Introduction
Pancreatic cancer is known to metastasize rapidly,
most commonly to the liver and peritoneum, followed by the lungs,
bones and brain (1,2). The occurrence of cutaneous metastases
is rare, predominantly presenting in close proximity to the
umbilicus and is termed Sister Mary Joseph’s nodule (SMJN)
(3). Non-umbilical cutaneous
metastases are rare, with only a small number of cases reported
(4,5). The present report describes a case of
multiple cutaneous metastatic lesions associated with pancreatic
cancer, reviews the published literature regarding cutaneous
metastasis from pancreatic cancer (by conducting a detailed PubMed
search). Furthermore, an analysis of 63 reported cases of cutaneous
metastasis from pancreatic cancer was conducted with regard to the
clinical and pathological characteristics, treatment strategies and
survival outcomes, thus providing an overview of this rare type of
disease manifestation. Written informed consent was obtained from
the patient’s family.
Case report
In May 2012, a 76-year-old female was referred to
the Department of Oncology (Shandong Cancer Hospital and Institute,
Jinan, China) with the complaint of nausea and a lack of appetite
for 10 days prior to referral. The patient had experienced
gallstones for several years. Upon physical examination,
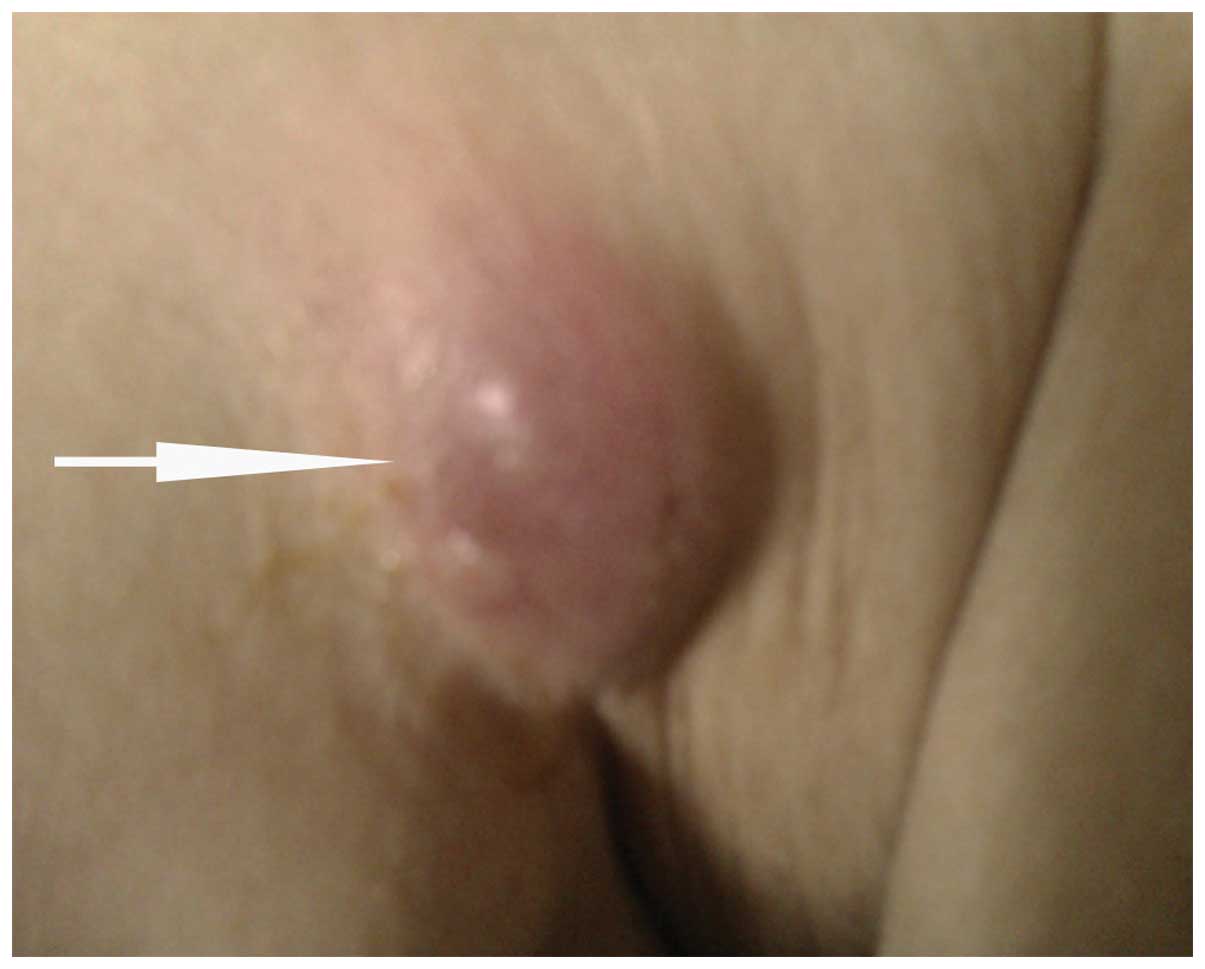
asymptomatic violaceous nodules were observed on the right anterior
axillary fold, occipital scalp, chest and abdomen (Fig. 1). Completion of the physical
examination identified no further abnormalities. Routine laboratory
testing revealed that renal and hepatic markers were within the
normal ranges, including white blood cell (9.2×109/l;
normal range, 3.5–9.5×109/l), platelet
(301×109/l; normal range, 125–350×109/l)
count, serum alanine aminotransferase (10 U/l; normal range, 7–40
U/l), blood urea nitrogen (3.98 mmol/l; normal range, 2.9–8.2
nmol/l), creatinine (45 μmol/l; normal range, 45–84 μmol/l) and
glucose levels (5.85 mmol/l; normal range, 5.85 mmol/l). The
hemoglobin (111 g/l; normal range, 115–150 g/l) levels were
slightly below the normal range, potentially due to the increased
age of the patient and low food uptake, the albumin (35.1 g/l;
normal range, 40–55 g/l) level may be lower than the normal range
due to a decrease in food uptake and decreased synthesized liver
function due to metastasis, potassium levels (3.4 mmol/l; normal
