Introduction
Breast cancer is the most common type of cancer in
females in developed and developing countries. Since 2004, a French
national screening program of breast cancer has been established
for females between 50–74 years old (1). Mass screening for breast cancer has
led to the identification of 14,500 novel breast cancers, with a
rate of 6.7 cancers identified per 1,000 females screened. In 2011,
breast cancer was the most common type of cancer among French
females, with 53,000 new cases identified, followed by colorectal
cancer (19,000 cases) and lung cancer (12,000 cases). In addition,
breast cancer continued to be the leading cause of cancer-related
mortality, with 11,500 mortalities, in 2011. However, the rate of
breast cancer-related mortality has actually decreased since 1946
(2).
Various studies have identified a number of risk
factors, including age, lymphovascular emboli or invasion (LVI),
menopause, hormone receptors and the type of treatments, which may
affect the survival of early-stage breast cancer patients (3–11).
Few studies has described early breast cancer and
therefore, the aim of the present study was to explore whether
other factors impact the survival and development of contralateral
breast cancer (CBC) at the early stage.
Materials and methods
Patients
This study retrospectively collected the clinical
and pathological data of a total of 85 early-stage breast cancer
patients (pT1aN0M0) who were treated at the Central Hospital of
Grenoble University (Grenoble, France) between January 2001 and
December 2008. All patients underwent surgery, however, the
post-operative treatments varied, including radiotherapy (RT),
hormone therapy, chemotherapy and observation. The follow-up time
ranged between three and 127 months. Overall, eight patients (9%)
were lost to follow-up. The pre-operative examinations included
annual breast cancer screening reports, taking a family history, a
physical examination, routine laboratory tests, tumor marker
analysis, bilateral mammography or chest radiograph, examination of
the sentinel lymph node, abdominal/pelvic contrast-enhanced
computed tomography and magnetic resonance imaging. This study was
approved by the ethics committee of the Central Hospital of
Grenoble University.
Inclusion and exclusion criteria
The criteria used to select the patients were as
follows: i) The patient must be diagnosed in the region of
Rhone-Alpes and treated at the Central Hospital of Grenoble, with
no history of breast cancer prior to January 2001. ii) an Eastern
Cooperative Oncology Group performance status of 0–2 prior to
surgery; iii) patients were included if they presented with other
types of systemic disease, including hypertension and diabetes, but
excluded if the condition was considered a contraindication of
surgery; iv) patients did not present with other primary tumors; v)
all tumors were ≤5 mm at the greatest dimension according to the
pathological report; and vi) microinvasion of the primary tumor of
1–3 mm in the longest diameter, determined as ductal carcinoma
in situ (DCIS) or lobular carcinoma in situ (LCIS),
according to the pathological reports, and not as a satellite
lesion or metastasis.
Treatments
The treatment strategies predominantly included
surgery, RT, hormone therapy, chemotherapy and observation. All
patients underwent surgery, including quadrantectomy, mastectomy or
lumpectomy. Examination of the lymph nodes included an examination
of the sentinel lymph node and dissection of the axillary lymph
nodes. In addition, all patients received RT (50 Gy/25 fractions
for five weeks), with the exception of patients with microinvasion
or without any trace of the tumor bed following biopsy. The
patients received 50 Gy of internal mammary chain RT if the tumor
was located in the internal quadrant of the breast. The
administration of 45 Gy to the supraclavicular area following
axillary dissection was insufficient. Either 6-MV photon X or
electrons were used to administer a boost of 10 Gy to the tumor bed
in patients with high-risk factors of relapse, including an age of
<60 years old, R1 (it has been observed that tumor cells remain
in the surgical margin when viewed under the microscope) and
high-risk family history (≥1 family members have breast cancer).
Patients with Her-2(+++) overexpression, according to the ASCO-CAP
HER2 Test Guideline Recommendations (12) where Her-2(+++) is defined as uniform
intense membrane staining of >30% of the invasive tumor cells,
were administered six 21 day cycles of a fluorouracil (intravenous,
500 mg/m2, days 1 and 8), epirubicin (75
mg/m2, day 1) and cyclophosphamide (500
mg/m2, day 1) regimen of chemotherapy, which lasted 4.5
months. Hormone therapy with tamoxifen or anti-aromatase inhibitors
was offered for hormone receptor-positive patients. Observation was
only recommended for the following patients: i) Those at low-risk
of recurrence, including those ≥60 years old, those with no
relevant family history and a performance state of 0; and ii) those
refusing any treatment following surgery. The medical
characteristics of the patients are presented in Table I.
 | Table IPatient characteristics (n=85). |
Table I
Patient characteristics (n=85).
