Introduction
Laryngeal cancer is a common malignancy in
otolaryngology, accounting for 1–5% of all cases of cancer,
worldwide. It is the eleventh most common type of cancer, accounts
for 35.4% of cases of head and neck cancer, and is the third most
common type of head and neck malignancy, worldwide (1). O6-methylguanine-DNA methyltransferase
(MGMT) is a key enzyme in the DNA repair network that removes
mutagenic and cytotoxic adducts from O6-guanine in the DNA.
Numerous carcinogens target O6-guanine, thus, the loss of MGMT gene
expression results in the accumulation of unrepaired DNA damage and
subsequent tumor development. MGMT is transcriptionally
downregulated via the hypermethylation of CpG islands in its
promoter region (2,3).
The average level of MGMT mRNA expression is
significantly lower in cancerous mucosa compared with the
corresponding non-cancerous mucosa. Histone modification is closely
associated with the DNA methylation status of a gene and is key for
gene regulation. DNA hypermethylation in the promoter CpG islands
of tumor suppressor genes (TSGs) inhibits transcriptional
initiation and results in permanent gene silencing, a key process
in carcinogenesis (4–6). Histone H3 lysine 9 (H3K9) acetylation
and histone H3 lysine 4 (H3K4) di-methylation are associated with
active gene transcription, however, H3K9 di-methylation is
associated with gene repression (7,8).
Studies investigating the interaction between DNA methylation
status and various histone modifications are currently ongoing.
To the best of our knowledge, no studies
investigating the pattern of histone modifications in the TSG, MGMT
in laryngeal carcinoma have been conducted. To establish a possible
function for such epigenetic modifications of the MGMT gene in
laryngeal carcinogenesis, the present report analyzed MGMT mRNA
expression levels, DNA methylation status, and the levels of
promoter region di-methyl-H3K9 (H3K9me2), H3K4me2 and acetyl-H3K9
(H3K9ac) following DNA methyltransferase inhibitor
5-aza-2′-deoxycytidine (Aza) and/or trichostatin A (TSA) treatment
of laryngeal carcinoma HEp-2 cells. Furthermore,
methylation-specific polymerase chain reaction (MSP) and reverse
transcription (RT)-quantitative polymerase chain reaction (qPCR)
were used to detect the association between MGMT gene expression
levels and DNA methylation status in laryngeal squamous cell
carcinoma (LSCC) tissues. Thus, the current report presents a
mechanism for the inactivation of the TSG, MGMT in LSCC
tissues.
Materials and methods
Cell line and tissue samples
HEp-2 cells were cultured in RPMI-1640 medium (pH
7.2; Gibco BRL, Life Technologies Inc., Grand Island, NY, USA)
supplemented with 10% fetal bovine serum (inactivated under 56°C
for 30 min), 100×103 U/l penicillin and
100×103 U/l streptomycin, and were cultured in a closed
incubator in a 5% humidified CO2 atmosphere at a
constant temperature of 37°C. Cells were required to reach the
logarithmic growth phase and a viable cell count of 95–100%
immediately prior to the experiments.
Fifty LSCC patients, who were diagnosed and treated
between January 2008 and May 2009 at the Shengjing Hospital of
China Medical University (Shenyang, China), were evaluated in the
present study. Prior to surgery, the patients were pathologically
diagnosed with LSCC; however, no chemotherapy or radiation was
administered. Control mucosa samples were obtained from the
patients who had received a total laryngectomy; the samples were
obtained from tissue >2.0 cm from the tumor margin. Field
cancerization may result in the tumor affecting a larger tissue
area; therefore, only histologically healthy control mucosa samples
were used in the present study. The samples were immediately
snap-frozen in liquid nitrogen and stored at −80°C. Relevant
clinical characteristics of the patients (age, gender, state of
nodal metastases, and clinical stage, T stage and differentiation
grade of the tumor) were extracted from the patients’ files. Tumor
staging was conducted according to the International Union against
Cancer 2002 tumor node metastasis classification (9). The patient tumor samples were
collected following receipt of informed consent.
Treatment of cells with Aza and TSA
HEp-2 cells were divided into three groups: (i) Aza
group, 5 μmol/l Aza (Sigma-Aldrich, St. Louis, MO, USA) was added
and cultured for 72 h; (ii) TSA group, 300 nmol/l TSA
(Sigma-Aldrich) was added and cultured for 24 h; and (iii) Aza plus
TSA group, 5 μmol/l Aza was added and cultured for 48 h prior to
adding 300 nmol/l TSA and continuing to culture for 24 h. Control
cells of the same batch, were not treated with any agent. The dose,
time period and sequence of Aza and/or TSA treatment were based on
similar preliminary studies (10–12).
RNA extraction and RT-qPCR
Total RNA was isolated from cultured cells and
tissues using TRIzol® reagent (Invitrogen Life
Technologies, Carlsbad, CA, USA) according to the manufacturer’s
instructions. Total RNA treated with 2 μg DNase I (Thermo Fisher
Scientific, Pittsburgh, PA, USA) was converted to complementary
(c)DNA using a Reverse Transcription System kit (Thermo Fisher
Scientific). RNA was excluded from the cDNA synthesis reactions and
served as a negative control. PCR was performed in a 25-μl reaction
volume using the Maxima SYBR® Green/ROX qPCR Master Mix
(Thermo Fisher Scientific) under the following conditions: 95°C for
10 min, followed by 40 cycles of 95°C for 30 sec (denaturation),
56°C for 30 sec (annealing), and 72°C for 30 sec (extension); and
an additional extension of 72°C for 10 min. The PCR product length
for the MGMT gene fragment was 151 bp and the MGMT primers were as
follows: Upstream, 5′-CGAAATAAAGCTCCTGGGCA-3′ and downstream,
5′-GAACTCTTCGATAGCCTCGGG-3′. The PCR product length for the GAPDH
gene fragment was 115 bp and the GAPDH primers were as follows:
Upstream, 5′-TCCCATCACCATCTTCCAG-3′ and downstream,
5′-ATGAGTCCTTCCACGATACC-3′. The 2−ΔΔct method was used
to calculate the MGMT gene expression levels relative to an
internal GAPDH control and, thus, the fold changes of the gene
expression levels. All experiments were repeated three times for
statistical analysis.
