Introduction
Nitric oxide (NO) is an important bioactive
signaling molecule that is significant in numerous physiological
processes in the cardiovascular, neurological and immune systems.
However, increased NO production may also contribute to the
pathogenesis of a variety of disorders, including various cancers,
such as breast, cervical, gastric, colorectal and head and neck
cancers (1). The formation of NO
from arginine is catalyzed by three types of NO synthase (NOS):
Endothelial NOS (eNOS), neuronal NOS (nNOS) and the inducible
isoenzyme (iNOS) (2). iNOS
expression is generally induced by inflammatory stimuli and is
responsible for the production of large quantities of NO. It has
been reported that the synthesis of NO is induced by cytokines in
certain human carcinoma cell lines (3). A previous study has suggested that a
high expression of iNOS is associated with the aggressive behavior
of colorectal adenocarcinomas (4),
however, the biological significance of NO in malignant tumors
remains unclear.
Cancer cell invasion and metastasis are complex
multi-step processes that involve cell adhesion, degradation of the
extracellular matrix by proteolytic enzymes and motility factors
that influence cell migration (5).
Matrix metalloproteinases (MMPs) are significant in the degradation
of the extracellular matrix and the MMP family consists of >20
proteolytic enzymes (6). MMP
production appears to be a marker for cancer cells with elevated
metastatic potential (7) and the
activation of MMP activity has been detected in colon carcinoma
(8).
Cnidii Rhizoma is the dried root of Cnidium
officinale Makino and has been reported to exhibit antitumor
activity in ddY mice (9), inhibit
liver and lung metastasis of tumor cells in vivo (10) and exhibit anti-angiogenic activity
in renal glomerular capillary endothelial cells, chick embryo
chorioallantoic membrane and rat cornea (11).
N-(3-(aminomethyl)benzyl)acetamidine (1400W), a
nontoxic novel NOS inhibitor, is the most selective inhibitor of
iNOS (12). 1400W has been reported
to be effictive in the treatment of colonic injury in an
experimental model of colitis in rats (13). Recently, the potency and selectivity
of 1400W, as an inhibitor of iNOS and cytokine release modifier,
have indicated a potential use for 1400W in cancer therapy
(14).
Colorectal cancer is the second most common cause of
cancer in women (9.2% of diagnoses) and the third most common in
men (10.0%) worldwide (15). It is
a multifactorial disease etiology, which includes genetic factors,
environmental exposures, such as diet, and inflammatory conditions
of the digestive tract. In Western Europe and the USA the most
common type of colon cancer is adenocarcinoma, which accounts for
98% of all cases. Lymphoma and squamous cell carcinoma occur less
frequently (16). Adenocarcinoma is
a malignant epithelial tumor, originating from the superficial
glandular epithelial cells lining the colon and rectum.
Conventional adenocarcinoma is characterized by glandular
formation, which is the basis for histological tumor grading
(17).
The present study investigates the ability of
pro-inflammatory cytokine-induced NO to modulate the invasiveness
of human colorectal adenocarcinoma HT-29 cells, which is a cell
line mainly used as an in vitro colon epithelial cell model
to investigate absorption, transport and secretion by intestinal
cells, and the effect of the extract from Cnidii Rhizoma on NO
production and invasiveness of HT-29 cells.
Materials and methods
Preparation of Cnidii Rhizoma
extract
Cnidium officinale Makino root was collected
in Jeong-seon, Republic of Korea. Specimens (no. 00C-37) were
preserved by air-drying the roots and were deposited in the
herbarium of the Intractable Disease Research Center (Dongguk
University, Gyeongju, Republic of Korea). Cnidii Rhizoma (60 g) was
extracted using 400 ml distilled water for 3 h. The extract was
filtered and the 200 ml filtrate was concentrated in vacuo,
lyophilized using a Freezezone Console Freeze Dry System (7755040;
Labconco, Kansas City, MO, USA) and stored at −20°C prior to use.