range, 3.5–5.3 mmol/l) were lower than the normal range potentially
due to decreased food uptake and increased fibrinogen levels (4.48
g/l; normal range, 2–4 g/l) may correlate with the end-stage of the
disease (6). Serum carbohydrate
antigen (CA)19-9 (2.1 U/l; normal range, 0–39 U/l), cancer antigen
72-4 (5.06 U/l; normal range, 0–6.9 U/l) and α-fetoprotein (2.52
ng/ml; normal range, 0–7 ng/ml) were also within normal limits,
however, carcinoembryonic antigen (CEA) was elevated to 27.54 ng/ml
(reference range, 0–3.4 ng/ml). A computed tomography (CT) scan of
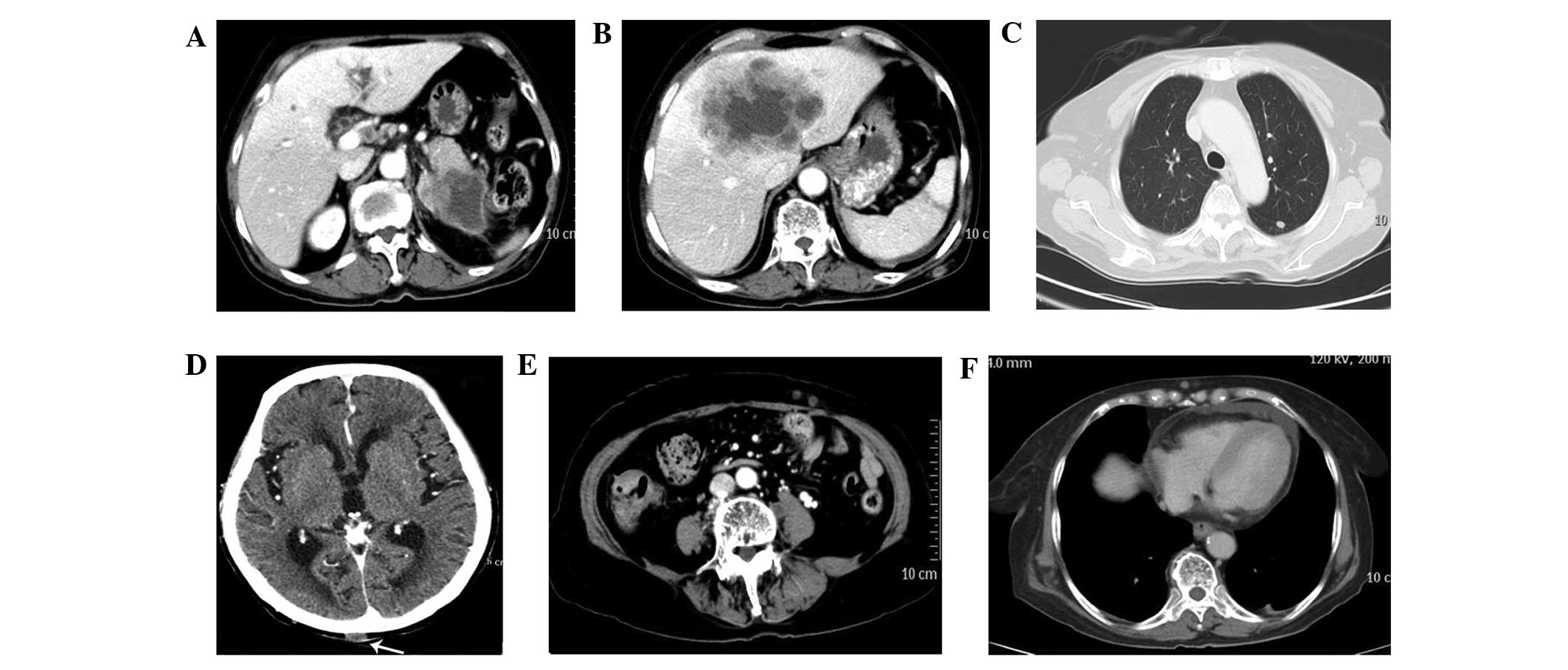
the abdomen, chest, pelvis and brain was performed, which revealed
an enlarged pancreatic tail containing a low-density soft tissue
mass measuring ~7 cm in diameter. Post-peritoneum lymph node
enlargement, lesions on the lungs, hepatic focal lesions (maximum
size, ~8×10 cm) and multiple subcutaneous nodules were also
identified on the right upper chest, occipital scalp, upper arm and
abdomen (Fig. 2). Examination and
thorough investigation did not detect metastases elsewhere (for
example in the ovaries or brain). Abdominal ultrasound and CT
findings were consistent with signs for cancer of the tail of the
pancreas with multiple metastatic lesions. The nodule of skin at
the right anterior axillary fold was removed for biopsy and
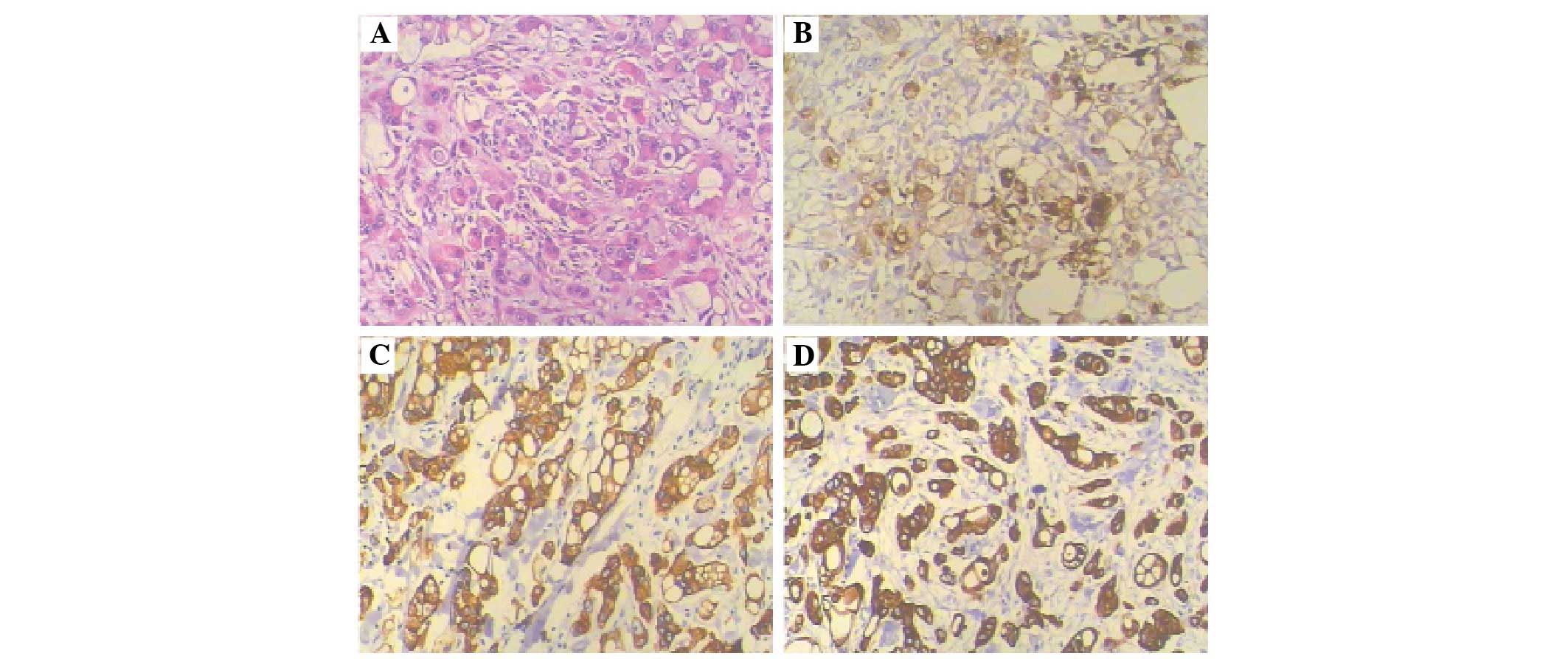
pathological examination of the excised lesion identified a poorly
differentiated metastatic adenocarcinoma involving the dermis and
subcutaneous tissue. Immunohistochemical staining (Fig. 3) revealed that the tumor was weakly
and focally positive for CEA (Fig.
3B) and strongly positive for cytokeratin (CK) 7 (Fig. 3C) and CK19 (Fig. 3D). Staining was negative for
estrogen and progesterone receptors, thyroid transcription
factor-1, and CK20 (not shown). Thus, the patient was diagnosed
with stage IV disease, according to the American Joint Committee on
Cancer TNM staging system for pancreatic cancer (7) and was subsequently treated with one
cycle of gemcitabine. Prior to administration of the second cycle
of gemcitabine (1.2 g, days 1 and 8, every three weeks), the lesion
at the occipital scalp enlarged due to a superficial ulceration. As
a result of disease progression, the patient was administered with
oxaliplatin (100 mg, day 1, every two weeks) combined with S-1
capsules; however, the patient rapidly deteriorated and succumbed