MSP
Genomic DNA was prepared from cell lines and tissues
using the phenol/chloroform extraction protocol and was modified by
bisulfite treatment. Briefly, DNA was denatured by incubating with
0.3 mol/l NaOH at 37°C for 30 min, followed by incubation with 10
mmol/l hydroquinone and 3 mol/l sodium bisulfite (Sigma-Aldrich) at
55°C for 16 h. Modified DNA was purified using the Wizard DNA
Clean-Up System (Promega Corporation, Madison, WI, USA) according
to the manufacturer’s instructions. The MGMT PCR product length was
121 bp for the methylated fragment and 122 bp for the unmethylated
fragment. The MSP primers are located in the promoter region of the
MGMT gene and were as follows: Upstream, 5′-GGTCGTTTGTACGTTCGC-3′
and downstream, 5′-GACCGATACAAACCGAACG-3′ for the methylation
primers; and upstream, 5′-GTAGGTTGTTTGTATGTTTGT-3′ and downstream,
5′-AACCAATACAAACCAAACA-3′ for the unmethylated primers. PCR was
performed under the following conditions: 95°C for 12 min, followed
by 32 cycles of 95°C for 30 sec (denaturation), 61°C for 45 sec
(annealing) and 72°C for 30 sec (extension). An additional
extension of 72°C for 7 min was performed prior to agarose gel
electrophoresis and ethidium bromide staining. A Chemilmanger 5500
automatic image analyzer (Alpha Innotech Corporation, San Leandro,
CA, USA) was used to obtain imaging data and analyze the
electrophoresis results. All experiments were repeated three times
for statistical analysis.
Chromatin immunoprecipitation assay
(ChIP)
ChIP was performed as previously described with
specific modifications (13).
Briefly, ~1.75×107 HEp-2 cells, treated as described
above, were fixed with 1% formaldehyde at 37°C for 20 min,
resuspended in lysis buffer (1% sodium dodecyl sulfate, 10 mmol/l
EDTA, 50 mmol/l Tris HCl; pH 8.1) and sonicated to generate ~500-bp
DNA fragments. Antibodies against H3K9me2, H3K4me2 or H3K9ac
(Upstate Biotechnology Inc., Lake Placid, NY, USA) were used to
immunoprecipitate the major soluble chromatin fraction. The
remaining soluble fraction was incubated with normal rabbit IgG
(negative control) and used as a DNA input control. DNA-protein
crosslinks were reversed by heating the samples to 65°C for 5 h and
digesting with proteinase K. DNA was then extracted using the
phenol/chloroform protocol. The ChIP experiments were repeated
three times.
PCR analysis of the immunoprecipitated
DNA
PCR reactions were performed using 2 μl
immunoprecipitated DNA, a negative control and a DNA input control.
The PCR product length for the MGMT gene fragment was 171 bp and
the ChIP-PCR primers, located in the MGMT promoter region, were as
follows: Upstream, 5′-CCCCATCTCCAAATAAGGTCA-3′ and downstream,
5′-CCTAGACACTGCCAGAGCCTG-3′. PCR products were resolved on 2%
agarose gels (Promega Corporation) and quantified using a GelDoc
1000 (Bio-Rad, Hercules, CA, USA) and Molecular Analyst software
(Alpha Innotech Corporation). The levels of H3K9, and H3K4
di-methylation and H3K9 acetylation in each immunoprecipitate were
determined by quantifying the intensities of the PCR products in
the immunoprecipitated versus input DNA. ChIP was repeated a
minimum of two times and three independent PCR analyses of each
sample were performed.
Wound healing assay
HEp-2 cells were seeded in six-well plates and
cultured in RPMI-1640 medium supplemented with 10% fetal bovine
serum. A wound was created in the center of the cell monolayer
using a sterile plastic pipette tip. The cells were cultured for 24
h to allow cell migration. To assess the ability of the cells to
migrate into the wound area, an inverted microscope was used to
capture images of the cells 0 and 24 h after wounding.
Matrigel® invasion assay
HEp-2 cells (5×104) cultured in 200 μl
serum-free RPMI-1640 medium were seeded onto the upper chambers of
Matrigel®-coated Transwell® filters (pore
size, 8 μm; Corning Life Sciences, Corning, NY, USA). RPMI-1640
(500 μl) supplemented with 10% fetal bovine serum was added to the
lower chambers as a chemoattractant. Cells were incubated at 37°C
in a humidified 5% CO2 atmosphere for 24 h. Cells that
had successfully invaded through the inserts were fixed in 4%
paraformaldehyde for 30 min and stained with methylrosanilinium
chloride. The invaded cells were counted from five preselected
microscopic fields (magnification, ×200). The mean result of the
assay was obtained from three independent experiments.
Statistical analysis
The ratio results were expressed as the mean ±
standard deviation. Student’s t-test was used to calculate the
significance between the treated and control samples. The Spearman
rank-correlation test was used to examine the association between
the MGMT expression level, and DNA hypermethylation and histone
modification. Furthermore, χ2 and Fisher’s exact tests
were adopted to analyze the aberrant DNA hypermethylation of MGMT
within the clinicopathological parameters. Statistical calculations
were performed using SPSS version 13.0 (SPSS Inc., Chicago, IL,
USA) and P<0.05 was considered to indicate a statistically
significant difference.
Results
Analysis of MGMT mRNA expression levels
and DNA methylation status in laryngeal carcinoma HEp-2 cells
before and after treatment with Aza and TSA
RT-qPCR was performed to assess the level of MGMT
mRNA expression in the control group (no Aza or TSA treatment;
relative mRNA expression level, 1) compared with the expression
level following treatment with TSA (relative mRNA expression level,
1.383±0.0417; P<0.05), Aza (relative mRNA expression level,
1.847±0.0.03844; P<0.01) and a combination of Aza and TSA
(relative mRNA expression level, 2.140±0.04509; P<0.01; Fig. 1A).
