
|
5
|
Fourquet A, Meunier M and Campana F:
Occult Primary Cancer with Axillary Metastases. Disease of the
Breast. Harris JR, Lippman ME, Morow M and Osborne CK: 3rd edition.
Lippincott Williams & Wilkins; Philadelphia, PA: pp. 1047–1052.
2004
|
|
6
|
Kaklamani V and Gradishar WJ: Axillary
node metastases with occult primary breast cancer. http://www.uptodate.com/.
Accessed December 2, 2013
|
|
7
|
Blanchard DK and Farley DR: Retrospective
study of women presenting with axillary metastases from occult
breast carcinoma. World J Surg. 28:535–539. 2004.
|
|
8
|
Copeland EM and McBride CM: Axillary
metastases from unknown primary sites. Ann Surg. 178:25–27.
1973.
|
|
9
|
Sobin LH, Gospodarowicz MK and Wittekind
C: International Union Against Cancer (UICC) TNM classification of
malignant tumours. 7th edition. Wiley-Liss; New York, NY: 2009
|
|
10
|
Tsubouchi H, Kumada H, Kiyosawa K, et al:
Prevention of immunosuppressive therapy or chemotherapy-induced
reactivation of hepatitis B virus infection: Joint report of the
Intractable Liver Diseases Study Group of Japan and the Japanese
Study Group of the Standard Antiviral Therapy for Viral Hepatitis.
Kanzo. 50:38–42. 2009.
|
|
11
|
Lüftner D, Cheli C, Mickelson K, et al:
ADVIA Centaur HER-2/neu shows value in monitoring patients with
metastatic breast cancer. Int J Biol Markers. 19:175–182. 2004.
|
|
12
|
Scaltriti M, Verma C, Guzman M, et al:
Lapatinib, a HER2 tyrosine kinase inhibitor, induces stabilization
and accumulation of HER2 and potentiates trastuzumab-dependent cell
cytotoxicity. Oncogene. 28:803–814. 2009.
|
|
13
|
Vogel CL, Cobleigh MA, Tripathy D, et al:
Efficacy and safety of trastuzumab as a single agent in first-line
treatment of HER2-overexpressing metastatic breast cancer. J Clin
Oncol. 20:719–726. 2002.
|
|
14
|
Baselga J, Carbonell X, Castañeda-Soto
N-J, et al: Phase II study of efficacy, safety, and
pharmacokinetics of trastuzumab monotherapy administered on a
3-weekly schedule. J Clin Oncol. 23:2162–2171. 2005.
|
|
15
|
Gomez HL, Doval DC, Chavez MA, et al:
Efficacy and safety of lapatinib as first-line therapy for
ErbB2-amplified locally advanced or metastatic breast cancer. J
Clin Oncol. 26:2999–3005. 2008.
|
|
16
|
Kaufman B, Mackey JR, Clemens MR, et al:
Trastuzumab plus anastrozole versus anastrozole alone for the
treatment of postmenopausal women with human epidermal growth
factor receptor 2-positive, hormone receptor-positive metastatic
breast cancer: results from the randomized phase III TAnDEM Study.
J Clin Oncol. 27:5529–5537. 2009.
|
|
17
|
Johnston S, Pippen J Jr, Pivot X, et al:
Lapatinib combined with letrozole versus letrozole and placebo as
first-line therapy for postmenopausal hormone receptor-positive
metastatic breast cancer. J Clin Oncol. 27:5538–5546. 2009.
|
|
18
|
Rimawi MF, Mayer IA, Forero A, et al:
Multicenter phase II study of neoadjuvant lapatinib and trastuzumab
with hormonal therapy and without chemotherapy in patients with
human epidermal growth factor receptor 2-overexpressing breast
cancer: TBCRC 006. J Clin Oncol. 31:1726–1731. 2013.
|
|
19
|
Baselga J, Cortés J, Kim SB, et al;
CLEOPATRA Study Group. Pertuzumab plus trastuzumab plus docetaxel
for metastatic breast cancer. N Engl J Med. 366:109–119. 2012.
|
|
20
|
Swain SM, Kim SB, Cortés J, et al:
Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic
breast cancer (CLEOPATRA study): overall survival results from a
randomised, double-blind, placebo-controlled, phase 3 study. Lancet
Oncol. 14:461–471. 2013.
|