following two months of treatment. An autopsy was not permitted for
this patient.
A literature search of the electronic PubMed
database (www.ncbi.nlm.nih.gov/pubmed; up to
July 2013) was conducted using Medical Subject Headings (www.nlm.nih.gov/mesh), keywords and by limiting the
search to human studies. The terms pancreatic cancer, pancreatic
neoplasm, cutaneous metastasis and SMJN were used. The abstracts
were reviewed, and articles that were not associated with to the
specific topic were excluded. Duplicate references as well as
repeated publications were discarded. All of the studies that were
considered to be eligible were retrieved and the final selection
was based on the full article. Only those patients with a confirmed
pathological diagnosis, as a result of a biopsy or an autopsy of
the skin lesion or pancreatic tumor, were included. Articles in
which skin lesions presented with paraneoplastic syndrome
associated with pancreatic cancer were excluded.
Furthermore, the reference lists were screened to
identify additional eligible articles. An analysis of the published
studies, concerning clinical and pathological characteristics,
treatment strategies and survival outcomes, was performed. Data
extraction included the following parameters: i) The age, gender
and the location of the pancreatic tumors of each patient; ii) the
site, number, appearance and association with any incision or
surgery of the cutaneous metastatic lesions; iii) the serum tumor
marker levels, histopathological types, grade and
immunohistochemistry of the primary tumor; iv) lymph node and
distal metastases; v) therapy, including chemotherapy, radiotherapy
and surgery; and vi) survival information. In addition, the
original authors were contacted for supplementary information and
long-term survival data. Overall survival from the time of the
initial diagnosis of the skin metastases to the date of last
contact (or the date the patient succumbed to the disease) was
calculated using the Kaplan-Meier method.
The literature search resulted in 62 cases of
cutaneous metastasis of pancreatic cancer. The data of all 63
patients (including the current case) are summarized in Table I.
 | Table IClinical characteristics of patients
with cutaneous metastasis from pancreatic cancer. |
Table I
Clinical characteristics of patients
with cutaneous metastasis from pancreatic cancer.