The mean yield of extract was 6.9% of the dried ingredient
weight.
Cell culture
The HT-29 human colon adenocarcinoma cell line
(American Type Culture Collection, Manassas, VA, USA) was cultured
at 37°C in a humidified atmosphere of 5% CO2 in
RPMI-1640 medium (Gibco-BRL, Carlsbad, CA, USA), supplemented with
10% (v/v) fetal bovine serum (Gibco-BRL).
iNOS induction
To induce iNOS expression, subconfluent monolayers
were cultured in serum-free medium for 24 h. Growth-arrested
cultures were treated with pro-inflammatory cytokines, 100 U/ml
interferon γ (IFN-γ) (Sigma-Aldrich, St. Louis, MO, USA), 10 ng/ml
interleukin-1 α (IL-1α) (PeproTech, Inc., Rocky Hill, NJ, USA) and
25 ng/ml tumor necrosis factor-α (TNF-α) (R&D Systems,
Minneapolis, MN, USA), pro-inflammatory cytokines and 0.1–5 mg/ml
water extract of Cnidii Rhizoma or 0.5 mM 1400W (Sigma-Aldrich) in
fresh medium without fetal bovine serum. After 48 h, the
supernatants were collected and the cells were harvested and lysed
as previously described (18).
Nitrite assay
Nitrite, a stable-end product of NO production in
HT-29 cells, was measured as previously described (19) in the supernatants obtained from the
cell culture. The protein concentration of the supernatant was
determined using a bicinchoninic acid protein assay kit
(Sigma-Aldrich) with bovine serum albumin as the standard.
Western blot analysis
Using a 7% SDS-polyacrylamide gel, electrophoresis
was performed to analyze the protein from cell lysates and
subsequently electrophoretically transferred to a polyvinylidene
difluoride membrane. The membrane was treated with 5% non-fat milk
for 1 h to block non-specific binding and probed with a rabbit
anti-human polyclonal iNOS antibody (sc-651; Santa Cruz
Biotechnology, Santa Cruz, CA, USA) at a final dilution of 1:1,000.
The primary antibodies were detected using biotin-rabbit anti-mouse
immunoglobulins G, A and M (heavy and light chains; Zymed, San
Francisco, CA, USA) and alkaline phosphate-conjugated streptavidin,
and were visualized using 4-nitro blue tetrazolium chloride or
5-bromo-4-chloro-3-indolyl-phosphate substrate (Promega, Madison,
WI, USA).
Invasion assay
Cell migration through Matrigel-coated filters was
measured using Transwell chambers (Corning Inc., Corning, New York,
NY, USA) with 8 μm-pore polycarbonate filters coated with Matrigel
matrix (BD Biosciences, Bedford, MA, USA) as previously described
(20). HT-29 cells were seeded at a
density of 0.5×104 cells/well in the upper compartment
of each invasion chamber and incubated for 24 h in the absence or
presence of 100 U/ml interferon (IFN)-γ (Sigma-Aldrich), 10 ng/ml
interleukin (IL)1-α (PeproTech, Inc.) or 25 ng/ml tumor necrosis
factor (TNF)-α (R&D Systems), plus extract of Cnidii Rhizoma
(0.1–5 mg/ml) or 1400W (0.5 mM). Non-migrating cells on the upper
surface of the membrane were gently scrubbed with a cotton swab,
and the invading cells on the lower surface were fixed with 100%
methanol and stained with hematoxylin and eosin Y solution (RICCA
Chemical Company, Charlotte, NC, USA). The number of cells was
counted under a microscope at a magnification of ×100.
