Introduction
Mesenchymal chondrosarcoma (MCS) is a rare and
highly aggressive pathological variant of CS arising from the soft
and hard tissues (1). MCS accounts
for 3–9% of all CS cases (2–7) and
was first identified by Lichtenstein and Bernstein (6) in 1959. In total, 30–50% of MCSs are
extraskeletal in origin. Extraskeletal MCS (EMCS) usually occurs in
the second and third decades of life (7) and affects females more frequently than
males (8). The neoplasm is rare and
aggressive, with a high likelihood for delayed distant metastasis
and late recurrence (3,9), and with a poor prognosis and survival
rate (10). The most common sites
of extraskeletal origin (11–13)
include the lower extremities, orbits and central nervous system,
among others. Only 6% of EMCSs arise from soft tissue of the head
and neck region (14).
Histopathological examination of EMCS reveals a tumor composed of
atypical undifferentiated small cells and islands of matured
chondroid matrix, typically with a bimorphic appearance. Computed
tomography scans usually reveal the presence of various patterns of
calcification within the lesion.
To the best of our knowledge, only one previous case
of primary EMCS involving the buccal region has been reported. In
the current study, a novel case of primary EMCS arising from the
right buccal region in a 26-year-old female is presented and the
literature is reviewed, with a focus on the management of this
tumor. The patient provided written informed consent.
Case report
A 26-year-old female, with no medical history,
presented to the surgical Out-patients Department of the Oral and
Maxillofacial Surgery of Peking University Medical College Hospital
(Chinese Academy of Medical Sciences, Beijing, China), with the
chief complaint of a mass in the right buccal region. The mass was
painless and had gradually increased in size for 12 months. Six
months previously, upon investigation at another hospital, apparent
upper mandibular lymph node swelling was detected in the right
buccal region. The patient was provisionally diagnosed with lymph
node inflammation and was treated with antibiotics. However, the
patient’s condition deteriorated two weeks prior to the
presentation to the Peking University Medical College Hospital.
Extraoral examination revealed a firm mass measuring ~3×2.5 cm in