| Characteristic | Patients, n (%) |
|---|
| Age, years | |
| ≤60 | 23 (36.5) |
| 60–80 | 34 (54) |
| >80 | 6 (9.5) |
| Gender | |
| Male | 39 (61.9) |
| Female | 24 (38.1) |
| Location | |
| Umbilicus only | 18 (28.6) |
| Non-umbilicus
only | 43 (68.3) |
| Umbilicus and
non-umbilicus concurrently | 2 (3.1) |
| Skin metastasis and
pancreatic cancer | |
| Skin lesion
first | 35 (55.6) |
| Pancreatic cancer
first | 18 (28.6) |
| Concurrently | 7 (11.1) |
| No details | 3 (4.8) |
| Appearance | |
| Nodule or mass | 46 (73.0) |
| Plaque, swelling or
thickening | 11 (17.5) |
| Cellulitis | 1 (1.6) |
| No details | 5 (7.9) |
| Lesions, n | |
| Single | 42 (66.7) |
| Multiple | 21 (33.3) |
| Association with
local surgery | |
| Yes | |
| Drainage site | 8 (12.7) |
| Incision site | 2 (3.2) |
| Needle tract | 7 (11.1) |
| Transplanted
skin | 1 (1.6) |
| No | 45 (71.4) |
| Primary tumor
site | |
| Head or uncus | 21 (33.3) |
| Body | 9 (14.3) |
| Tail | 16 (25.4) |
| Body and tail | 8 (12.7) |
| Majority of the
pancreas | 1 (1.6) |
| No details | 7 (11.1) |
| Histology | |
| Adenocarcinoma | 53 (84.1) |
| Mucin-secreting
adenocarcinoma | 4 (6.3) |
| Adenosquamous cell
carcinoma | 1 (1.6) |
| Large cell
undifferentiated carcinoma | 1 (1.6) |
| Vasoactive
intestinal polypeptide tumor | 1 (1.6) |
| Metastatic islet
cell amphicrine carcinoma | 1 (1.6) |
| Mucinous
cystadenocarcinoma | 1 (1.6) |
| Intraductal
papillary mucinous carcinoma | 1 (1.6) |
| Lobular
panniculitis | 1 (1.6) |
| No details | 3 (4.7) |
| Grade (32 cases
available) | |
|
Well-differentiated | 7 (21.8) |
| Moderately or poorly
differentiated | 25 (78.1) |
| Immunochemistry | |
| CK7-positive | 11/11 (100.0) |
| CK19-positive | 5/5 (100.0) |
| CA19-9-positive | 11/12 (91.7) |
| CEA-positive | 6/8 (75.0) |
| CK20-negative | 4/5 (80.0) |
| PSA-positive | 2/6 (33.3) |
| Concurrent
metastasis | |
| Lymphoma metastasis
(25 cases available) | |
| Yes | 20 (80.0) |
| No | 5 (20.0) |
| Distal metastasis
(43 cases available) | |
| Liver | 26 (60.5) |
| Peritoneum | 21 (48.8) |
| Lung | 11 (25.6) |
| No distal
metastasis | 3 (7.0) |
| Therapy (34 cases
available) | |
| Chemotherapy | 20 (58.8) |
| Surgery | 15 (44.1) |
| Radiation | 6 (17.6) |
| No therapy | 10 (29.4) |
| Survival time after
skin metastasis (47 cases available) | |
| <6 months | 25 (53.2) |
| ≥6 months | 22 (46.8) |
The average patient age was 62.9 years (range, 40–85
years). Males constituted a marginally greater proportion of the
cohort (61.9%; 39/63), however, no significant difference was
identified in patient gender.
Among the locations of the cutaneous metastases,
skin lesions at non-umbilicus sites were more common than at
umbilicus sites (43 vs. 18), and only two cases exhibited
non-umbilicus and umbilicus metastases concurrently. The majority
of the skin lesions were singular (66.7%; 42/63), particularly in
patients exhibiting SMJN (90.0%; 18/20; data not shown). The
predominant manifestation of the cutaneous skin metastases was a
nodule or mass (73.0%; 46/63). Other manifestations included
plaques, swelling or thickening (17.5%; 11/63) and one case of
cellulitis. Over a quarter (28.6%; 18/63) of the skin lesions were
located at local puncture or surgery locations, including surgical
or biliary drainage sites (12.7%; 8/18), incision sites (3.2%;
2/18), the needle tract (11.1%, 7/18) or sites of skin
transplantation due to burn (1.6%; 1/18).
Among the sites of the primary tumor, the pancreatic
head or uncus accounted for 21/63 of cases (33.3%), the body for
nine cases (14.3%), the tail for 16 cases (25.4%), the body and
tail for eight cases (12.7%), and the majority of the pancreas in
one case. Seven of the cases did not provide detailed
information.
A wide range of histological subtypes were
presented, with a predominance of adenocarcinoma (84.1%; 53/63),
including four cases of mucin-secreting adenocarcinoma. Each of the
following histological subtypes were observed in just one case of
those evaluated in this report: Adenosquamous carcinoma, large-cell
undifferentiated carcinoma, vasoactive intestinal polypeptide
tumor, metastatic islet cell amphicrine carcinoma, mucinous
cystadenocarcinoma and intraductal papillary mucinous carcinoma.
The tumor histology was described without specifying the subtype in
three cases.
The differentiation grade of the tumor cells was
described in 32 cases and the majority of the known pathologies
were graded as moderately or poorly differentiated (78.2%; 25/32).
However, 31 cases did not provide detailed information regarding
the differentiation grade.
Immunohistochemical analysis of CK7 was performed in
11 cases and was positive in 100% of cases, CK19 was also positive
in 100% of cases (5/5), CA19-9 was positive in 91.7% of cases
(11/12), CEA was positive in 75% of cases (6/8) and
prostate-specific antigen was positive in 33.3% of cases (2/6).
CK20 was weakly positive in one case and negative in four cases.
Forty cases did not provide detailed information regarding the
immunohistochemistry of the tumor cells.
Concurrent local lymph node metastasis was noted in
20/25 cases, however, the relevant information was not provided by
38 cases. Concurrent distal metastases (other than the skin) was
observed in 43 cases (67.2%) including the liver (26 cases), the
peritoneum (21 cases) and the lungs (11 cases). Only three cases
did not develop concurrent metastases.