Gelatin zymography
A gelatin zymography assay was performed as
previously described (20). The
HT-29 cells were plated at a density of 5×106 cells/well
in 6-well plates. After 18 h, the monolayers were rinsed three
times with phosphate-buffered saline followed by exposure to 100
U/ml IFN-γ, 10 ng/ml IL-1α and 25 ng/ml TNF-α, and extract of
Cnidii Rhizoma (0.1–5 mg/ml) or 1400W (0.5 mM) under serum-free
conditions for 24 h. The conditioned media was collected,
normalized to the cell number, mixed with 10× non-reducing sample
buffer (EZ BioResearch LLC, St Louis, MO, USA), consisting of 120
mM Tris-HCl (pH 6.8), 50% (v/v) glycerol, 4% (w/v) SDS, 28.8 mM
2-mercaptoethanol and 0.2% (w/v) bromophenol blue, and SDS-PAGE was
subsequently performed using a gel containing 10% SDS and 0.1%
(w/v) gelatin. The resulting gel was rinsed in 2.5% (v/v) Triton
X-100 for 1 h and enzyme degradation was performed at 37°C for 18 h
in 50 mM Tris-HCl (pH 7.5), 5 mm CaCl2 and 0.04%
NaN3. The gel was subsequently stained for 30 min using
0.05% Coomassie Blue in 45% (v/v) methanol combined with 1% (v/v)
acetic acid, and destained in a solution containing 10% acetic acid
(v/v) and 25% methanol (v/v).
Statistical analysis
The data were analyzed for statistical significance
using Student’s t-test. P<0.05 was considered to indicate
a statistically significant difference.
Results and Discussion
Induction of NO production in HT-29
cells
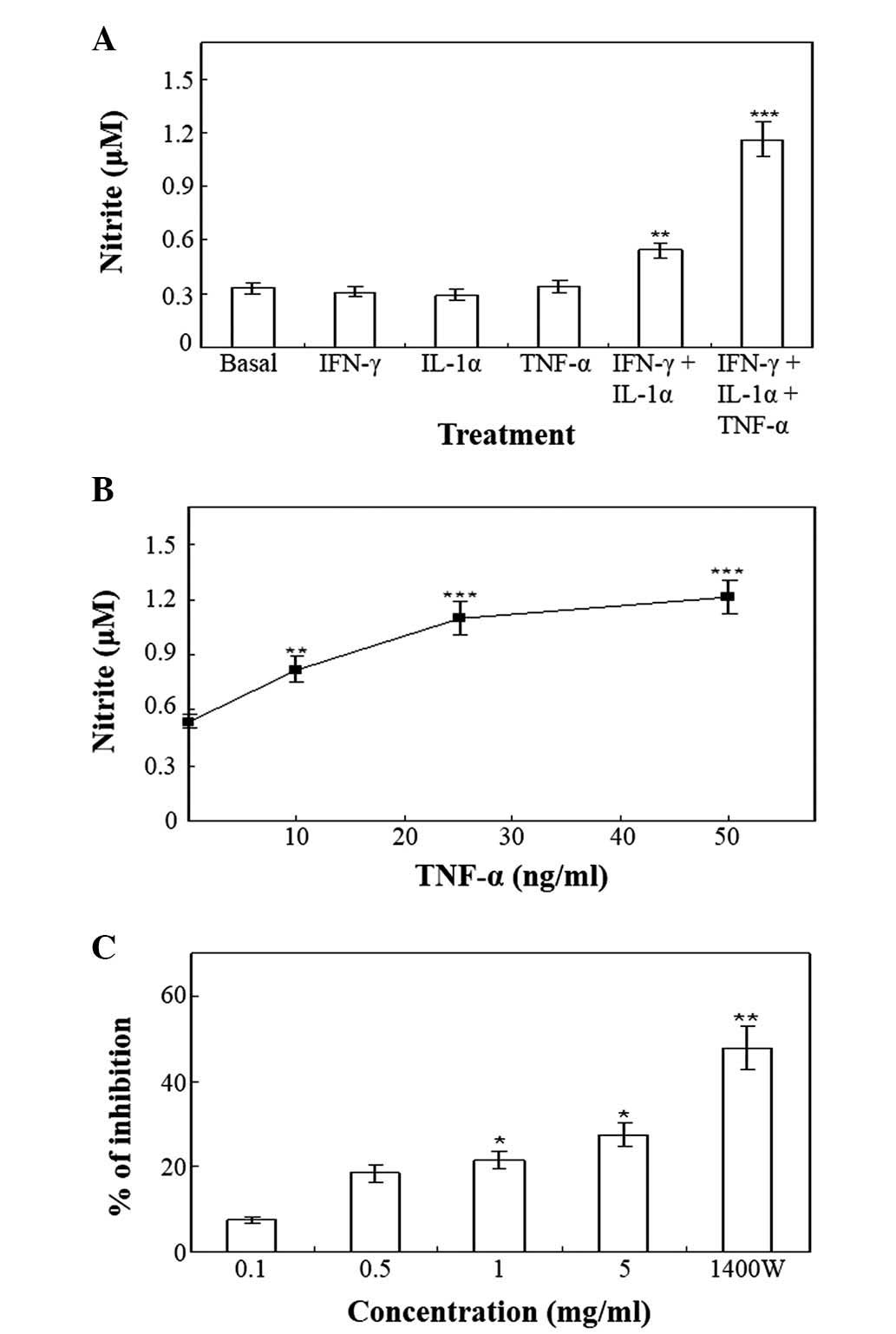
Upon stimulation with the vehicle for 48 h, the
resting HT-29 cells produced basal levels of nitrite (Fig. 1A). The pro-inflammatory cytokines,