size, without fixation to the mandible. The overlying skin color
and texture was normal. The mass was well defined and lobulated on
palpation. The facial nerve was not involved with the tumor. A
physical examination and chest X-ray revealed no clinical evidence
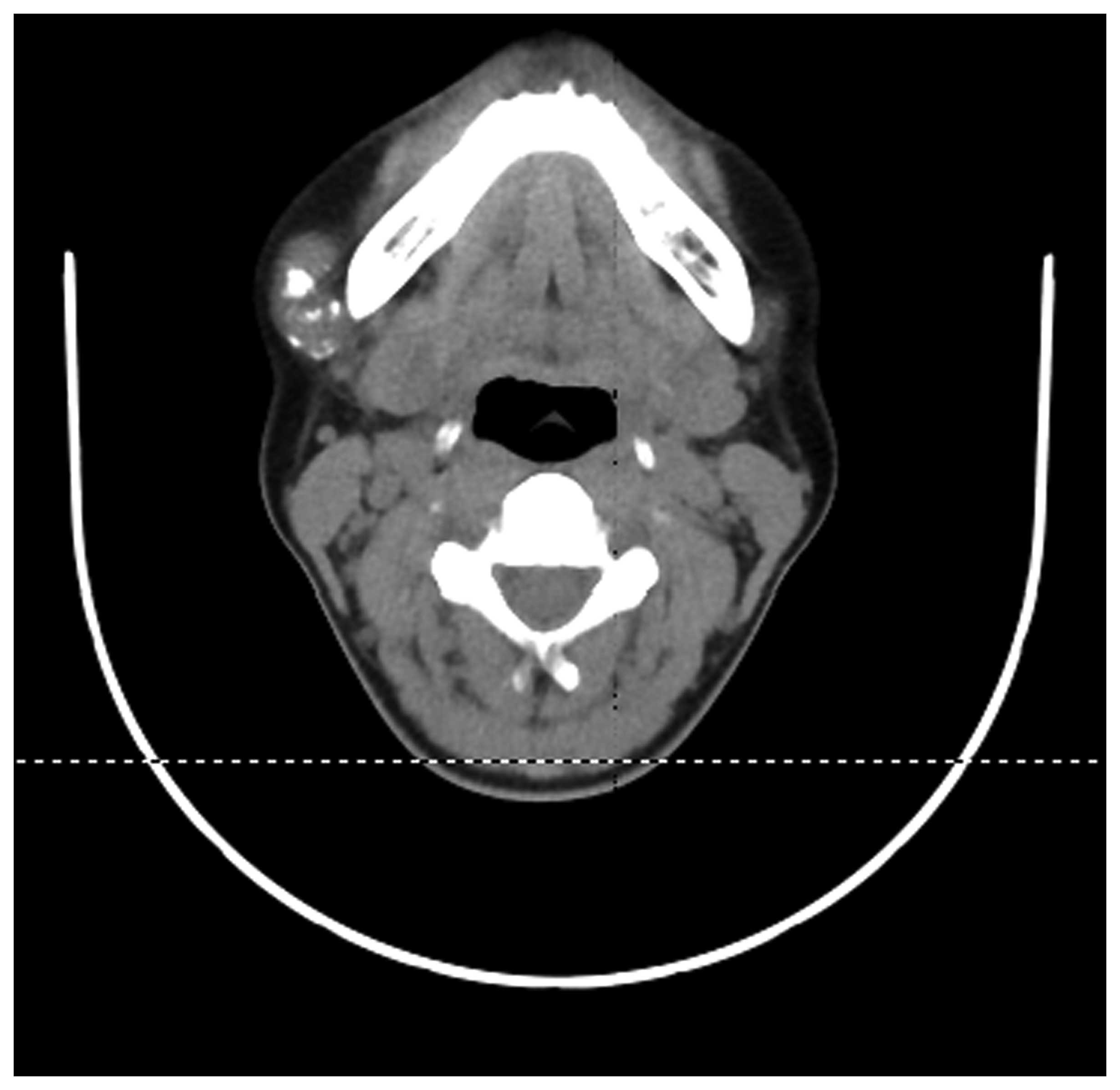
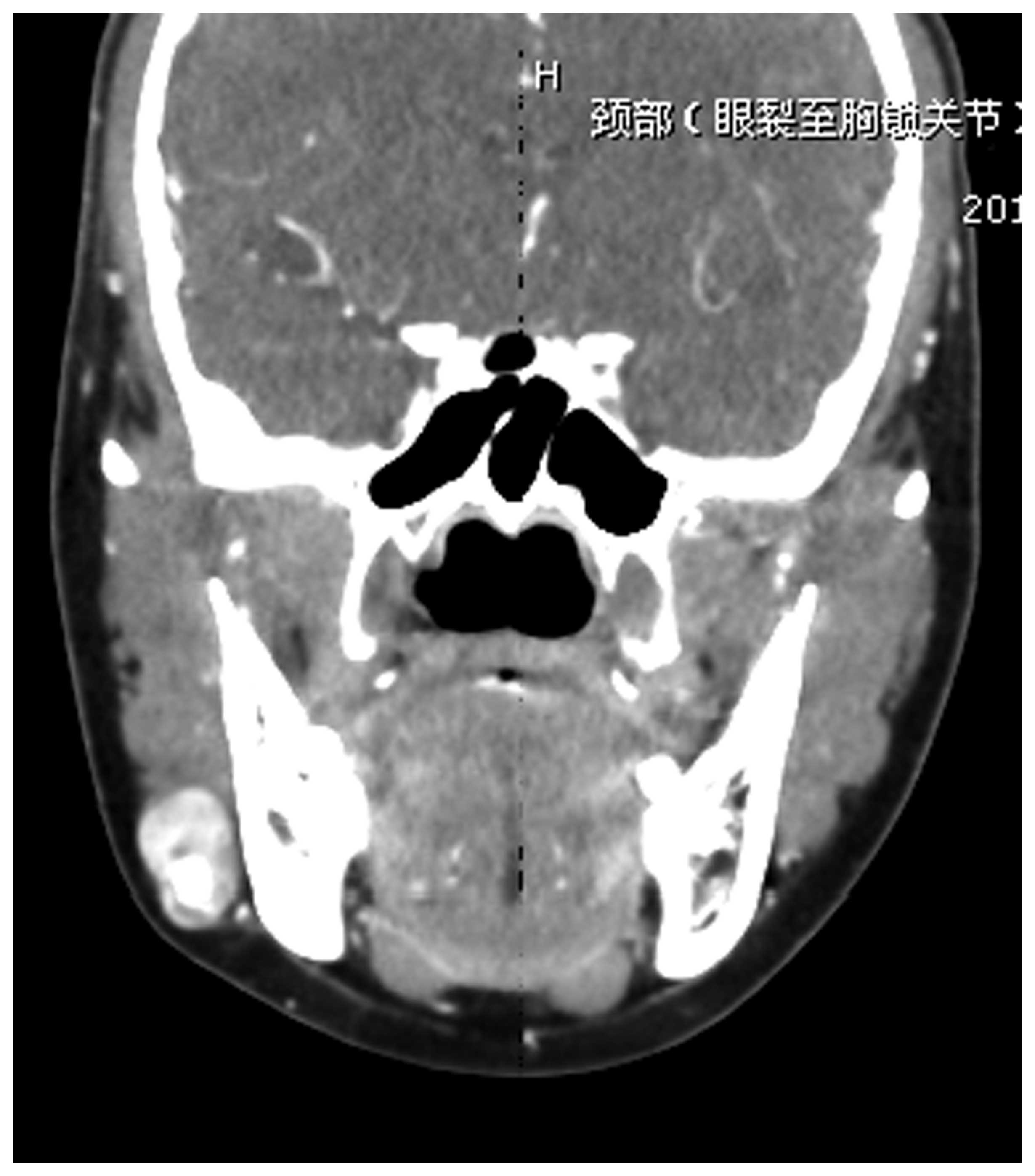
of distant metastasis. Computed tomography (CT) scans revealed a
well-defined mass, without involvement of the mandible (Figs. 1 and 2). The mass was widely resected under
general anesthesia and was subsequently histopathologically
investigated. The post-operative course was uneventful. The gross
appearance of the mass was typically grey or tan in color and
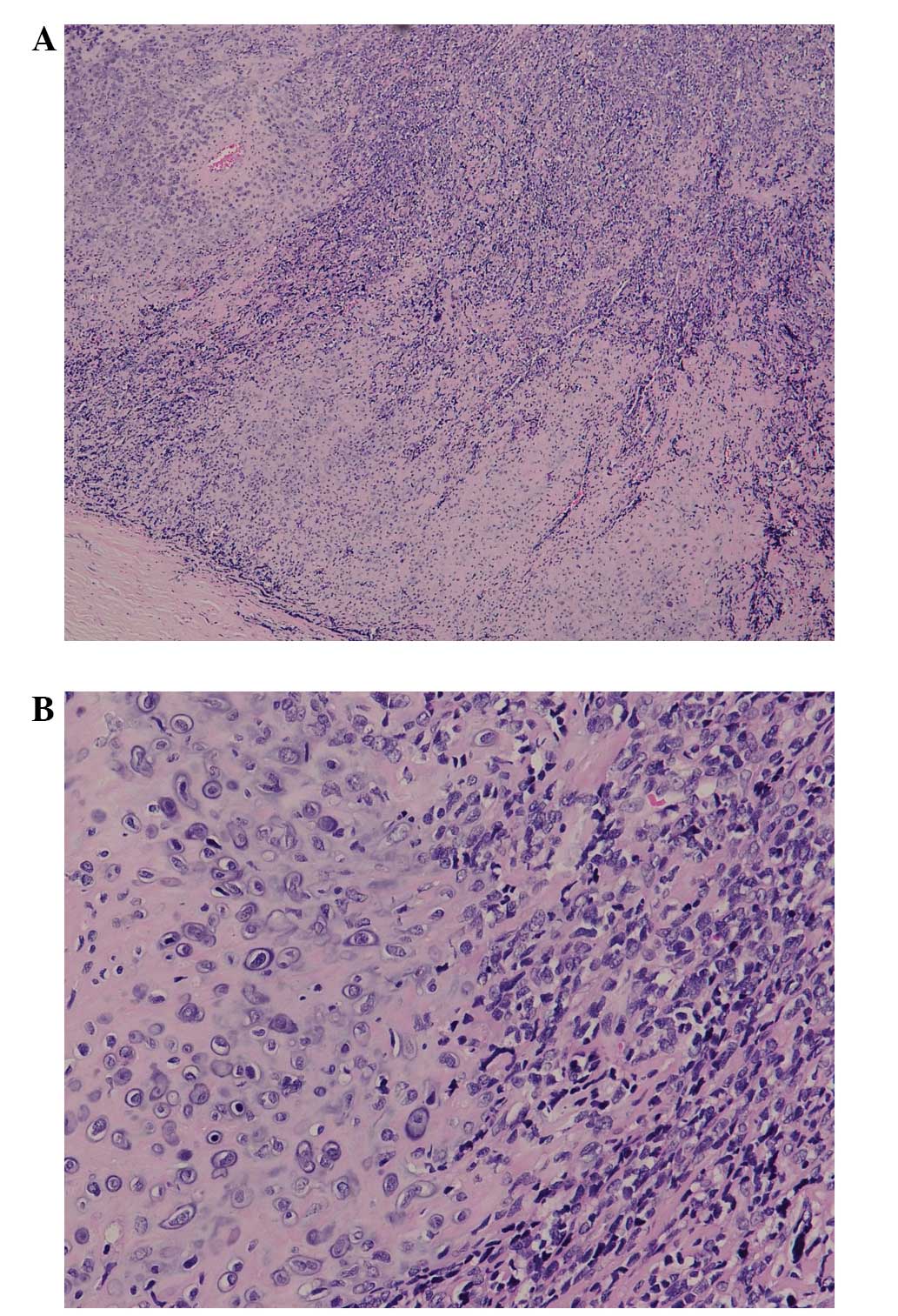
poorly circumscribed. Microscopy revealed a tumor composed of
islands of well-differentiated cartilage surrounded by areas of
ovoid- and spindle-shaped cells exhibiting a hemangiopericytomatous
pattern (Figs. 3 and 4). Focal
calcification was observed in chondroid areas. Immunohistochemical
analysis of the tumor cells revealed positivity for vimentin,
S-100, AE1/AE3, B-cell lymphoma-2, cluster of differentiation
(CD)99 and leukocyte common antigen, and negativity for desmin,
epithelial membrane antigen (EMA) and CD34. Histology and
immunohistochemistry indicated the diagnosis of EMCS. The patient
declined chemotherapy and radiotherapy, but continued to attend
follow-up appointments. No local recurrence or metastasis was
identified following surgery. To date, no local reccurence or
metastasis has been identified in the patient.
Discussion
MCS is a rare and highly aggressive pathological
variant of CS arising from the soft and hard tissues. MCS accounts
for 3–9% of all CSs. In total, 30–50% of MCSs are extraskeletal in
origin. The most common sites of extraskeletal origin are the lower
extremities, orbits and central nervous system, among others. Rare
occurrences have also been identified in the lungs, ribs, pleura,
spleen and kidneys. EMCS accounts for only 2% of all soft-tissue
sarcomas, and only 6% arise from the soft tissue of the head and
neck region. EMCS exhibits two peaks of incidence in adults,
depending on its location. EMCS of the head and neck usually
affects patients in the second or third decades of life, whereas
EMCS of the deep muscles usually affects patients in the fifth
decade of life (3,14,15).