Treatment consisted of chemotherapy in 20 cases,
surgery in 15 cases and radiotherapy in six cases. The survival
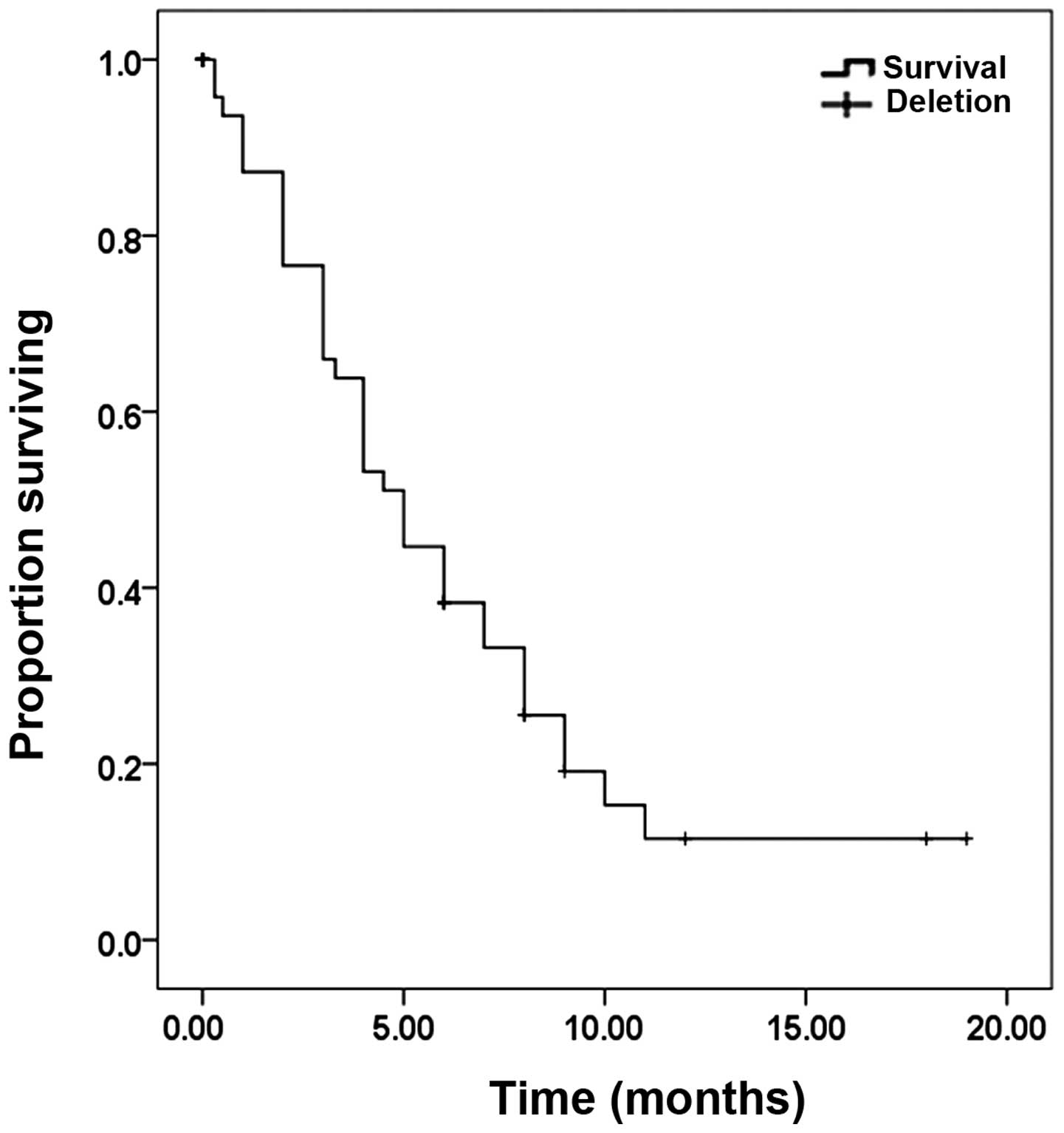
period was evaluated in 42 patients and ranged from a few days to
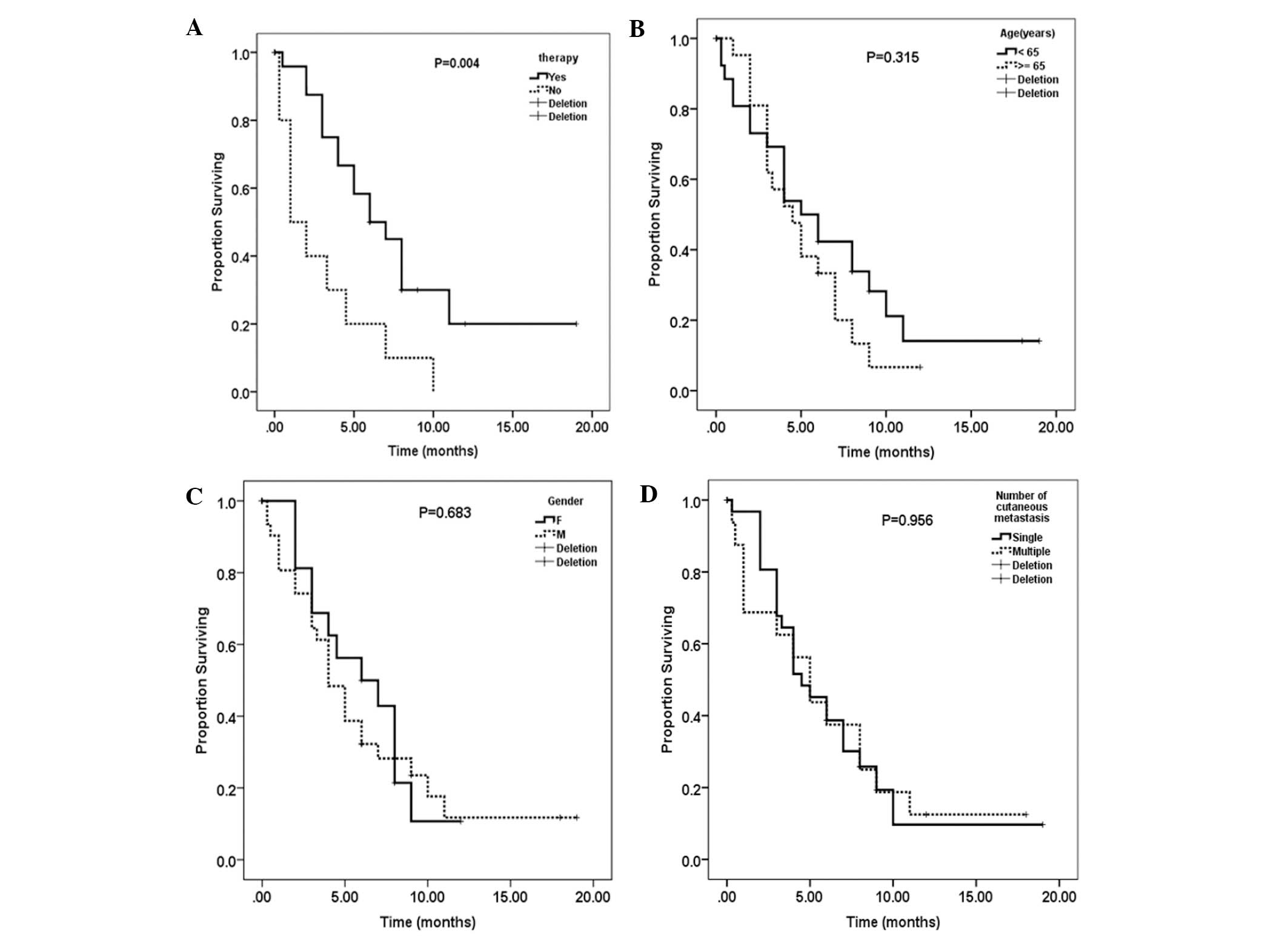
~19 months, with a median value of five months (Fig. 4). Therapy, including surgery,
chemotherapy, radiation or a combination improved the survival time
from 3.0 to 8.3 months (P=0.004; Fig.
5A). Younger patients (aged <65 years) exhibited improved
survival when compared with those aged >65 years, with a mean
survival time of 7.0 vs. 5.1 months, respectively; however, the
difference was not identified to be significant (P=0.315; Fig. 5B). Gender and the number of skin
lesions had no influence on overall patient survival (Fig. 5C and D).
Discussion
Metastasis of pancreatic cancer is the leading cause
if disease worldwide, and the pattern of metastasis commonly
includes the regional lymph nodes, liver, peritoneum, lungs and
brain. Uncommon sites of metastases from pancreatic cancer include
the muscle, skin, heart, pleura and stomach (8). Cutaneous metastasis from pancreatic
carcinoma is considered to be particularly rare (3,4). In a
study of 420 patients with cutaneous metastases, Lookingbill et
al (9) identified just two
cases (0.48%) originating in the pancreas. Cubilla and Fitzgerald
(10) reported that 9/119 patients
(7.6%) with pancreatic cancer exhibited cutaneous metastases at
autopsy. In the present report 63 cases of cutaneous metastases
originating from pancreatic cancer were identified during a
systematic literature review and are discussed in detail.
The review revealed that cutaneous metastatic
disease from pancreatic cancer occurs in all ages, ranging from 40
to 85 years, however, more commonly occurs in older patients, with
a mean age of 62.9 years and 63.5% patients aged >60 years. The
gender ratio of males to females was 1.63:1 (39:24), demonstrating
a male preponderance. This was consistent with a recent report by
Yendluri et al (11), in
which the male to female ratio was 1.3:1.
Horino et al (12) reported 49 cases of metastatic
pancreatic carcinoma to the skin and revealed that skin lesions
were the initial sign of pancreatic cancer in 46 cases (93.9%),
with umbilical lesions being the most common metastatic location on
the skin (45.5%). Miyahara et al (13) also reported that cutaneous
metastases were present prior to the diagnosis of pancreatic cancer
in 20/22 patients (90.1%). Metastatic lesions in the skin were the
first symptoms of pancreatic cancer in 11 cases and the lesions