IFN-γ (100 U/ml), IL-1α (10 ng/ml) and TNF-α (50 ng/ml), did not
affect the production of nitrite when added alone to HT-29 cells
(Fig. 1A). The minimum requirement
for enhanced nitrite production was the combination of IFN-γ and
IL-1α (Fig. 1A), while other
combinations of cytokines were ineffective. The addition of TNF-α
to the combination of IFN-γ and IL-1α produced an approximately
two-fold enhancement in IFN-γ and IL-1α-induced nitrite production
at 48 h (Fig. 1A). Various
concentrations of TNF-α (0–50 ng/ml) in the presence of the
combination of 100 U/ml IFN-γ and 10 ng/ml IL-1α, induced a
concentration-dependent enhancement of nitrite production (Fig. 1B). Pretreatment of the cells with ≥5
mg/ml Cnidii Rhizoma or 0.5 mM 1400W, exerted an inhibitory effect
on the production of nitrite induced by treatment with 100 U/ml
IFN-γ, 10 ng/ml IL-1α and 25 ng/ml TNF-α (Fig. 1C). NO has been previously implicated
in tumor biology. Previous studies have demonstrated that the
expression level and activity of iNOS correlates with the
histological grade of malignancy in human gynecological (21), breast (22), central nervous system (23) and lung cancers (24). iNOS activity and the resulting NO
concentrations have been demonstrated to contribute to tumor
progression by mediating tumor vascularization and tumor blood flow
(21). iNOS was also induced in
human colon adenocarcinoma, ovarian and glioblastoma cell lines in
response to cytokine stimulation (21). The enhanced expression of iNOS in
human colon carcinoma correlates with tumor growth and vascular
invasion and may be indicative of the survival potential of cells
(8).