EMCS exerts a slight predominance in females. In individuals >30
years old, the disease more frequently affects the trunk or the
soft tissues of the limbs. At present, only one previous case of
primary EMCS involving the buccal region has been reported. To the
best of our knowledge, the present study is the second case of
primary EMCS arising from the right buccal region to be
reported.
The clinical signs and symptoms of EMCS include pain
and the presence of a soft mass. A variety of signs and symptoms
are associated with the primary site of involvement, including
facial paresthesia and lip paresis. In certain cases, local surgery
or biopsy may lead to rapid growth. EMSCs are also occasionally
misdiagnosed. The main differential diagnoses of EMCS include
rhabdomyosarcoma, Ewing’s sarcoma, hemangiopericytoma, synovial
sarcoma and other variants of CS, as these also exhibit small round
blue cells (14,16), particularly in biopsy specimens and
fine-needle aspiration cytology (FNAC). Therefore, it is
hypothesized that a pre-operative FNAC pathological examination is
indicative for the correct therapy. However, researchers from
Modena University reported a case of pleural MCS that was
incorrectly diagnosed by FNAC (17). Therefore, the diagnosis of EMCS on
the basis of FNAC remains controversial. This is due to the
difficulty in sampling the two phases of the tumor, the lack of
distinctive clinical and radiographical features and the rarity of
the tumor. Buchon et al (18) suggested wide sampling of the tumor
mass, to detect the cartilaginous component, which is diagnostic of
EMCS.
Typical histological features of MCS, consisting of
small, round or spindled mesenchymal cells interspersed with
islands of hyaline cartilage, have been identified (10,19).
Immunohistochemistry is important, particularly in small biopsies.
Typical immunohistochemistry results include the positivity of the
mesenchymal portion for vimentin, Leu-7 and CD99 (20), and the positivity of the
cartilaginous areas for S-100 protein. However, S-100 positivity is
not specific for CSs and may be observed in other tumors, including
melanomas, malignant peripheral nerve sheath tumors, schwannomas
and histiocytomas. Immunonegativity for cytokeratin, EMA and CD34
is observed in EMCS (21,22). Focal positivity for cytokeratin and
EMA is usually exhibitedby Synovial sarcomas, while
hemangiopericytomas exhibit immunopositivity for CD34 and EMA.
Hoang et al (23) found Sox9
to be consistently positive in the cartilaginous and primitive
mesenchymal cells in EMCS, thus aiding in the differentiation from
other small round blue cell tumors. Collagen type II (24) and friend leukemia virus
integration-1 (25) may prove to be
diagnostic tools, even in MCS samples lacking cartilage
islands.
Although the radiographical appearance is generally
not specific, the two component structures of EMCS with
differentiated cartilage islands interspersed within vascular
undifferentiated mesenchyme are also frequently observed in EMCS
(2,26). Previously, it was reported that
50–100% of EMCSs demonstrate calcification on plain radiography and
CT scans (15,27,28).
The uncalcified areas exhibit low attenuation similar to muscle on
CT. T2-weighted magnetic resonance imaging (MRI) clearly show a
two-component structure composed of calcified and uncalcified areas
(4,25,26),
and enhanced MRI reveals heterogeneous enhancement of each
area.
At present, early surgery is the standard local
treatment, with studies demonstrating improved survival in patients
who undergo wide surgical resection (29). Zakkak et al (30) reviewed various treatment modalities
in the maxilla and mandible and found that the best outcome was
observed following radical surgery. Radiotherapy may be important
(31), however, certain studies
have indicated that EMCS is a radioresistant tumor (32–35).
Previous studies have reported the use of pre-operative radiation
therapy to reduce the tumor bulk prior to radical resection and
prevent further extension and micrometastasis. However, this does
not affect the pre-operative approach. No improvement in prognosis
has been identified with post-operative radiotherapy, even when
there is evidence demonstrating a trend toward increased survival.