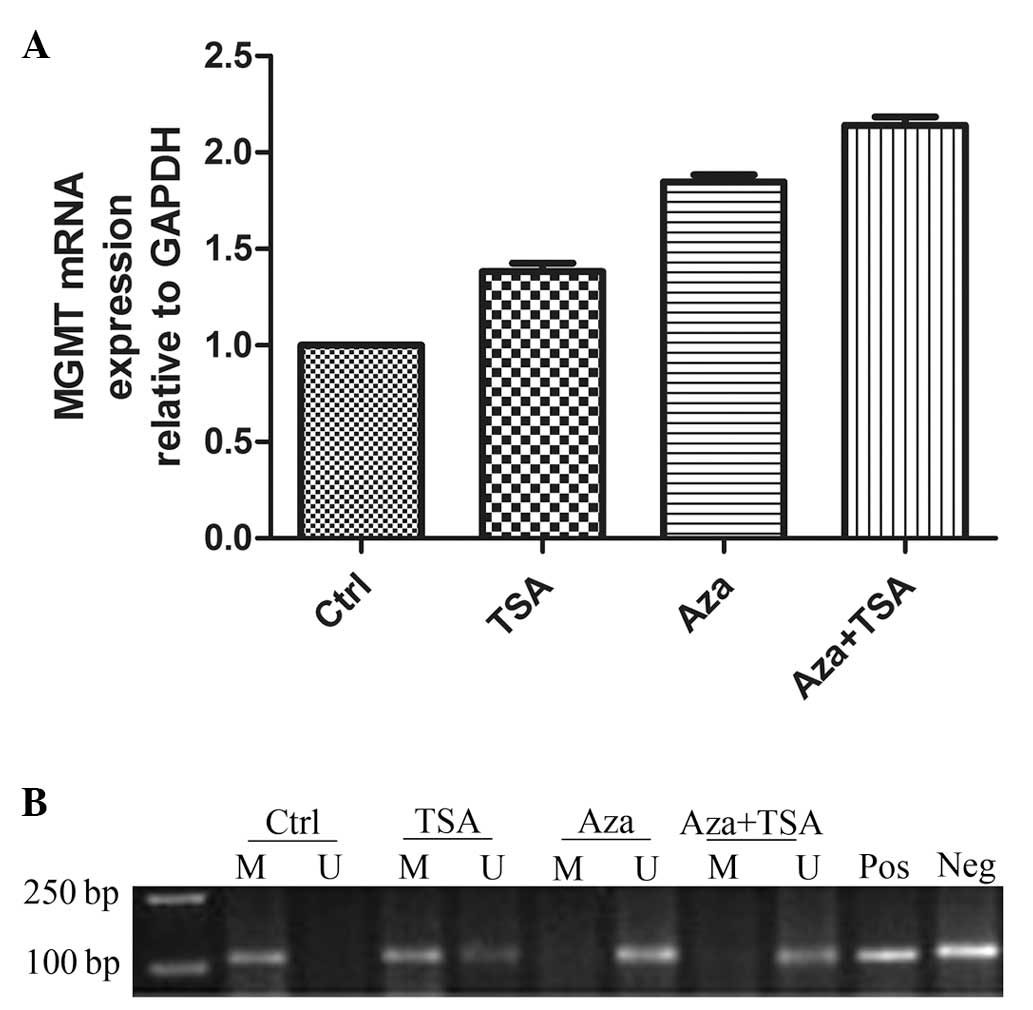 | Figure 1MGMT mRNA expression levels and DNA
methylation status in laryngeal carcinoma HEp-2 cells before and
after treatments with Aza and TSA. (A) RT-qPCR analysis of MGMT
mRNA expression in laryngeal carcinoma HEp-2 before (Ctrl) and
after treatment with Aza and/or TSA. (B) Methylation-specific PCR
analysis of DNA methylation at the MGMT promoter region in
laryngeal carcinoma HEp-2 cells before and after treatment with Aza
and/or TSA. Lane M, methylated alleles (121 bp); lane U,
unmethylated alleles (122 bp); Pos, positive control; Neg, negative
control. A minimum of three independent experiments were performed,
which all produced similar results. MGMT, O6-methylguanine-DNA
methyltransferase; Ctrl, control; TSA, trichostatin A; Aza,
5-aza-2′-deoxycytidine; RT-qPCR, reverse transcription-quantitative
polymerase chain reaction. |
To identify whether DNA methylation was associated
with the changes in MGMT mRNA expression level, the present study
used MSP to assess the DNA methylation status of MGMT in HEp-2
cells. DNA methylation in the MGMT gene promoter region (presence
of a methylation band only) was apparent prior to treatment. TSA
treatment induced hemi-methylation of the MGMT promoter CpG islands
(co-presence of methylation and non-methylation bands) and Aza
treatment resulted in total DNA demethylation (presence of a
methylation band only). The effect of Aza and TSA combination
treatment was similar to that of Aza alone (Fig. 1B).
Differential effects of Aza and TSA
treatment on histone modification in laryngeal carcinoma HEp-2
cells
To elucidate the effect of epigenetic agents on
histone modifications, HEp-2 cells were treated with Aza and TSA.
Aza treatment resulted in a significant decrease in the levels of
H3K9 di-methylation in the MGMT promoter region (0.343±0.003 vs.
0.236±0.001; P<0.01) and TSA treatment resulted in a marginal
reduction in H3K9 di-methylation (0.343±0.003 vs. 0.330±0.003;
P>0.05). The effect of combined Aza and TSA treatment on H3K9
di-methylation was similar to that of Aza alone (0.343±0.003 vs.
0.220±0.001; P<0.01; Fig. 2). To
analyze the level of H3K4 di-methylation at MGMT promoter regions,
ChIP assays were performed on HEp-2 cells. Aza treatment
significantly increased the level of H3K4 di-methylation at the
MGMT promoter (0.484±0.007 vs. 0.631±0.002; P<0.01); however,
TSA treatment did not affect H3K4 di-methylation (0.484±0.007 vs.
0.510±0.002; P>0.05; Fig. 3).
The effect of combined Aza and TSA treatment on the level of H3K4
di-methylation was similar to that of Aza alone (0.484±0.007 vs.
0.677±0.006; P<0.01). Furthermore, H3K9 acetylation was
significantly increased at MGMT promoter regions following
treatment with TSA (0.470±0.001 vs. 0.612±0.003; P<0.01),
however, Aza exhibited no marked effect on H3K9 acetylation
(0.470±0.001 vs. 0.452±0.002, P>0.05). The effect of combined
Aza and TSA treatment on H3K9 acetylation levels was similar to
that of TSA alone (0.470±0.0007 vs. 0.621±0.0023, P<0.05;
Fig. 4).