| Characteristics | n |
|---|
| Age, years |
| ≤55 | 37 |
| >55 | 48 |
| Postmenopausal |
| Yes | 55 |
| No | 22 |
| Unknown | 8 |
| Family history |
| 1st degree | 12 |
| 2nd degree | 24 |
| No | 26 |
| Unknown | 23 |
| Surgery type |
| Lumpectomy | 2 |
| Mastectomy | 33 |
| Qyadrantectomy | 50 |
| Histology type |
| ILC | 12 |
| IDC | 71 |
| Others | 2 |
| SBR |
| Grade I | 37 |
| Grade II | 23 |
| Grade III | 9 |
| Unknown | 16 |
| Microinvasion |
| High-grade | 16 |
| Medium-grade | 15 |
| Low-grade | 3 |
| None | 26 |
| Unknown | 25 |
| Adjuvant
treatment |
| HT | 3 |
| HT+RT | 17 |
| RT | 54 |
| CT+RT | 1 |
| CT+HT | 1 |
| Observation | 9 |
| Hormone receptor |
|
ER+/PR+ | 50 |
|
ER−/PR− | 7 |
|
ER+/PR− | 19 |
|
ER−/PR+ | 5 |
| Unknown | 4 |
| Boost technique |
| Yes | 45 |
| No | 40 |
| Her-2(+++) |
| Yes | 9 |
| No | 39 |
| Unknown | 37 |
Follow-up
Every three months, the patients were followed up
and obtained results from mammography and biochemistry analyses. In
total, 30% of patients returned to the Central Hospital of Grenoble
University, while 61% visited their family doctors. The period of
follow-up was from the date of surgery to the October 15, 2011.
Local recurrence or new breast cancer were confirmed by
histological examination.
Statistical analysis
Data are presented as the mean ± standard deviation
or as n (%). To assess the differences between the groups,
Student’s t-test was used for continuous variables, and the
χ2 test was used for categorical variables. P<0.05
was considered to indicate a statistically significant difference.
Statistical analysis was performed using STATA software (version
10; Stata Corporation, College Station, TX, USA). Disease-free
survival (DFS) time was estimated using Kaplan-Meier analysis. The
log-rank test was used to evaluate the effects of different
individual variable factors on the relapse-free survival time. The
overall survival (OS) time was defined as the elapsed interval
between the date of the initial surgery to mortality, loss to
follow-up or October 15, 2011. The DFS time was defined as the time
from the date of surgery to the date of local recurrence or new
CBC.
Results
Treatment
In total, 85 patients underwent surgery and 72
patients received RT, of which, 40 were administered boost
irradiation. Furthermore, 21 patients received hormone therapy and
two patients received chemotherapy, which was followed by an
additional herceptin treatment for one year in one patient.
Herceptin dosages were dependent on the weight of the patient:
herceptin dose for the first cycle (mg/kg) = 6 mg × weight of
patient (kg); herceptin dosage for the second to final cycle
(mg/kg) = 4 mg × weight of the patient (kg), each cycle lasts for
21 days and the overall treatment lasts for a year. Seven patients
underwent observation only. No local recurrence or mortalities were
observed during the follow-up period, however, 11 secondary cancers
were identified in 10 patients. This consisted of five cases of
secondary CBC and one each of thyroid, bladder, tongue and colon
cancer. One patient was identified with cervical and breast cancer
on the contralateral side.
OS and DFS analysis
A complete follow-up was achieved in 91% (n=77) of
patients, while 9% (n=8) were lost to follow-up. The follow-up
period varied between three and 127 months. The median follow-up
period was 60 months. No mortalities occurred during the study
period. The corresponding rates of DFS by variable prognostic
factors are shown in Table II.
 | Table IIDFS by variable prognostic factors
(n=85). |
Table II
DFS by variable prognostic factors
(n=85).