were identified by physical examination in nine cases. The present
review identified that skin lesions were the first sign of
pancreatic cancer in 35 cases (55.6%). Concurrent physical
examination identified seven cases (10.9%) and just 18 cases were
identified subsequent to the diagnosis of pancreatic carcinoma.
Miyahara et al (13) reported that skin lesions were
present in the umbilicus in 16/22 cases (72.7%) of cutaneous
metastasis of pancreatic cancer. However, in the present review, a
high proportion of non-umbilicus (vs. umbilicus) lesions were
recorded, with a ratio of 43:18 (2.4:1).
Pancreatic cancer has the ability to metastasize to
all cutaneous tissue, most frequently to the umbilicus.
Non-umbilical cutaneous nodules have also been observed on the
scalp (4), neck (14), temple (5), thorax, epigastric region and axilla
(15,16). Local surgery is closely associated
with the site of the cutaneous metastases of pancreatic cancer.
Data from the present review concurred with this, identifying 18
cases (28.6%) in which the metastatic lesion was closely associated
with the site of local drainage, incision, surgery, needle puncture
or skin transplantation (17–23).
Therefore, careful management should be taken during surgery to
avoid the implantation of tumor cells that results in
metastasis.
The locations of the primary pancreatic carcinoma in
the present review were the head (33.3%), body (14.3%), tail
(25.4%), and the body and the tail (12.7%) of the pancreas. This
corresponds with a previous report by Horino et al (12), which identified that the locations
of the primary pancreatic carcinoma were the head (32.3%), body
(12.9%), tail (32.3%) and the body and the tail (19.4%) of the
pancreas. Yendluri et al (11) stated that although 70–80% of
pancreatic cancer arises in the head of the pancreas, in patients
presenting with an SMJN, the majority (91%) were in the tail and
body of the pancreas. The present review was consistent with this,
with 76.2% of pancreatic tumors present in the tail and body of the
pancreas of the SMJN patients. However, in the present report, skin
lesions resulting from a primary tumor in the head of the pancreas
account for a relatively smaller proportion (36.6%; 9/25) of
non-umbilical cutaneous metastases from pancreatic carcinoma. Hafez
(24) reviewed 17 cases of
non-umbilical cutaneous metastasis from pancreatic carcinoma and
identified that the site of the pancreatic tumor was commonly in
the head of the pancreas (52.8%). We hypothesize that this may be
due to tumors of the pancreatic head having a different metastatic
route from tumors of the pancreatic tail.