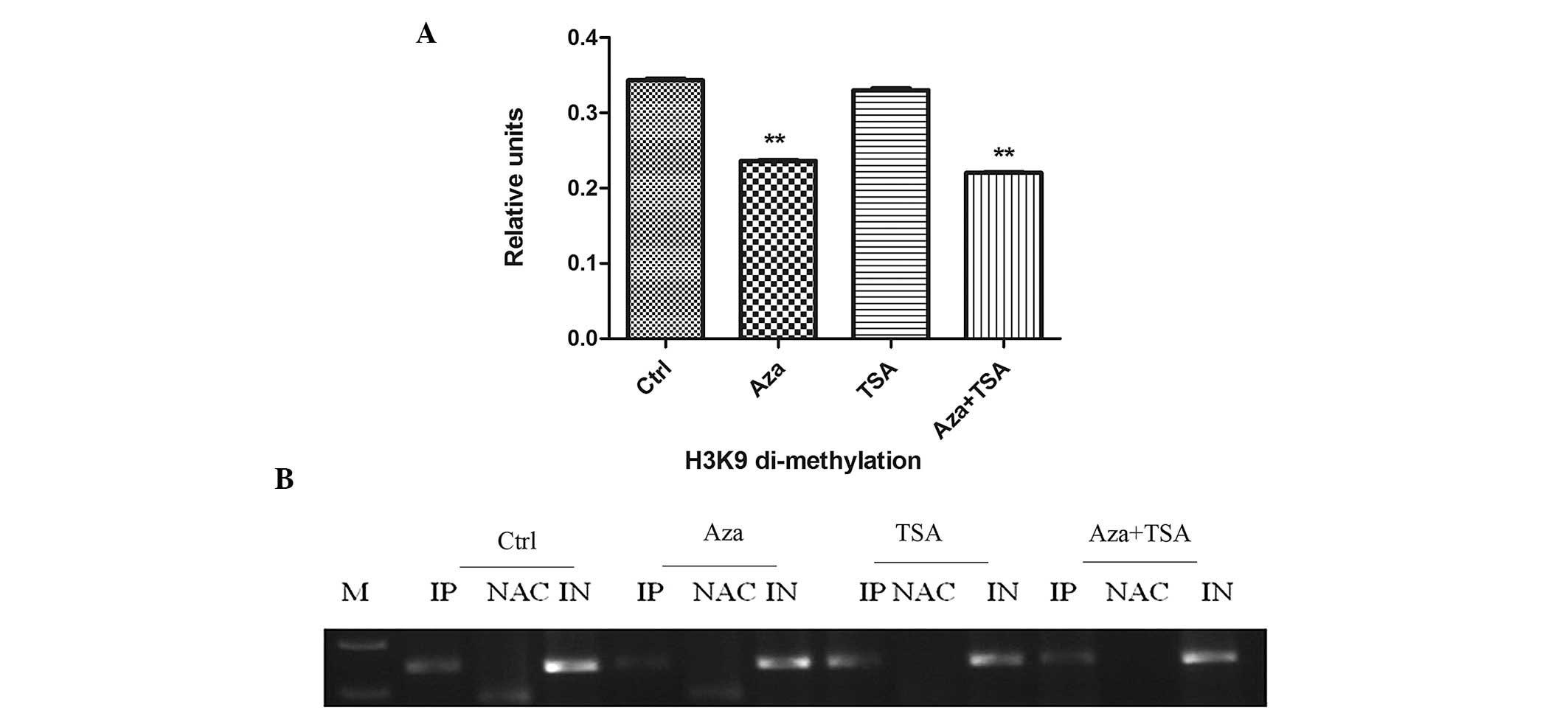 | Figure 2ChIP analysis of H3K9 di-methylation
before and after treatment of HEp-2 cells with Aza, TSA, or Aza and
TSA. Three independent ChIP assays were performed using an antibody
that recognizes di-methyl H3K9 at the O6-methylguanine-DNA
methyltransferase promoter region. (A) Summary of PCR analyses of
ChIP assays. The mean precipitated DNA/input DNA ratios
demonstrated on the y-axis represent the relative values of H3K9
di-methylation. Mean H3K9 di-methylation levels are demonstrated by
the standard error bars and **P<0.01. (B) PCR assay.
Ctrl, prior to treatment; Aza, following treatment with Aza; TSA,
following treatment with TSA; Asa + TSA, following treatment with
Aza and TSA. ChIP, chromatin immunoprecipitation; H3K9, histone 3
lysine 9; Aza, 5-aza-2′-deoxycytidine; TSA, trichostatin A; PCR,
polymerase chain reaction; IP, immunoprecipitated DNA; NAC, no
antibody control; IN, input DNA from whole cell lysate. |
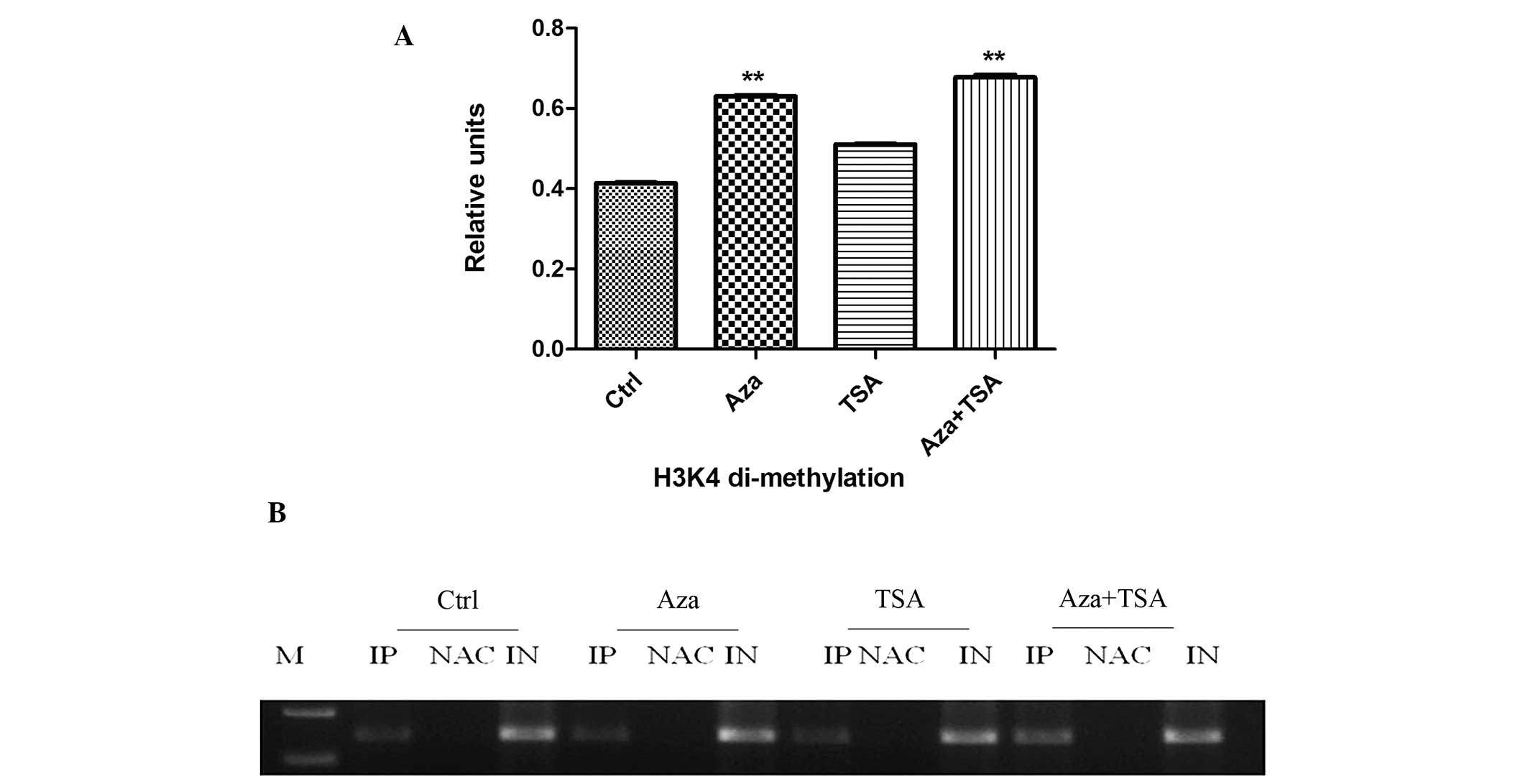 | Figure 3ChIP analysis of H3K4 di-methylation
before and after treatment of HEp-2 cells with Aza, TSA, or Aza and
TSA. Three independent ChIP assays were performed using an antibody