| DFS, % |
|---|
|
|
|---|
| Factor | n | 1-year (95%
CI) | 3-year (95%
CI) | 5-year (95%
CI) |
|---|
| Age, years |
| ≤55 | 37 | 97.2
(81.8–99.6) | 97.2
(81.8–99.6) | 87.0
(63.7–95.8) |
| >55 | 48 | 97.9
(85.8–99.7) | 95.6
(83.5–98.9) | 95.6
(83.5–98.9) |
| Postmenopausal |
| Yes | 55 | 98.2
(87.6–99.7) | 96.1
(85.2–99.0) | 92.4
(77.0–97.7) |
| No | 22 | 95.2
(70.7–99.3) | 95.2
(70.7–99.3) | 95.2
(70.7–99.3) |
| Unknown | 8 | 100.0 | 100.0 | 83.3
(27.3–97.5) |
| Family history |
| 1st degree | 12 | 91.0
(50.8–98.7) | 91.0
(50.8–98.7) | 72.7
(24.1–93.1) |
| 2nd degree | 24 | 100.0 | 95.5
(71.9–99.3) | 95.5
(71.9–99.3) |
| No | 26 | 100.0 | 100.0 | 92.3
(56.6–98.9) |
| Unknown | 23 | 95.7
(72.9–99.4) | 95.7
(72.9–99.4) | 95.7
(72.9–99.4) |
| Surgery type |
| Lumpectomy | 2 | 100.0 | 100.0 | 100.0 |
| Mastectomy | 33 | 96.9
(79.8–99.6) | 96.9
(79.8–99.6) | 92.0
(70.8–98.0) |
|
Quadrantectomy | 50 | 98.0
(86.4–99.7) | 95.7
(83.8–98.9) | 91.1
(72.9–97.3) |
| Histology type |
| ILC | 12 | 100.0 | 90.9
(50.8–98.7) | 90.9
(50.8–98.7) |
| IDC | 71 | 97.1
(88.9+99.3) | 97.1
(88.9+99.3) | 91.7
(79.5+97.0) |
| Others | 2 | 100.0 | 100.0 | 100.0 |
| SBR |
| I | 37 | 97.2
(81.9–99.6) | 97.2
(81.9–99.6) | 90 (71.9–96.7) |
| II | 23 | 100.0 | 94.7
(68.1–99.2) | 94.7
(68.1–99.2) |
| III | 9 | 88.9
(44.3–98.4) | 88.9
(44.3–98.4) | 88.9
(44.3–98.4) |
| Unknown | 16 | 100.0 | 100.0 | 100.0 |
| Microinvasion |
| High-grade | 16 | 100.0 | 100.0 | 100.0 |
| Medium-grade | 15 | 85.7
(53.9–96.2) | 85.7
(53.9–96.2) | 73.5
(35.9–91.1) |
| Low-grade | 3 | 100.0 | 100.0 | 0.0 |
| None | 26 | 100.0 | 96.0
(74.8–99.4) | 96 (74.8–99.4) |
| Unknown | 25 | 100.0 | 100.0 | 92.9
(59.1–99.0) |
| Adjuvant
treatment |
| HT | 3 | 100.0 | 100.0 | 100.0 |
| HT+RT | 17 | 100.0 | 100.0 | 100.0 |
| RT | 54 | 98.1
(87.4–99.7) | 96.1
(85.3–99.0) | 89.7
(74.1–96.1) |
| CT | 1 | 100.0 | 100.0 | 100.0 |
| CT+HT | 1 | 100.0 | 100.0 | 100.0 |
| Observation | 9 | 88.9