The present report identified that the histological
subtype of pancreatic cancer varies, although adenocarcinoma
accounts for 84.1% of cases. Moderately or poorly differentiated
grade tumors constitute 78.1% (25/32) of those cases with a known
pathology. This is due to poorly differentiated tumor cells
exhibiting a more aggressively invasive phenotype (25).
Immunohistochemistry demonstrating positivity for
CK7, CK19 and CA19-9 indicated that these markers had high
specificity in the diagnosis of pancreatic cancer. This is
important, particularly in cases where the skin lesion is observed
prior to the primary tumor. The expression of CK20 can be variable;
Matros et al (26) used
tissue specimens from 103 patients and demonstrated that CK20
expression was present in 63% of pancreatic adenocarcinoma.
However, in the present review CK20 was weakly positive in one case
and negative in four cases, indicating that CK20 negativity may
serve as a specific diagnostic marker of pancreatic carcinoma. The
case presented in the current report identified that the tumor
cells were positive for CK7 and CK19, and negative for CK20,
estrogen and progesterone receptors, as well as thyroid
transcription factor-1. This immunophenotype was consistent with
metastasis from the patient’s primary diagnosis of pancreatic
adenocarcinoma.
In the present study 70% of cases exhibited elevated
leveks of CA19-9 and CEA. However, in a study by Gui et al
(27), the proportion of patients
with elevated CA19-9 and CEA levels was 80.3%.
When cutaneous metastases are present, pancreatic
cancer is usually widely disseminated. Takeuchi et al
(16) reported the involvement of
other organs at the time of diagnosis of the skin metastases in
eight out of nine patients. Horino et al (12) reviewed 49 reported cases of
pancreatic metastasis and found that 90.3% of the cases exhibited
multiple organ metastases or peritoneal seeding. The present review
demonstrated that in 43/46 (93.5%) cases that were evaluated,
distal metastasis had occurred. The majority of distal metastases
occurred in the liver, followed by the peritoneum and the lungs,
which is consistent with the above-mentioned reports.
Various theories of cutaneous metastasis have been
proposed, however, no specific mechanism has been elucidated. These
theories include the soil and seed hypothesis, direct invasion,
lymphatic or hematogenous dissemination and the chemotaxis
hypothesis (11,12,28,29).
Tumor seeding during resection is a feared complication as
recurrence within the peritoneal cavity commonly occurs following
resection with curative intent. Consistent with this theory, the
present review identified 18 cases in which tumor seeding was
associated with trauma.
According to Yendluri et al (11), the average survival time for SMJN
patients was less than four months, which is the time scale
expected for stage IV pancreatic cancer. Takeuchi et al
(16) reported that seven out of
nine cases of skin metastasis from pancreatic cancer succumbed
within seven months of the diagnosis. The findings of the present
report demonstrated that survival time ranged from a few days to
~19 months with a median value of five months, which is consistent
with the above-mentioned reports. Therapy, including surgery,
chemotherapy, radiation or a combination improved the survival time
from 3.0 to 8.3 months (P=0.004). This significant difference may
be explained by certain patients having a low Karnofsky score
(30), therefore, not being
physically able to undergo curative surgery. Younger patients (aged
<65 years) had a longer survival time when compared with those
patients aged >65 years, with a mean survival time of 7.0 vs.
5.1 months, respectively; however, the difference was not
significant (P=0.315). Only 63 patients were evaluated in the
present review and a larger sample size may have led to a
significant difference. By contrast, there was no difference in
survival between males and females or between patients with a
single skin lesion and multiple skin lesions.
In conclusion, pancreatic cancer rarely presents as
cutaneous metastases, however, this possibility should be
considered in the differential diagnosis, particularly when the
malignant skin lesion is of unknown origin. Immunohistochemical
staining with a specific antibody (for example CK19 or CK7) may aid
in the elucidation of the origin of the underlying tumor, which may
guide further management of the treatment strategy. Although the
patients exhibited stage IV pancreatic cancer with cutaneous
metastasis, they appeared to demonstrate improved outcomes as a
result of treatment with a combination of surgery, radiotherapy and
chemotherapy.
Acknowledgements
The authors would like to thank Dr Agustin for the
translation from Spanish to English. The authors would also like to
thank Dr Ilario de Sio for providing the patient follow-up
information.
References
|
1
|
Cascinu S, Falconi M, Valentini V and
Jelic S; ESMO Guidelines Working Group. Pancreatic cancer: ESMO
Clinical Practice Guidelines for diagnosis, treatment and
follow-up. Ann Oncol. 21(Suppl 5): v55–v58. 2010.
|
|
2
|
Satoh K and Shimosegawa T: Pancreatic
tumor: progress in diagnosis and treatment. Topics: 1. Pancreatic
carcinoma: 2. Pathogenesis and pathobiology in pancreatic cancer. -
The molecular mechanisms of carcinogenesis, and invasion and
metastasis in pancreatic cancer. Nihon Naika Gakkai Zasshi.
101:7–16. 2012.(In Japanese).
|
|
3
|
Targarona Soler EM and Trias Folch M:
Sister Mary Joseph’s nodule. Med Clin (Barc). 113:118–119. 1999.(In
Spanish).
|
|
4
|
Kaoutzanis C, Chang MC, Abdul Khalek FJ
and Kreske E: Non-umbilical cutaneous metastasis of a pancreatic
adenocarcinoma. BMJ Case Rep. 2013.bcr2012007931. 2013.
|
|
5
|
Takemura N, Fujii N and Tanaka T:
Cutaneous metastasis as the first clinical manifestation of
pancreatic adenocarcinoma: a case treated with gemcitabine. J
Dermatol. 34:662–664. 2007.
|
|
6
|
Khorana AA: Cancer-associated thrombosis:
updates and controversies. Hematology Am Soc Hematol Educ Program.