that recognizes di-methyl H3K4 at the O6-methylguanine-DNA
methyltransferase promoter region. (A) Summary of PCR analyses of
ChIP assays. The mean precipitated DNA/input DNA ratios
demonstrated on the y-axis represent the relative values of H3K4
di-methylation. Mean H3K4 di-methylation levels are demonstrated by
the standard error bars and **P<0.01 vs. control
group. (B) PCR assay. Ctrl, prior to treatment; Asa, following
treatment with Aza; TSA, following treatment with TSA; Asa + TSA,
following treatment with Aza and TSA. ChIP, chromatin
immunoprecipitation; H3K4, histone 3 lysine 4; Aza,
5-aza-2′-deoxycytidine; TSA, trichostatin A; PCR, polymerase chain
reaction; IP, immunoprecipitated DNA; NAC, no antibody control; IN,
input DNA from whole cell lysate. |
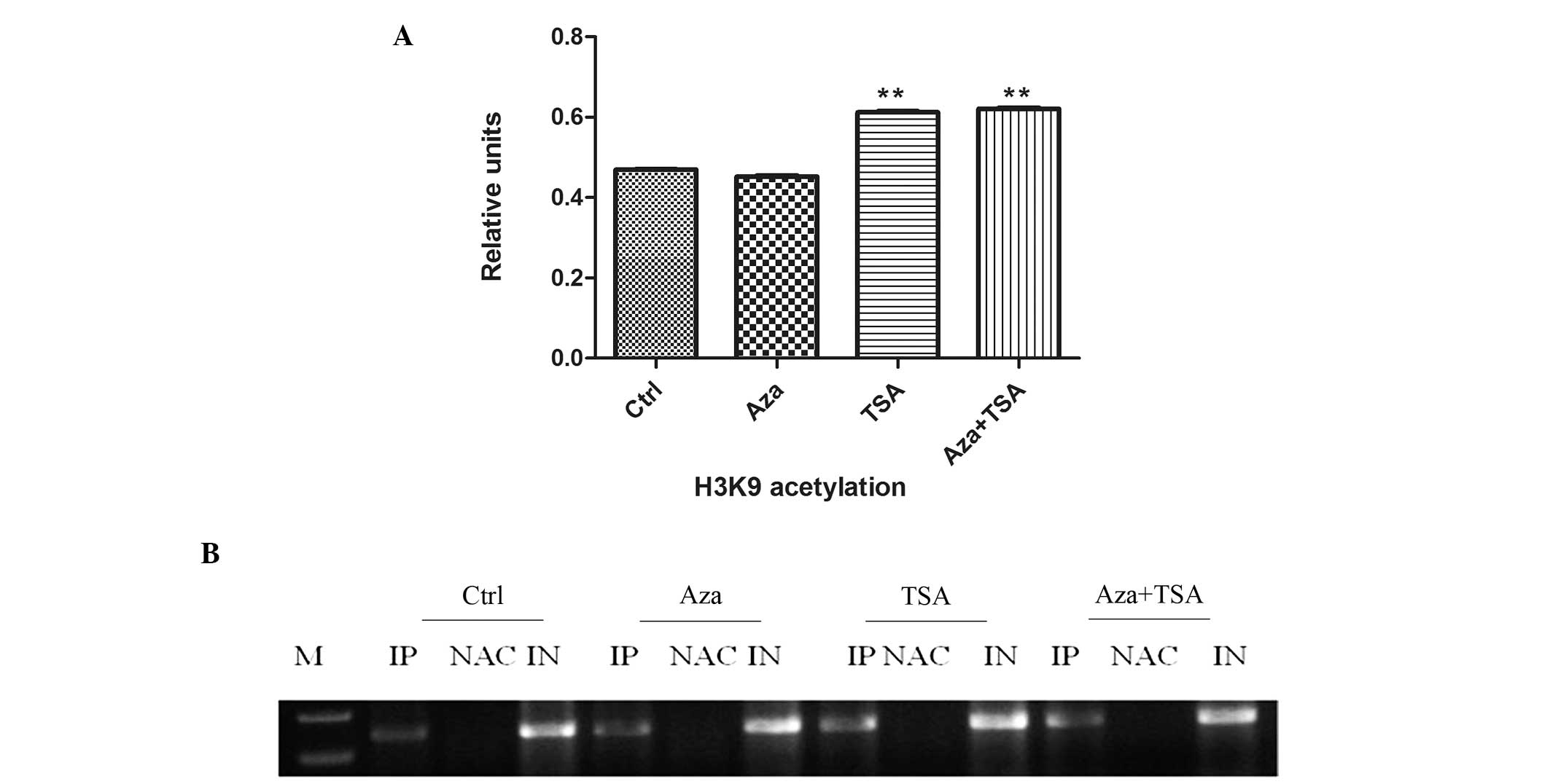 | Figure 4ChIP analysis of H3K9 acetylation
before and after treatment of HEp-2 cells with Aza, TSA, or Aza and
TSA. Three independent ChIP assays were performed using an antibody
that recognizes H3K9 acetylation at the O6-methylguanine-DNA
methyltransferase promoter region. (A) Summary of PCR analyses of
ChIP assays. The mean precipitated DNA/inputDNA ratios demonstrated
on the y-axis represent the relative values of H3K9 acetylation.