(43.3–98.4) | 88.9
(43.3–98.4) | 44.4
(1.0–86.6) |
| Hormone
receptor |
|
ER+/PR+ | 50 | 97.9
(86.1–99.7) | 97.9
(86.1–99.7) | 89.4
(69.3–96.6) |
|
ER+/PR− | 19 | 100.0 | 94.1
(65.0–99.2) | 94.1
(65.0–99.2) |
|
ER−/PR− | 7 | 100.0 | 100.0 | 100.0 |
|
ER−/PR+ | 5 | 80.0
(20.4–96.9) | 80.0
(20.4–96.9) | 80.0
(20.4–96.9) |
| Unknown | 4 | 100.0 | 100.0 | 100.0 |
| Boost
technique |
| Yes | 45 | 100.0 | 97.1±2.9 | 91±6.5 |
| No | 40 | 95.6±3.1 | 95.6±3.1 | 92±4.6 |
| Her-2(+++) |
| Yes | 9 | 100.0 | 100.0 | 100.0 |
| No | 39 | 94.9
(81.0–98.7) | 94.9
(81.0–98.7) | 88.6
(65.0–96.7) |
| Unknown | 37 | 100.0 | 97.0
(80.3–99.6) | 93.2
(75.4–98.3) |
Prognostic factors for DFS (univariate
analysis)
As no mortalities were observed in this study, the
appearance of CBC was regarded as the evolution of primary breast
cancer. The cumulative recurrence for DFS was univariately affected
by the known parameters of family history and microinvasion
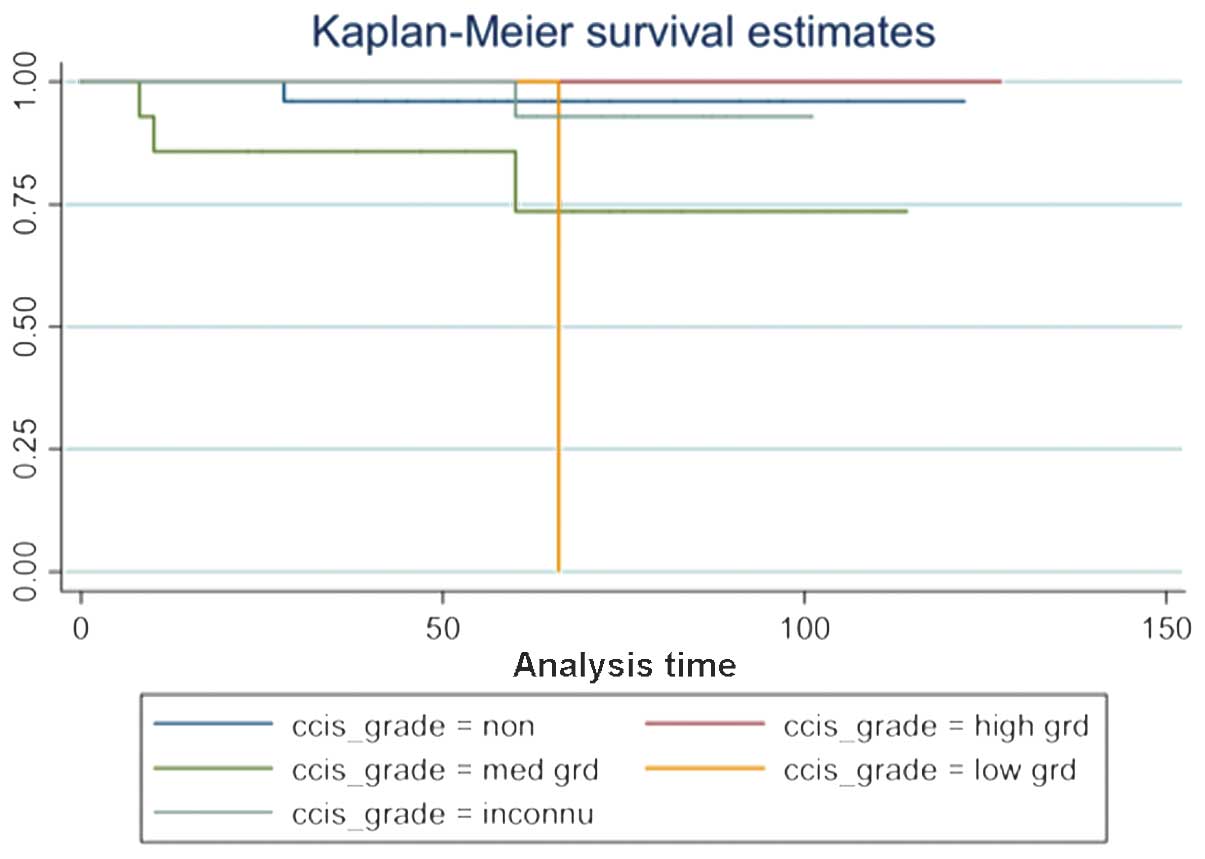
(summarized in Table III and
Figs. 1 and 2).
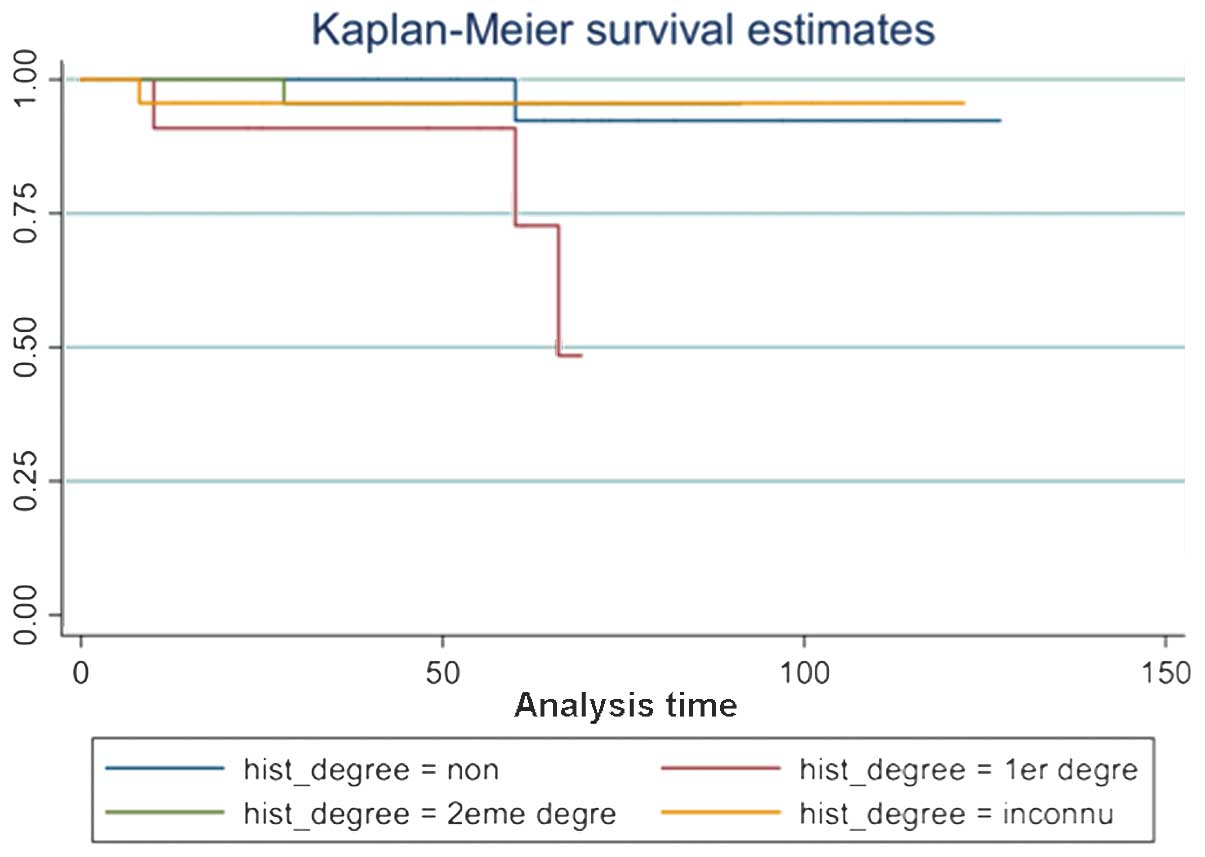 | Figure 1Disease-free survival by family
history (Kaplan-Meier). The Kaplan-Meier estimates revealed that
the first degree of family history, which describes the family
member with breast cancer (daughter, mother or sibling), is the
most important for the occurrence of new contralateral breast
cancer. Whilst the second degree of family history (aunt or niece),
without family history or other status, lessens the revolution.
Hist_degree, degree of family history; non, no family history; 1ere
degree, first degree family history; 2eme degree, second degree
family history; inconnu, unknown. |
 | Table IIIUnivariate analysis by multiple
potential factors for DFS (log-rank test). |
Table III
Univariate analysis by multiple
potential factors for DFS (log-rank test).
| Factor | n | P-value |
|---|
| Age, years |
| ≤55 | 37 | |
| >55 | 48 | 0.6006 |
| Postmenopausal |
| Yes | 55 | |