2012:626–30. 2012.
|
|
7
|
Klöppel G, Rindi G, Perren A, Komminoth P
and Klimstra DS: The ENETS and AJCC/UICC TNM classifications of the
neuroendocrine tumors of the gastrointestinal tract and the
pancreas: a statement. Virchows Arch. 456:595–597. 2012.
|
|
8
|
Ahmed K, Sussman JJ, Wang J and
Schmulewitz N: A case of EUS-guided FNA-related pancreatic cancer
metastasis to the stomach. Gastrointest Endosc. 74:231–233.
2011.
|
|
9
|
Lookingbill DP, Spangler N and Helm KF:
Cutaneous metastases in patients with metastatic carcinoma: a
retrospective study of 4020 patients. J Am Acad Dermatol.
29:228–236. 1993.
|
|
10
|
Cubilla A and Fitzgerald PJ: Pancreas
cancer. I Duct adenocarcinoma A clinical-pathologic study of 380
patients. Pathol Annu. 13:241–289. 1978.
|
|
11
|
Yendluri V, Centeno B and Springett GM:
Pancreatic cancer presenting as a Sister Mary Joseph’s nodule: case
report and update of the literature. Pancreas. 34:161–164.
2007.
|
|
12
|
Horino K, Hiraoka T, Kanemitsu K, Tsuji T,
Inoue K, Tanabe D, et al: Subcutaneous metastases after curative
resection for pancreatic carcinoma: a case report and review of the
literature. Pancreas. 19:406–408. 1999.
|
|
13
|
Miyahara M, Hamanaka Y, Kawabata A, Sato
Y, Tanaka A, Yamamoto A, et al: Cutaneous metastasis from
pancreatic cancer. Int J Pancreatol. 20:127–130. 1996.
|
|
14
|
Abdel-Hafez HZ: Cutaneous pancreatic
metastasis: a case report and review of literature. Dermatol Surg.
34:1580–1583. 2008.
|
|
15
|
Bordel Gómez MT and Used Aznar MM:
Cutaneous metastases from adenocarcinoma of unknown primary site.
Actas Dermosifiliogr. 97:662–665. 2006.(In Spanish).
|
|
16
|
Takeuchi H, Kawano T, Toda T, Minamisono
Y, Nagasaki S, Yao T and Sugimachi K: Cutaneous metastasis from
pancreatic adenocarcinoma: a case report and a review of the
literature. Hepatogastroenterology. 50:275–277. 2003.
|
|
17
|
Fiori E, Galati G, Bononi M, De Cesare A,
Binda B, Ciardi A, Volpino P, Cangemi V and Izzo L: Subcutaneous
metastasis of pancreatic cancer in the site of percutaneous biliary
drainage. J Exp Clin Cancer Res. 22:151–154. 2003.
|
|
18
|
Bonenti G, Zanon E and Righi D: The
cutaneous metastasis of a pancreatic carcinoma in a patient with
biliary drainage: a case. Radiol Med. 94:264–266. 1997.(In
Italian).
|
|
19
|
Pontinen T, Melin A, Varadi G, Khanmoradi
K, Chewaproug D, Kung SC, Zaki R and Ortiz J: Cutaneous metastasis
of pancreatic adenocarcinoma after kidney transplant: a case report
and review of the literature. Exp Clin Transplant. 8:273–276.
2010.
|
|
20
|
Siriwardena A and Samarji WN: Cutaneous
tumour seeding from a previously undiagnosed pancreatic carcinoma
after laparoscopic cholecystectomy. Ann R Coll Surg Engl.
75:199–200. 1993.
|
|
21
|
Bergenfeldt M, Genell S, Lindholm K,
Ekberg O and Aspelin P: Needle-tract seeding after percutaneous
fine-needle biopsy of pancreatic carcinoma. Case Report Acta Chir
Scand. 154:77–79. 1988.
|
|
22
|
Habscheid W and Kirchner T: Skin
metastases following ultrasound-guided fine-needle puncture of
pancreatic cancer. Dtsch Med Wochenschr. 112:283–284. 1987.(In
German).
|
|
23
|
Akkooi AC, Dokter J and Boxma H: Unusual
first presentation of metastatic pancreatic cancer as skin
metastases in a burn patient. Burns. 36:e111–e114. 2010.
|
|
24
|
Hafez H: Cutaneous pancreatic metastasis:
a case report and review of literature. Indian J Cancer.
44:111–114. 2007.
|
|
25
|
Pinho AV, Rooman I and Real FX:
p53-dependent regulation of growth, epithelial-mesenchymal
transition and stemness in normal pancreatic epithelial cells. Cell
Cycle. 10:1312–1321. 2011.
|
|
26
|
Matros E, Bailey G, Clancy T, et al:
Cytokeratin 20 expression identifies a subtype of pancreatic
adenocarcinoma with decreased overall survival. Cancer.
106:693–702. 2006.
|
|
27
|
Gui JC, Yan WL and Liu XD: CA19-9 and
CA242 as tumor markers for the diagnosis of pancreatic cancer: a
meta-analysis. Clin Exp Med. 14:225–233. 2014.
|
|
28
|
Wang Z and Ma Q: Beta-catenin is a
promising key factor in the SDF-1/CXCR4 axis on metastasis of
pancreatic cancer. Med Hypotheses. 69:816–820. 2007.
|
|
29
|
Lookingbill DP, Spangler N and Sexton FM:
Skin involvement as the presenting sign of internal carcinoma. A
retrospective study of 7316 cancer patients. J Am Acad Dermatol.
22:19–26. 1990.
|
|
30
|
Guo JC, Li J, Hu Y, Zhang TP, Liao Q, Dai
MH and Zhao YP: The role of perioperative enteral and parenteral
nutrition treatment inpancreatic cancer: a multicenter, prospective
randomized controlled trial. Zhonghua Wai Ke Za Zhi. 51:987–990.
2013.(In Chinese).
|