Mean H3K9 acetylation levels are demonstrated by the standard error
bars and **P<0.05 vs. control group. (B) PCR assay.
Ctrl, prior to treatment; Asa following treatment with Aza; TSA,
following treatment with TSA; Asa + TSA, following treatment with
Aza and TSA. ChIP, chromatin immunoprecipitation; H3K9, histone 3
lysine 9; Aza, 5-aza-2′-deoxycytidine; TSA, trichostatin A; PCR,
polymerase chain reaction; IP, immunoprecipitated DNA; NAC, no
antibody control; IN, input DNA from whole cell lysate.. |
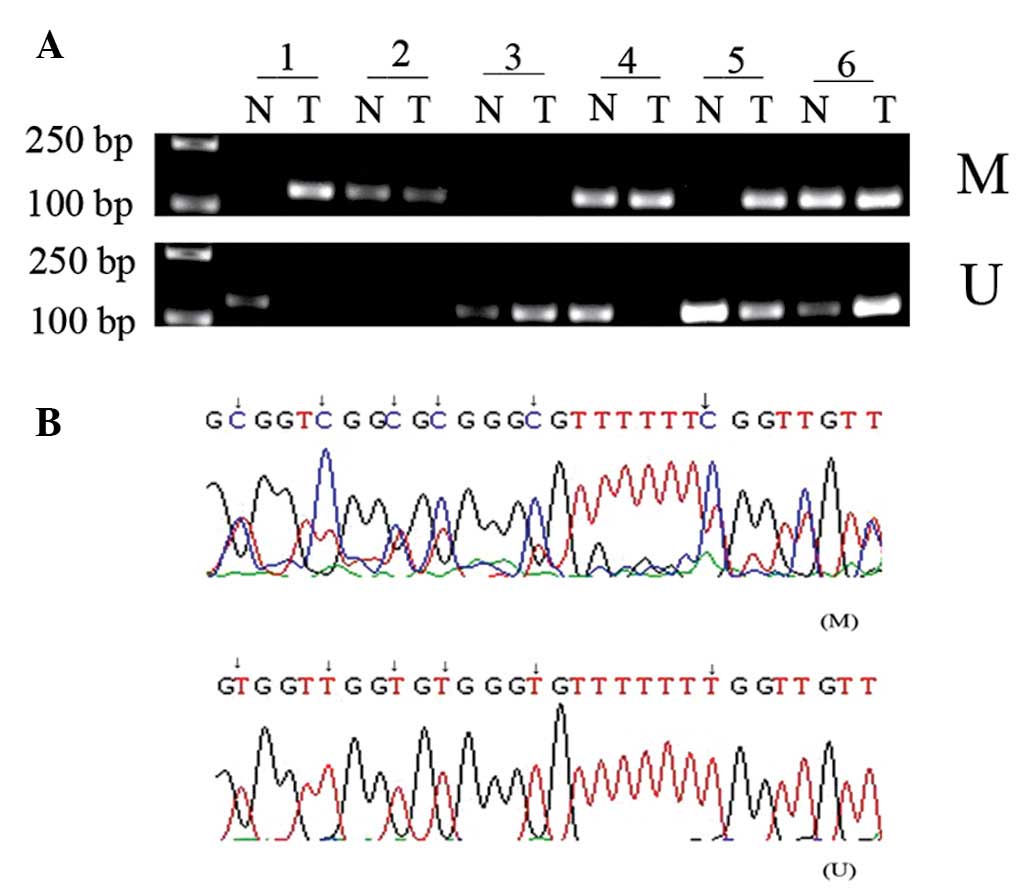
Frequent DNA methylation of MGMT in LSCC
and its clinical significance
The methylation status of MGMT in LSCC tissue and
paired adjacent healthy tissue samples was detected using MSP. The
DNA methylation rate of MGMT was significantly higher in laryngeal
cancer (54%) compared with 24% in the healthy control group
(P<0.05; Fig. 5A; data not
shown). The methylated and unmethylated products of MSP were
sequenced, confirming that sodium bisulfite modification was
sufficient for the DNA (Fig. 5B).
The present study also identified that the DNA methylation status
of MGMT exhibited no correlation with the age or gender of the
patient, the degree of tumor differentiation, the tumor T stage or
lymph node metastasis in LSCC tissues (P>0.05; Table I).
 | Table IClinicopathological parameters and
MGMT methylation status of tissue samples from laryngeal squamous
cell carcinoma patients. |
Table I
Clinicopathological parameters and
MGMT methylation status of tissue samples from laryngeal squamous
cell carcinoma patients.