| No | 22 | |
| Unknown | 8 | 0.8589 |
| Family history |
| 1st degree | 12 | |
| 2nd degree | 24 | |
| No | 26 | |
| Unknown | 23 | 0.0352a |
| Surgery type |
| Lumpectomy | 2 | |
| Mastectomy | 33 | |
|
Quadrantectomy | 50 | 0.7785 |
| Histology type |
| ILC | 12 | |
| IDC | 71 | |
| Others | 2 | 0.9317 |
| SBR |
| I | 37 | |
| II | 23 | |
| III | 9 | |
| Unknown | 16 | 0.5814 |
| Microinvasion |
| High-grade | 16 | |
| Medium-grade | 15 | |
| Low-grade | 3 | |
| None | 26 | |
| Unknown | 25 | 0.0425a |
| Adjuvant
treatment |
| HT | 3 | |
| HT+RT | 17 | |
| RT | 54 | |
| CT | 1 | |
| CT+HT | 1 | |
| Observation | 9 | 0.1916 |
| Hormone
receptor |
|
ER+/PR+ | 50 | |
|
ER−/PR− | 7 | |
|
ER+/PR− | 19 | |
|
ER−/PR+ | 5 | |
| Unknown | 4 | 0.6019 |
| Boost
technique |
| Yes | 45 | |
| No | 40 | 0.6546 |
| Her-2(+++) |
| Yes | 9 | |
| No | 39 | |
| Unknown | 37 | 0.3722 |
Patients with secondary CBC
In this study, six patients were identified with a
secondary primary CBC. The patient characteristics are presented in
Table IV. Only one patient was
>55 years old and four had a relevant family history, with three
being due to first degree relatives. The rate of CBC was 2.35, 3.53
and 7.06% at one, two and five years, respectively. Of the six
females, five were menopausal, four were identified with invasive
ductal carcinoma at the first diagnosis, two with invasive lobular
carcinoma, five with low-grade tumors (I+II) and only one with
high-grade tumors (III) according to the Bloom-Richardson grading
system. By contrast, the histology of the contralateral tumor was
reversed; four patients exhibited ILC, while two exhibited IDC. All
of these patients received RT, however, none received hormone
therapy due to a number of personal reasons. In addition, no
necrosis or LVI was identified.
 | Table IVCharacteristics of patients with new
contralateral breast cancer. |
Table IV
Characteristics of patients with new
contralateral breast cancer.