Induction of iNOS expression in HT-29
cells by a mixture of IFN-γ, IL-1α, and TNF-α
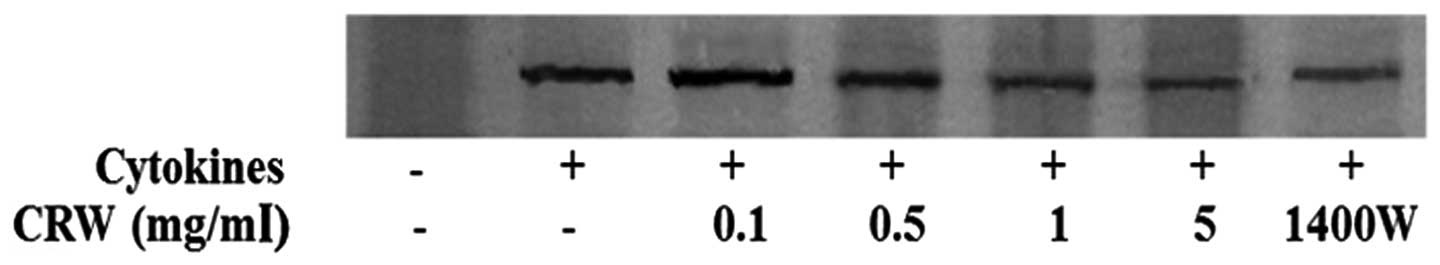
Fig. 2 shows that
the induction of iNOS expression in HT-29 cells by 100 U/ml IFN-γ,
10 ng/ml IL-1α and 25 ng/ml TNF-α. iNOS was not detected in the
absence of the cytokines. Treatment of the cells with Cnidii
Rhizoma reduced the expression of iNOS in a dose-dependent manner
(Fig. 2). The present study also
demonstrated that 1400W inhibited cytokine-induced iNOS
expression.
Invasiveness of HT-29 cells
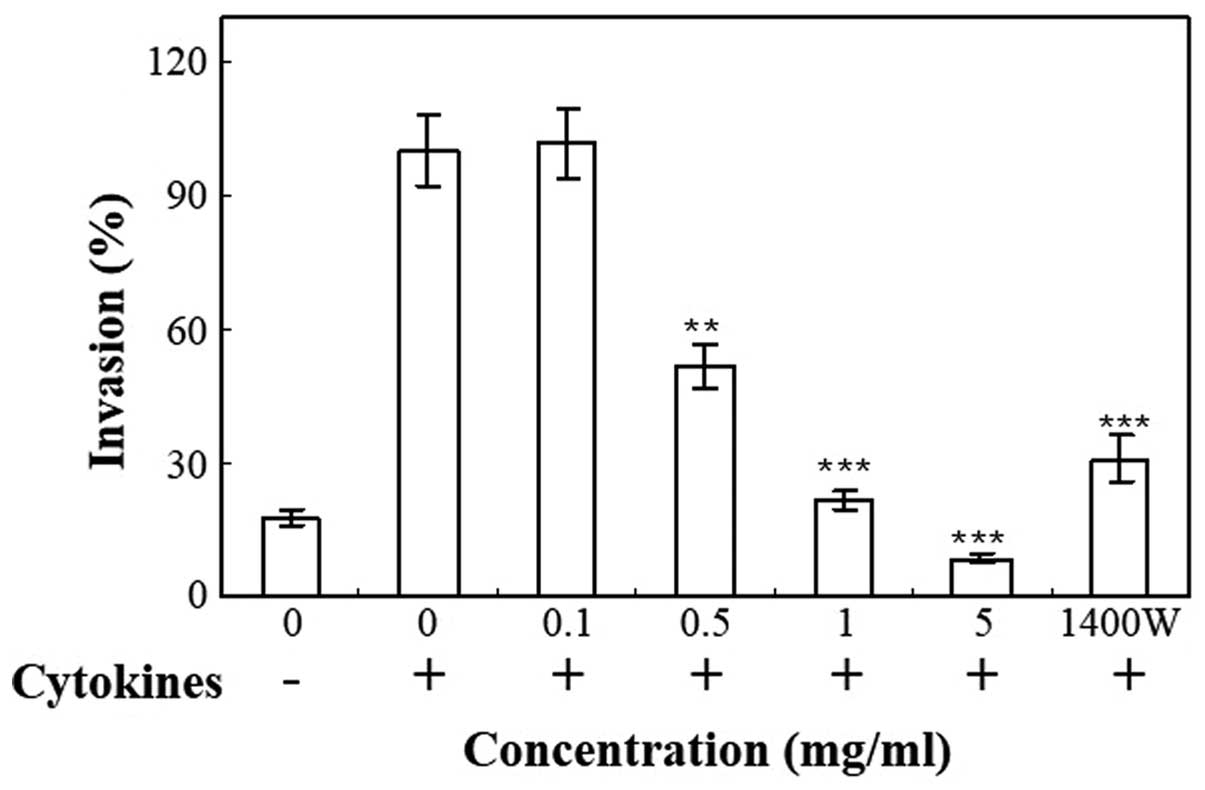
Transwell plates were used to measure the invasive
properties of cells following stimulation with 100 U/ml IFN-γ, 10
ng/ml IL-1α and 25 ng/ml TNF-α. The invasion of HT-29 cells through
Matrigel was significantly increased by treatment with cytokines
(Fig. 3). Cnidii Rhizoma inhibited
the invasiveness of cytokine-treated HT-29 cells through the
Matrigel-coated membrane in a concentration-dependent manner
(Fig. 3). The invasiveness of cells
was inhibited by treatment with 1400W, an iNOS inhibitor, which
confirms that iNOS contributes to the process of tumor cell
invasion. 1400W is an irreversible inhibitor of human iNOS, as well
as a weaker and reversible inhibitor of human nNOS and eNOS. The
potency and selectivity of 1400W to iNOS in vitro and in
vivo is increased compared with any other described iNOS
inhibitor (25). The present study
demonstrated that 1400W inhibited NO production (Fig. 1C) and iNOS expression (Fig. 2) in HT-29 cells. Inhibition of NO
production by 1400W was accompanied by a reduction of HT-29 cell