| Variable | Patients, n | MGMT methylation
status, n (%) | P-value |
|---|
|
|---|
| M | U |
|---|
| Gender | | | | 0.517 |
| Male | 44 | 23 (52.3) | 21 (47.7) | |
| Female | 6 | 4 (66.7) | 2 (33.3) | |
| Age, years | | | | 0.162 |
| <60 | 24 | 16 (66.7) | 8 (33.3) | |
| ≥60 | 26 | 11 (42.3) | 15 (57.7) | |
| Tumor T stage | | | | 0.982 |
| T1 + T2 | 24 | 13 (54.2) | 11 (45.8) | |
| T3 + T4 | 26 | 14 (53.8) | 12 (46.2) | |
| Lymphatic
metastasis | | | | 0.968 |
| Positive | 11 | 6 (54.5) | 5 (45.5) | |
| Negative | 39 | 21 (53.8) | 18 (46.2) | |
| Tumor Grade | | | | 0.586 |
|
Well-differentiated | 29 | 18 (62.0) | 11(38.0) | |
| Moderately and
poorly differentiated | 21 | 9 (42.9) | 12 (57.1) | |
| Type | | | | 0.747 |
| Glottic | 23 | 13 (56.5) | 10 (43.5) | |
| Supraglottic | 27 | 14 (51.9) | 13 (47.1) | |
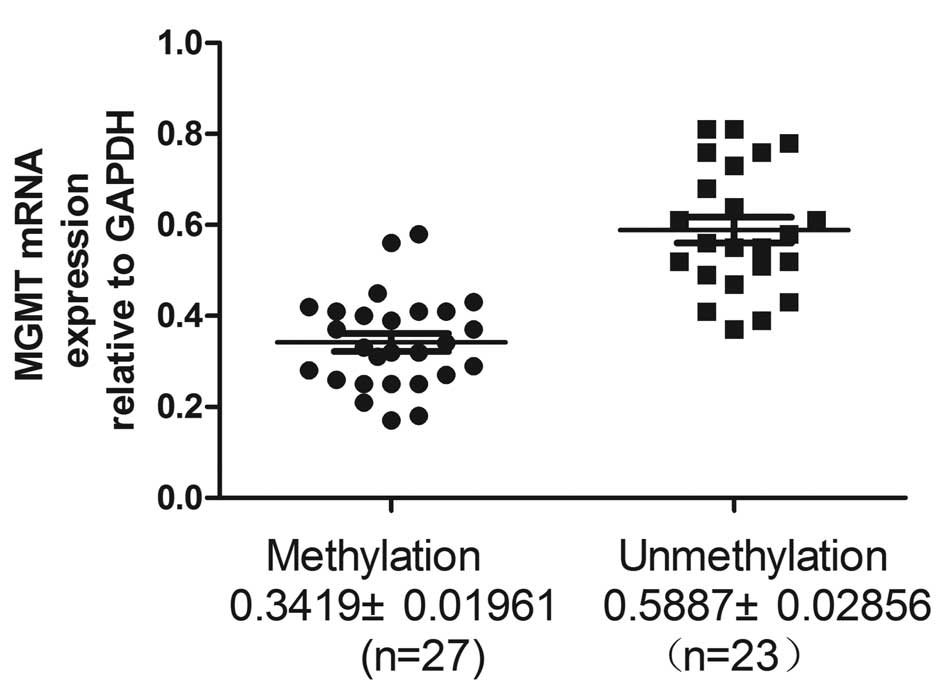
Low expression level of MGMT is
associated with DNA methylation status in LSCC tissues
RT-qPCR was performed to assess the MGMT mRNA
expression level in LSCC tissues. The present study identified that
MGMT mRNA expression levels were significantly lower in LSCC
tissues exhibiting MGMT DNA methylation compared with unmethylated
DNA (0.3419±0.01916 vs. 0.5887±0.02856; P<0.0001; Fig. 6 and Table II).
 | Table IIAssociation between MGMT mRNA
expression levels and DNA methylation status in laryngeal squamous
cell carcinoma tissues. |
Table II
Association between MGMT mRNA
expression levels and DNA methylation status in laryngeal squamous
cell carcinoma tissues.
| DNA methylation
status | Cases, n | MGMT mRNA
expression level |
|---|
| Methylated | 27 | 0.3419±0.01961 |
| Unmethylated | 23 | 0.5887±0.02856 |
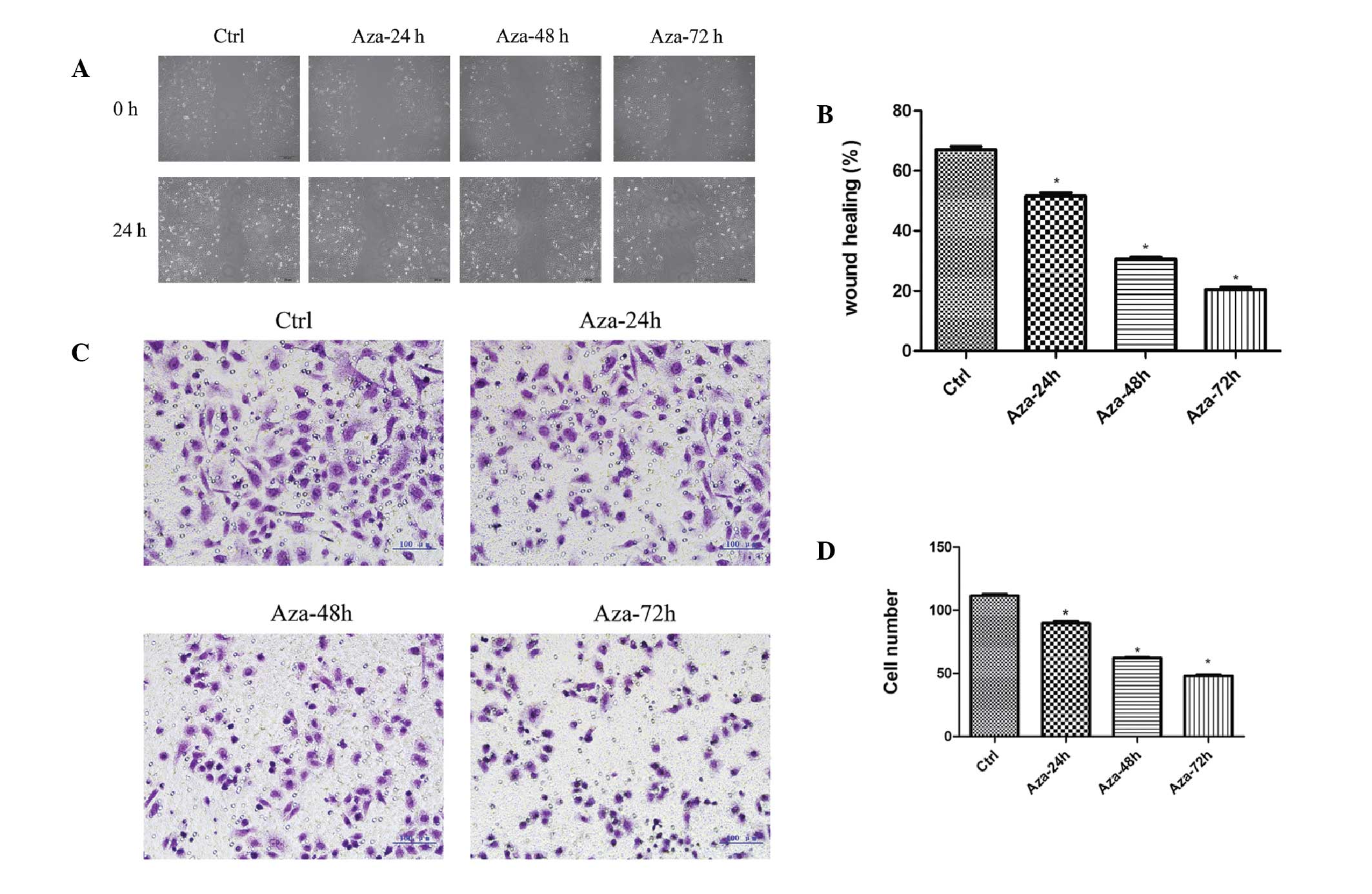
Migration and invasion inhibition of
HEp-2 cells following treatment with Aza
To investigate the inhibitory effect of Aza on the
migration and invasion of HEp-2 cells, a wound-healing and
Matrigel® invasion assay was performed. The present
study demonstrated that 24 h after establishing the wound, the
control group achieved the most wound closure (67.00±1.080%),
compared with the Aza 24- (51.50±1.190%), 48- (30.50±0.6455%) and
72-h (20.50±0.6455%) groups (F=510.9; P<0.0001; Fig. 7A and B). The Matrigel®
invasion assay demonstrated that the number of invading HEp-2 cells
was 111.5±1.190, 89.75±1.250, 62.25±0.4787, and 48.00±0.9129% in
the control, Aza 24-, 48- and 72-h groups, respectively. The number
of invading HEp-2 cells was significantly reduced following Aza
treatment when compared with the control group (F=794.5;
P<0.0001; Fig. 7C and D). Thus,
Aza significantly reduced HEp-2 cell migration and invasion.