| Patients | 1 | 2 | 3 | 4 | 5 | 6 |
|---|
| Age, years | 47 | 74 | 50 | 53 | 52 | 50 |
| Family history,
degree | 1st | 2nd | 1st | No | No | 1st |
| Interval of new
tumor, yearsa | 1 | 2 | 5 | 5 | 1 | 5 |
| Menopausal | No | Yes | Yes | Yes | Yes | Yes |
| ER/PR | +/− | +/+ | +/+ | +/+ | +/+ | +/+ |
| Primary
histology | IDC | ILC | ILC | IDC | IDC | IDC |
| Primary histology
grade | III | II | I | I | I | I |
| Contralateral
cancer histology | IDC | ILC | ILC | ILC | IDC | ILC |
| Size of the second
tumor, mm | Unknown | Unknown | 26 | 9 | 7 | 26 |
| Initial
surgery | MT | QT | QT | QT | QT | MT |
| Initial RT | No | Yes | Yes | Yes | Yes | Yes |
| Initial HT | No | No | No | No | No | No |
| Necrosis/LVI | No | No | No | No | No | No |
| Microinvasion,
grade | Medium | No | Low | Medium | Medium | Unknown |
| First margin of
surgery | (−) | (−) | (−) | (−) | (−) | (−) |
Discussion
As all patients in the present study were diagnosed
with early-stage breast cancer, the study aimed to understand why
the rates of CBC remained so high.
CBC is considered to be the most common type of
secondary cancer for those whose primary cancers are located in the
breast, accounting for almost half of all secondary tumors
(10). Therefore, the analysis of
CBC is becoming an important public issue. The overall incidence
rates of CBC vary between 4 and 8 per 1,000 individuals per year,
with different stages and treatment strategies (11). With regard to the incidence of
secondary CBC of early-stage breast cancer, Gao et al
(13) observed that the rates of
CBC were 2.9, 6.1, 9.1 and 12% at five, 10, 15 and 20 years,
respectively. Comparatively, the incidences of 2.35, 3.53 and 7.06%
at one, two and five years that were identified in the current
study were marginally lower.
Among the CBC patients in the present study, five
out of six were <55 years old. Although the study did not report
that age impacts the rates of CBC, a number of studies have
revealed that females of a young age suffer a greater risk of
secondary primary breast cancer. Broët et al (14) identified that patients <55 years
old [relative risk (RR), 1.40; 95% confidence interval (CI),
1.10–1.78] were associated with an increased risk of CBC. However,
another study considered an age of ≤45 years to be a risk factor
(15). By contrast, compared with
the ages of between 45 and 55 years, Gao et al (13) found an age of >55 years to be a
risk factor.
In the present study, family history was a key
potential risk factor of CBC; this has been confirmed by a number
of studies. Reiner et al (16) considered females of <45 years old
with first degree relatives to be at the highest risk (RR, 2.5; 95%
CI, 1.1–5.3). A study by Yadav et al (17) also showed that females with a family
history had the highest incidence rates of CBC (15.3%; RR, 1.6; 95%
CI, 1.12–1.27) at 20 years old. Additionally Lizarraga et al
(18) found that having multiple
first and second degree relatives appeared to increase the risk of
CBC by two- or three-fold.
The impact of microinvasion on the rate of CBC
remains controversial, and has been investigated by few studies
(19). It is not easy to determine
whether microinvasion is a risk factor for CBC or metastasis.
However, in this study, the statistical analyses revealed that
microinvasion does impact the rates of CBC. A study by Claus et
al (20) demonstrated that
patients whose primary tumor was diagnosed as LCIS were 2.6 times
(95% CI, 2.0–3.4%) more likely to develop CBC within the first six
months of the initial primary tumor compared with females with
DCIS. If the period of follow-up can be extended or more
early-stage breast cancer patients are excluded, the potential
effect of microinvasion may be observed.
As shown in Table
IV, none of the CBC patients received hormone therapy, however,
all the patients exhibited indicators of suitability for hormone
therapy according to their positive status of ER/PR, which markedly
increases the incidence of CBC. Tamoxifen, as a representative of
hormone therapy, is well known to reduce the risk of CBC (21,22).
Furthermore, in the latest large multiple center study (23), 1,583 patients with BRCA1 mutations
and 881 with BRCA2 mutations, 383 (24%) and 454 (52%) of patients
were administered tamoxifen, respectively, following the initial
breast cancer diagnosis. This cohort study revealed that the use of
tamoxifen may reduce the risk of CBC for BRCA1 and BRCA2 mutation
carriers. However, a study among elderly patients (≥65 years old)
who were classified as T1N0M0 and treated with breast-conserving
surgery and RT showed no significant differences in the 10-year
survival of CBC patients or OS between the tamoxifen and
non-tamoxifen cohorts (24).
The serine protease urokinase-type plasminogen
activator (uPA) and its inhibitor, PAI-1, are considered to be
independent, statistically prognostic factors in primary breast
cancer. The NNCB-3 trial (23)
confirmed the highest level of evidence for the clinical utility of
uPA and PAI-1. Furthermore, uPA and PAI-1 also serve as predictive
factors of response to adjuvant therapy and the early relapse of
breast cancer. At present, examination of UPA/PAI-1 has been
essential in the pathological surgical studies of breast cancer in
France (25–30), and may be investigated in our future
retrospective studies.