invasion through the Matrigel (Fig.
3). The present study revealed that cytokine treatment
increased NO production in HT-29 cells. Cytokines further enhanced
the invasiveness of the HT-29 cells. The present results suggest
that endogenous NO production induced by cytokines increases the
invasion of the human colorectal adenocarcinoma HT-29 cells. In the
HT-29 cell Matrigel assay, the inhibition of invasion caused by
treatment with 1400W, the most selective iNOS inhibitor,
demonstrated the involvement of iNOS. The cytokine-stimulated
invasion of HT-29 cells was not completely abolished by 1400W,
which indicates that other mechanisms may also induce invasion, in
addition to the iNOS/NO pathway.
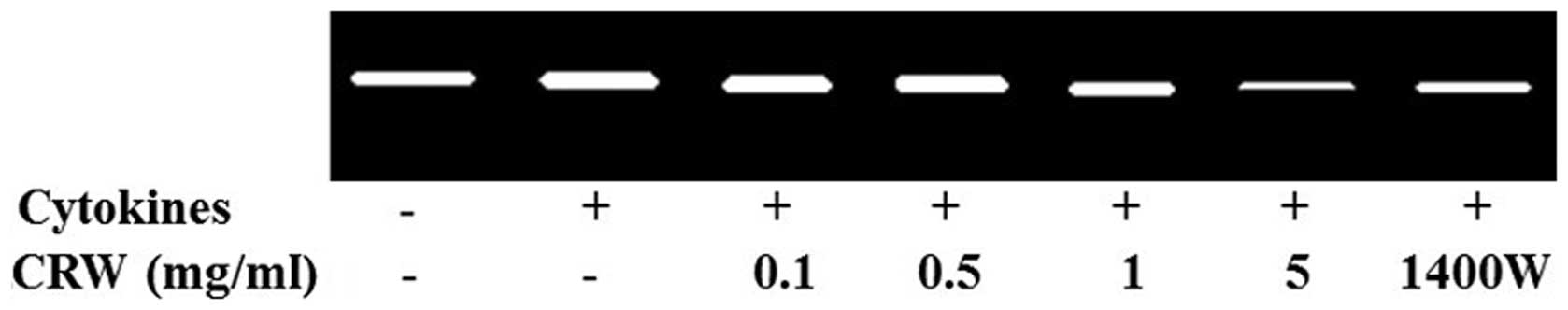
MMP-2 activity in HT-29 cells
MMP-2 is one of the key enzymes involved in the
degradation of the backbone of the cellular basement membrane, type
IV collagen (26). To determine the
activity of MMP-2, gelatin zymography was conducted using the
conditioned medium, which was collected and measured following the
treatment of cells for 24 h with the cytokines, and pretreatment
with Cnidii Rhizoma or 1400W. MMP-2 activity in HT-29 cells was
increased by the treatment of cytokines (Fig. 4). At a concentration of 5 mg/ml,
Cnidii Rhizoma pretreatment inhibited cytokine-induced MMP-2
activity (Fig. 4). An association
was observed between a decrease in MMP-2 levels in HT-29 cells and
a reduction in invasiveness. MMP-2 activity was also inhibited by
1400W (0.5 mm), which indicates that iNOS contributes to the
induction of MMP-2 activity (Fig.