Chemotherapy plays a limited role and thus, should be used for
high-grade mesenchymal tumors, local recurrence with aggressive
behavior or in cases with potential for metastasis. A study of 35
cases demonstrated that poorly-differentiated MCSs responds to
combined chemotherapy and radiotherapy, while combined neoadjuvant
chemotherapy and surgery are more effective against more
differentiated tumors, such as the hemangiopericytoma-like variant
(32). Cesari et al
(29) demonstrated clear results
supporting the use of chemotherapy, whereby the disease-free
survival rate of patients at 5–10 years after surgical remission of
the disease was 76 and 17% with and without chemotherapy,
respectively (29). However, two
additional studies contradict these results with regard to
chemotherapy and radiotherapy, reporting that only radical surgery
significantly improves survival, while radiotherapy and
chemotherapy may be of palliative use, independent of the age of
the patient, the site of the tumor and the histological variant
(36,37).
The prognosis is extremely variable, with published
five-year survival rates ranging between 42 and 80.7% (38,39)
and 10-year survival rates ranging between 21 and 67%
(3,13,29,41,42).
In the present study, a rare case of primary EMCS in
the buccal region, with aggressive clinical, radiographic and
histological features was presented. Early radical removal of the
tumor is the standard treatment for EMCS. Adjuvant radiation
therapy and chemotherapy must be considered, due to the
histologically aggressive nature of the tumor. EMCS is a rare
entity that must be considered in the differential diagnosis of
soft-tissue neoplasms with calcification in the oral and
maxillofacial region, particularly in young adults.
References
|
1
|
Lockhart R, Menard P, Martin JP, Auriol M,
Vaillant JM and Bertrand J: Mesenchymal chondrosarcoma of the jaws:
report of four cases. Int J Oral Maxillofac Surg. 27:258–262.
1998.
|
|
2
|
Huvos AG, Rosen G, Dabska M and Marcove
RC: Mesenchymal chondrosarcoma: a clinicopathologic analysis of 35
patients with empasis on treatment. Cancer. 51:1230–1237. 1983.
|
|
3
|
Nakashima Y, Unni KK, Shives TC, Swee RG
and Dahlin DC: Mesenchymal chondrosarcoma of bone and soft tissue:
a review of 111 cases. Cancer. 57:2444–2453. 1986.
|
|
4
|
Salvador AH, Beabout JW and Dahlin DC:
Mesenchymal chondrosarcoma: observations on 30 new cases. Cancer.
28:605–615. 1971.
|
|
5
|
Bertoni F, Picci P, Bacchini P, Capanna R,
Innao V, Bacci G and Campanacci M: Mesenchymal chondrosarcoma of
bone and soft tissues. Cancer. 52:533–541. 1983.
|
|
6
|
Lichtenstein L and Bernstein D: Unusual
benign and chondroid tumors of the bone. A survey of some
mesenchymal cartilage tumors and a malignant chondroblastic tumor
including a few multicentric ones, as well as many atypical benign
chondroblastomas and chondromyxoid fibromas. Cancer. 12:1142–1157.
1959.
|
|
7
|
Vencio EF, Reeve CM, Unni KK and
Nascimento AG: Mesenchymal chondrosarcoma of the jaw bones:
clinicopathologic study of 19 cases. Cancer. 82:2350–2355.
1998.
|
|
8
|
Williams HK, Edwards MB and Adekeye EO:
Mesenchymal chondrosarcoma. Int J Oral Maxillofac Surg. 16:119–124.