In the present study, five other secondary primary
cancers were identified, including thyroid, bladder, tongue, colon
and cervical cancer. It is likely that multiple factors, including
genetic effects, endogenous hormones, pollution, environmental
exposure, age and the initial treatments for primary breast cancer,
resulted in variations between the standardized incidence ratios in
cancer of the digestive system, lungs, uterus, ovaries, kidneys and
bladder, soft tissue sarcoma, melanoma and certain types of
hematological malignancy (31,32).
In the current study, family history and
microinvasion were poor prognostic factors. The most likely reason
for this result was insufficient systemic treatment, particularly
from hormone therapy. At present, although a consensus has not been
reached on the use of adjuvant treatment for early-stage breast
cancer, a large quantity of observational and follow-up
examinations are being conducted by family doctors, and regularly
communication between family doctors and oncologists should be
encouraged and regarded as a routine procedure. Oncologists or
family doctors should persuade the patients who exhibit indicators
of suitability for hormone therapy (positive ER/PR status) to
continue the treatment. Furthermore, an increased period of
follow-up must be implemented and other significant biomarkers
investigated to continue this study further.
References
|
1
|
Balu-Maestro C, Chapellier C, Souci J,
Caramella T and Marcotte-Blonch C: Breast cancer screening imaging:
what do we do. J Gynecol Obstet Biol Reprod (Paris). 39:3–10.
2010.(In French). View Article : Google Scholar
|
|
2
|
French Institute for Public Health
Surveillance; National Cancer Institute France. Projection of the
incidence and mortality of cancer in France in 2011. Technical
report. French Institute for Public Health Surveillance;
Saint-Maurice: pp. 782011
|
|
3
|
Dinshaw KA, Budrukkar AN, Chinoy RF, Sarin
R, Badwe R, Hawaldar R and Shrivastava SK: Profile of prognostic
factors in 1022 Indian women with early-stage breast cancer treated
with breast-conserving therapy. Int J Radiat Oncol Biol Phys.
63:1132–1141. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sánchez-Muñoz A, Ribelles N and Alba E:
Optimal adjuvant hormonal therapy in postmenopausal women with
hormone-receptor-positive early breast cancer: have we answered the
question? Clin Transl Oncol. 12:614–620. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mouridsen HT and Robert NJ: The role of
aromatase inhibitors as adjuvant therapy for early breast cancer in
postmenopausal women. Eur J Cancer. 41:1678–1689. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Grau JJ, Zanon G, Caso C, Gonzalez X,
Rodriguez A, Caballero M and Biete A: Prognosis in women with
breast cancer and private extra insurance coverage. Ann Surg Oncol.
20:2822–2827. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mereno-Aspitia A, Hillman DW, Dyar SH,
Tenner KS, Gralow J, Kaufman PA, et al: Soluble human epidermal
growth factor receptor 2 (HER2) levels in patients with
HER2-positive breast cacner receiving chemotherapy with or without
trastuzumab: results from North Central Cancer Treatment Group
adjuvant trail N9831. Cancer. 119:2675–2682. 2013. View Article : Google Scholar
|
|
8
|
Swenson KK, Decher L, Haselow R, Farrell
JB and Sperduto PW: Prognostic factors after conservative surgery
and radiation therapy for early stage breast cancer. Am J Clin
Oncol. 21:111–116. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cianfrocca M and Goldstein LJ: Prognostic
and predictive factors in early-stage breast cancer. Oncologist.
9:606–616. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Harvey EB and Brinton LA: Second cancer
following cancer of the breast in Connecticut, 1935-82. Natl Cancer
Inst Monogr. 68:99–112. 1985.PubMed/NCBI
|
|
11
|
Chen Y, Thompson W, Semenciw R and Mao Y:
Epidemiology of contralateral breast cancer. Cancer Epidemio
Biomakers Prev. 8:855–861. 1999.
|
|
12
|
Wolff AC, Hammond ME, Hicks DG, et al:
Recommendations for human epidermal growth factor receptor 2
testing in breast cancer: American Society of Clinical
Oncology/College of American Pathologists clinical practice
guideline update. J Clin Oncol. 31:3997–4013. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gao X, Fisher SG and Emami B: Risk of
second primary cancer in the contralateral breast in women treated
for early-stage breast cancer: a population-based study. Int J
Radiat Oncol Biol Phys. 56:1038–1045. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Broët P, de la Rochefordière A, Scholl SM,
Fourquet A, Mosseri V, Durand JC, et al: Contralateral breast
cancer: annual incidence and risk parameters. J Clin Oncol.