4). MMPs are significant in tumor invasion and metastasis
through the proteolysis of several extracellular matrix proteins
and are overexpressed during tumor progression. The present study
reveals that pro-inflammatory cytokines induce NO production, iNOS
expression and the invasiveness of human colorectal adenocarcinoma
HT-29 cells, whilst pretreatment with Cnidii Rhizoma inhibited
these processes. The present results may provide sufficient
information for the further development of Cnidii Rhizoma as an
antitumor metastatic agent against colon cancer in animal studies
and later in human clinical trials.
In conclusion, this study revealed that
pro-inflammatory cytokines induce NO production, iNOS expression
and invasiveness of human colorectal adenocarcinoma HT-29 cells. In
addition, pretreatment with Cnidii Rhizoma inhibited
cytokine-mediated NO production, iNOS expression and invasiveness
of HT-29 cells. Therefore, future animal studies and subsequent
human clinical trails are required to investigate the potential
antitumor and antimetastatic effects of Cnidii Rhizoma against
colon cancer. In addition, considering the designing of appropriate
strategies for an intervention, further studies regarding the
active components of Cnidii Rhizoma are also required to
investigate the mechanisms regulated by Cnidii Rhizoma.
Acknowledgements
This study was supported by the Korea Research
Foundation Grant funded by the Korean Government (MOEHRD, Basic
Research Promotion Fund; no. KRF-2005-075-C00024).
References
|
1
|
Choudhari SK, Chaudhary M, Bagde S,
Gadbail AR and Joshi V: Nitric oxide and cancer: a review. World J
Surg Oncol. 11:1182013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Förstermann U and Sessa WC: Nitric oxide
synthases: regulation and function. Eur Heart J. 33:829–837.
837a–837d. 2012. View Article : Google Scholar :
|
|
3
|
Tanese K, Grimm EA and Ekmekcioglu S: The
role of melanoma tumor-derived nitric oxide in the tumor
inflammatory microenvironment: its impact on the chemokine
expression profile, including suppression of CXCL10. Int J Cancer.
131:891–901. 2012. View Article : Google Scholar :
|
|
4
|
Raina K, Agarwal C and Agarwal R: Effect
of silibinin in human colorectal cancer cells: targeting the
activation of NF-kappaB signaling. Mol Carcinog. 52:195–206. 2013.
View Article : Google Scholar :
|
|
5
|
Robert J: Biology of cancer metastasis.
Bull Cancer. 100:333–342. 2013.(In French). PubMed/NCBI
|
|
6
|
Khokha R, Murthy A and Weiss A:
Metalloproteinases and their natural inhibitors in inflammation and
immunity. Nat Rev Immunol. 13:649–665. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chaudhary AK, Pandya S, Ghosh K and
Nadkarni A: Matrix metalloproteinase and its drug targets therapy
in solid and hematological malignancies: an overview. Mutat Res.