1987.
|
|
9
|
Craford JG, Oda D, Egbert M and Myall R:
Mesenchymal chondrosarcoma of the maxilla in a child. Int J Oral
Maxillofac Surg. 53:938–941. 1995.
|
|
10
|
Knott PD, Gannon FH and Thompson LD:
Mesenchymal chondrosarcoma of the sinonasal tract: a
clinicopathological study of 13 cases with a review of the
literature. Laryngoscope. 113:783–790. 2003.
|
|
11
|
Johnson DB, Breidahl W, Newman JS, Devaney
K and Yahanda A: Extraskeletal mesenchymal chondrosarcoma of the
rectus sheath. Skeletal Radiol. 26:501–504. 1997.
|
|
12
|
Hashimoto N, Ueda T, Joyama S, Araki N,
Beppu Y, Tatezaki S, Matsumoto S, Nakanishi K, Tomita Y and
Yoshikawa H: Extraskeletal mesenchymal chondrosarcoma: an imaging
review of ten new patients. Skeletal Radiol. 34:785–792. 2005.
|
|
13
|
Miettinen M: Cartilage and bone-forming
tumors and tumor-like lesions. Diagnostic Soft Tissue Pathology
Philadelphia: Churchill-Livingstone; pp. 407–409. 2003
|
|
14
|
Shapeero LG, Vanel D, Couanet D, Contesso
G and Ackerman LV: Extraskeletal mesenchymal chondrosarcoma.
Radiology. 186:819–826. 1993.
|
|
15
|
Okamoto Y, Minami M, Ueda T, Inadome Y,
Tatsumura M and Sakane M: Extraskeletal mesenchymal chondrosarcoma
of the cervical meninx. Radiat Med. 25:355–358. 2007.
|
|
16
|
Neff B, Sataloff RT, Storey L, Hawkshaw M
and Spiegel JR: Chondrosarcoma of the skull base. Laryngoscope.
112:134–139. 2002.
|
|
17
|
Luppi G, Cesinaro AM, Zoboli A, Morandi U
and Piccinini L: Mesenchymal chondrosarcoma of the pleura. Eur
Respir J. 9:840–843. 1996.
|
|
18
|
Buchon R, Thoumas D, Talarmin F, Vicens JL
and Flageat J: Mesenchymal extra-skeletal chondrosarcoma. Apropos
of a case. Ann Radiol (Paris). 33:205–208. 1990.(In French).
|
|
19
|
Fletcher DM and Unni KK: World Health
Organization Classification of Tumors Pathology Genetics of Tumors
of Soft Tissue and Bone. Lyon: IARC Press; pp. 247–257. 2002
|
|
20
|
Granter SR, Renshaw AA, Fletcher CD, Bhan
AK and Rosenberg AE: CD99 reactivity in mesenchymal chondrosarcoma.
Hum Pathol. 27:1273–1276. 1996.
|
|
21
|
Swanson PE, Lillemoe TJ, Manivel JC and
Wick MR: Mesenchymal chondrosarcoma: an immunohistochemical study.
Arch Pathol Lab Med. 114:943–948. 1990.
|
|
22
|
Hoang MP, Suarez PA, Donner LR, Ro JY,
Ordónẽz NG, Ayala AG and Czerniak B: Mesenchymal chondrosarcoma: a
small cell neoplasm with polyphenotypic differentiation. Int J Surg
Pathol. 8:291–301. 2000.
|
|
23
|
Wehrli BM, Huang W, De Crombrugghe B,
Ayala AG and Czerniak B: Sox9, a master regulator of
chondrogenesis, distinguishes mesenchymal chondrosarcoma from other
small blue round cell tumors. Hum Pathol. 34:263–269. 2003.
|
|
24
|
Muller S, Soder S, Oliveira AM, Inwards CY
and Aigner T: Type II collagen as specific marker for mesenchymal
chondrosarcoma compared to other small cell sarcomas of the
skeleton. Mod Pathol. 18:1088–1094. 2005.
|
|
25
|
Lee AF, Hayes MM, LeBrun D, Espinosal,
Nielsen GP, Rosenberg AE and Lee CH: FLI-1 distinguishes Ewing
sarcoma from small cell osteosarcoma and mesenchymal
chondrosarcoma. Appl Immunohistoche Mol Morphol. 19:233–238.