13:1578–1583. 1995.PubMed/NCBI
|
|
15
|
Mariani L, Coradini D, Biganzoli E,
Boracchi P, Marubini E, Pilotti S, et al: Prognostic factors for
metachronous contralateral breast cancer: a comparison of the
linear Cox regression model and its artificial neural network
extension. Breast Cancer Res Treat. 44:167–178. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Reiner AS, John EM, Brooks JD, Lynch CF,
Bernstein L, Mellemkjær L, et al: Risk of asynchronous
contralateral breast cancer in noncarriers of BRCA1 and BRCA2
mutations with a family history of breast cancer: a report from the
Women’s Environmental Cancer and Radiation Epidemiology Study. J
Clin Oncol. 31:433–439. 2013. View Article : Google Scholar :
|
|
17
|
Yadav BS, Sharma SC, Patel FD, Ghoshal S
and Kapoor RK: Second primary in the contralateral breast after
treatment of breast cancer. Radiother Oncol. 86:171–176. 2008.
View Article : Google Scholar
|
|
18
|
Lizarraga IM, Sugg SL, Weigel RJ and
Scott-Conner CE: Review of risk factors for the development of
contralateral breast cancer. Am J Surg. 206:704–708. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sutherland CM and Mather FJ: Long-term
survival and prognostic factors in patients with regional breast
cancer (skin, muscle, and/or chest wall attachment). Cancer.
55:1389–1397. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Claus EB, Stowe M, Carter D and Holford T:
The risk of a contralateral breast cancer among women diagnosed
with ductal and lobular breast carcinoma in situ: data from the
Connecticut Tumor Registry. Breast. 12:451–456. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dalberg K, Johansson H, Johansson U and
Rutqvist LE: A randomized trial of long term adjuvant tamoxifen
plus postoperative radiation therapy versus radiation therapy alone
for patients with early stage breast carcinoma treated with
breast-conserving surgery. Stockholm Breast Cancer Study Group.
Camcer. 82:2204–2211. 1998.
|
|
22
|
Clarke MJ: WITHDRAWN: Tamoxifen for early
breast cancer. Cochrane Database Syst Rev. 8:CD0004862008.
|
|
23
|
Phillips KA, Milne RL, Rookus MA, Daly MB,
Antoniou AC, Peock S, et al: Tamoxifen and risk of contralateral
breast cancer for BRCA1 and BRCA2 mutation carriers. J Clin Oncol.
31:3091–3099. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Khan AJ, Parikh RR, Neboori HJ, Goyal S,
Haffty BG and Moran MS: The relative benefits of tamoxifen in older
women with T1 early-stage breast cancertreated with
breast-conserving surgery and radiation therapy. Breast J.
19:490–495. 2013.PubMed/NCBI
|
|
25
|
Annecke K, Schmitt M, Euler U, Zerm M,
Paepke D, Paepke S, et al: uPA and PAI-1 in breast cancer: review
of their clinical utility and current validation in the prospective
NNBC-3 trial. Adv Clin Chem. 45:31–45. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lamy PJ, Romieu G and Jacot W: UPA/PAI-1:
a tool for breast cancer treatment individualization. Biology,
clinical implications and quantification assays. Bull Cancer.
97:341–348. 2010.(In French). PubMed/NCBI
|
|
27
|
Eljuga D, Razumovic JJ, Bulic K,
Petrovecki M, Draca N and Bulic SO: Prognostic importance of PAI-1
in node negative breast cancer patients - results after 10 years of
follow up. Pathol Res Pract. 207:290–294. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Schmitt M, Mengele K, Napieralski R,
Magdolen V, Reuning U, Gkazepis A, et al: Clinical utility of
level-of-evidence-1 disease forecast cancer biomakers uPA and its
inhibitor PAI-1. Expert Rev Mol Diagn. 10:1051–1067. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kantelhardt EJ, Vetter M, Schmidt M,
Veyret C, Augustin D, Hanf V, et al: Prospective evaluation of
prognostic factors uPA/PAI-1 in node-negative breast cancer: phase
III NNBC3-Europe trial (AGO, GBG, EORTC-PBG) comparing 6xFEC versus
3×FEC/3×Docetaxel. BMC Cancer. 11:1402011. View Article : Google Scholar
|
|
30
|
Dazzi C, Cariello A, Maioli P, Magi S,
Rosti G, Giovanis P, et al: A high cytosol value of urokinase-type
plasminogen activator (uPA) may be predictive of early relapse in
primary breast cancer. Cancer Invest. 21:208–216. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rubino C, de Vathaire F, Diallo I,
Shamsaldin A and Lê MG: Increased risk of second cancers following
breast cancer: role of the initial treatment. Breast Cancer Res
Treat. 61:183–195. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Schaapveld M, Visser O, Louwman MJ, de
Vries EG, Willemse PH, Otter R, et al: Risk of new primary
nonbreast cancers after breast cancer treatment: a Dutch
population-based study. J Clin Oncol. 26:1239–1246. 2008.
View Article : Google Scholar : PubMed/NCBI
|