753:7–23. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Langenskiöld M, Ivarsson ML, Holmdahl L,
et al: Intestinal mucosal MMP-1 - a prognostic factor in colon
cancer. Scand J Gastroenterol. 48:563–569. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Haranaka K, Satomi N, Sakurai A, et al:
Antitumor activities and tumor necrosis factor producibility of
traditional Chinese medicines and crude drugs. Cancer Immunol
Immunother. 20:1–5. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Onishi Y, Yamaura T, Tauchi K, et al:
Expression of the anti-metastatic effect induced by Juzen-taiho-to
is based on the content of Shimotsu-to constituents. Biol Pharm
Bull. 21:761–765. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kwak DH, Kim JK, Kim JY, et al:
Anti-angiogenic activities of Cnidium officinale Makino and Tabanus
bovinus. J Ethnopharmacol. 81:373–379. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Garvey EP, Oplinger JA, Furfine ES, et al:
1400W is a slow, tight binding, and highly selective inhibitor of
inducible nitric-oxide synthase in vitro and in vivo. J Biol Chem.
272:4959–4963. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Menchén LA, Colón AL, Moro MA, et al:
N-(3-(aminomethyl) benzyl)acetamidine, an inducible nitric oxide
synthase inhibitor, decreases colonic inflammation induced by
trinitrobenzene sulphonic acid in rats. Life Sci. 69:479–791. 2001.
View Article : Google Scholar
|
|
14
|
Mertas A, Duliban H, Szliszka E, et al:
N-[3-(aminomethyl)benzyl]acetamidine (1400W) as a potential
immunomodulatory agent. Oxid Med Cell Longev. 2014:4912142014.
View Article : Google Scholar
|
|
15
|
Bernard WS and Christopher PW: World
Cancer Report 2014. International Agency for Research on Cancer,
World Health Organization; Lyon, France: 2014
|
|
16
|
Center MM, Jemal A, Smith RA and Ward E:
Worldwide variations in colorectal cancer. CA Cancer J Clin.
59:366–378. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fleming M, Ravula S, Tatishchev SF and
Wang HL: Colorectal carcinoma: Pathologic aspects. J Gastrointest
Oncol. 3:153–173. 2012.PubMed/NCBI
|
|
18
|
Kim M, Li YX, Dewapriya P, et al:
Floridoside suppresses pro-inflammatory responses by blocking MAPK
signaling in activated microglia. BMB Rep. 46:398–403. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Green LC, Wagner DA, Glogowski J, et al:
Analysis of nitrate, nitrite, and [15N]nitrate in biological
fluids. Anal Biochem. 126:131–138. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hwang BM, Chae HS, Jeong YJ, et al:
Protein tyrosine phosphatase controls breast cancer invasion
through the expression of matrix metalloproteinase-9. BMB Rep.
46:533–538. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Thomsen LL and Miles DW: Role of nitric
oxide in tumour progression: lessons from human tumours. Cancer
Metastasis Rev. 17:107–118. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Oktem G, Bilir A, Selvi N, et al:
Chemotherapy influences inducible nitric oxide synthase (iNOS) and
endothelial nitric oxide synthase (eNOS) activity on 3D breast
cancer cell line. Oncol Res. 16:195–203. 2006.PubMed/NCBI
|
|
23
|
Broholm H, Rubin I, Kruse A, et al: Nitric
oxide synthase expression and enzymatic activity in human brain
tumors. Clin Neuropathol. 22:273–281. 2003.PubMed/NCBI
|
|
24
|
Ramasamy K, Dwyer-Nield LD, Serkova NJ, et
al: Silibinin prevents lung tumorigenesis in wild-type but not in
iNOS−/− mice: potential of real-time micro-CT in lung cancer
chemoprevention studies. Clin Cancer Res. 17:753–761. 2011.
View Article : Google Scholar :
|
|
25
|
Babu BR and Griffith OW: Design of
isoform-selective inhibitors of nitric oxide synthase. Curr Opin
Chem Biol. 2:491–500. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bauvois B: New facets of matrix
metalloproteinases MMP-2 and MMP-9 as cell surface transducers:
outside-in signaling and relationship to tumor progression. Biochim
Biophys Acta. 1825:29–36. 2012.
|