2011.
|
|
26
|
Hashimoto N, Ueda T, Joyama S, Araki N,
Beppu Y, Tatezaki S, Matsumoto S, Nakanishi K, Tomita Y and
Yoshikawa H: Extraskeletal mesenchymal chondrosarcoma: an imaging
review of ten new patients. Skeletal Radiol. 34:785–792. 2005.
|
|
27
|
Shakked RJ, Geller DS, Gorlick R and
Dorfman HD: Mesenchymal chondrosarcoma: clinicopathologic study of
20 cases. Arch Pathol Lab Med. 136:61–75. 2012.
|
|
28
|
Yang BT, Wang ZC, Liu S, Xian JF, Zhang
ZY, Liu ZL and Lan BS: CT and MRI diagnosis of chondrosarcoma in
sinonasal and orbital region. Chin J Radiol. 40:572–576. 2006.
|
|
29
|
Cesari M, Bertoni F, Bacchini P, Mercuri
M, Palmerini E and Ferrari S: Mesenchymal chondrosarcoma: an
analysis of patients treated at a single institution. Tumori.
93:423–427. 2007.
|
|
30
|
Zakkak TB, Flynn TR, Boguslaw B and Adamo
AK: Mesenchymal chondrosarcoma of the mandible: case report and
review of the literature. J Oral Maxillofac Surg. 56:84–91.
1998.
|
|
31
|
Vencio EF, Reev CM, Unni KK and Nascimento
AG: Mesenchymal chondrosarcoma of the jaw bones: clinicopathologic
study of 19 cases. Cancer. 82:2350–2355. 1998.
|
|
32
|
Herrera A, Ortega C, Reyes G, Alvarez MA
and Tellez D: Primary orbital mesenchymal chondrosarcoma: case
report and review of the literature. Case Rep Med.
2012:2921472012.
|
|
33
|
Ruark DS, Schlehaider UK and Shah JP:
Chondrosarcomas of the head and neck. World J Surg. 16:1010–1016.
1992.
|
|
34
|
Angiero F, Vinci R, Sidoni A and Stefani
M: Mesenchymal chondrosarcoma of the left coronoid process: report
of a unique case with clinical, histopathologic, and
immunohistochemical findings, and a review of the literature.
Quintessence Int. 38:349–355. 2007.
|
|
35
|
Gallego L, Junquera L, Fresno MF and de
Vicente JC: Chondrosarcoma of the temporomandibular joint: A case
report and review of the literature. Med Oral Patol Oral Cir Bucal.
14:E39–E43. 2009.
|
|
36
|
Kawaguchi S, Weiss I, Lin PP, Huh WW and
Lewis VO: Radiation therapy is associated with fewer recurrences in
mesenchymal chondrosarcoma. Clin Orthop Relat Res. 472:856–864.
2014.
|
|
37
|
Burkey BB, Hoffman HT, Baker SR, Thornton
AF and McClatchy KD: Chondrosarcoma of the head and neck.
Laryngoscope. 100:1301–1305. 1990.
|
|
38
|
Geirnaerdt MJ, Bloem JL, Eulderink F,
Hogendoorn PC and Taminiau AH: Cartilaginous tumors: correlation of
gadolinium-enhanced MR imaging and histopathologic findings.
Radiology. 186:813–817. 1993.
|
|
39
|
Varma DG, Ayala AG, Carrasco CH, Guo SQ,
Kumar R and Edeiken J: Chondrosarcoma: MR imaging with pathologic
correlation. Radiographics. 12:687–704. 1992.
|
|
40
|
Dantonello TM, Int-Veen C and Leuschner I:
Mesenchymal chondrosarcoma of soft tissues and bone in children,
adolescents, and young adults: experiences of the CWS and COSS
study groups. Cancer. 112:2424–2431. 2008.
|
|
41
|
Miettinen M: From morphological to
molecular diagnosis of soft tissue tumors. Adv Exp Med Biol.
587:99–113. 2006.
|