Discussion
A growing number of TSGs are being identified that
are inactivated by epigenetic, rather than classic,
mutation/deletion events (14,15).
Unlike mutational inactivation, methylation is reversible, thus,
demethylating agents and inhibitors of histone deacetylases (HDACs)
have been evaluated in clinical trials (16–19).
Aza, a pyrimidine analogue with the 2′-deoxycytidine fifth carbon
atom replaced by a nitrogen atom, binds to DNA molecules during
replication and subsequently forms a complex with DNA
methyltransferase (DNMT1). This complex inhibits the
transmethylation activity of DNMT1. TSA is a HDAC inhibitor that
causes DNA histone hyperacetylation and subsequently induces p21
(WAF1/CIP1) gene expression. Upregulation of p21 (WAF1/CIP1) gene
transcription may result in cell cycle arrest and inhibition of
cell growth by regulating cell cycle regulatory factors and the
expression levels of apoptosis-associated proteins (17,20).
In the present study, it was identified that DNA
hypermethylation-silenced MGMT in laryngeal carcinoma HEp-2 cells
is characterized by H3K9 hypermethylation, and H3K9 and H3K4
hypomethylation at the promoter. Following treatment with Aza alone
or in combination with TSA, H3K9 di-methylation decreased and H3K4
di-methylation increased at the promoter, consistent with DNA
demethylation and reactivation of MGMT expression levels. In
addition, the effect of combined Aza and TSA treatment on MGMT
expression levels was stronger than that of Aza treatment alone.
Treatment of laryngeal carcinoma HEp-2 cells with Aza appeared to
reverse the MGMT gene methylation status and demethylate the
promoter region. Furthermore, the effect of combined Aza and TSA
treatment on MGMT expression levels was stronger than that of Aza
treatment alone. In the present study, the MGMT methylation status
was not affected by TSA alone as TSA is an HDAC inhibitor, which
does not affect the gene promoter methylation status. The primary
effect of epigenetic agents on MGMT gene expression levels was
promoter hypermethylation: MGMT gene expression level was
significantly upregulated by Aza, marginally upregulated by TSA,
and synergistically upregulated using a combination of the two
epigenetic agents. The results indicate that the primary epigenetic
factor influencing gene expression levels and downregulating the
TSG transcription was MGMT gene DNA methylation, however, histone
modifications were also key. ChIP assays were conducted to
investigate the association between the DNA methylation status and
histone modifications. ChIP is a technique used to identify the
presence of particular DNA-binding proteins that may modulate
chromatin structure and/or transcriptional characteristics of the
specific region of DNA with which the DNA-binding proteins are
associated. The present study demonstrated that H3K9 di-methylation
levels in the MGMT promoter region correlated with the DNA
methylation status. MGMT reactivation by Aza was accompanied by DNA
demethylation and a decrease in H3K9 di-methylation levels. In
contrast to H3K9 di-methylation, H3K4 di-methylation in the
promoter region was inversely correlated with the DNA methylation
status. Furthermore, H3K4 di-methylation may be associated with an
open chromatin configuration and transcriptional activation
(21). Aza increased the level of
H3K4 di-methylation, however, there was no significant affect on
H3K9 acetylation. TSA significantly increased the level of H3K9
acetylation in HEp-2 cells, although it produced a marginal effect
on MGMT gene expression levels. The present study proposes that DNA
methylation may be important in gene silencing and the maintenance
of repressive histone modifications at hypermethylated gene
promoters in laryngeal carcinogenesis. It has previously been
reported that DNA modification itself, or components of the DNA
methylation machinery, such as DNMTs or methyl CpG-binding
proteins, may directly interact with histone methyltransferases or
proteins in regions of DNA methylation. This interaction allows the
DNA methylation machinery to assemble an alterative histone
modification, demonstrating that histone methylation depends on DNA
methylation (22,23).
The DNA methylation status of a gene has previously
been reported as a promising biomarker in the early diagnosis and
prognosis of cancer (24,25). Aberrant DNA hypermethylation of gene
promoter regions is an important epigenetic mechanism that
regulates gene expression levels, resulting in the downregulation
and silencing of various TSGs (26–28).
In the present report, the MGMT gene was identified to exhibit a
frequent methylation rate in LSCC, which may indicate that the
occurrence of laryngeal cancer is associated with promoter
methylation of the MGMT gene. The MGMT mRNA expression level is
significantly lower when the promoter is methylated compared with
when the promoter is unmethylated. Therefore, the mRNA expression
level of the TSG, MGMT exhibits a negative correlation with CpG
island methylation in LSCC. Furthermore, the MGMT methylation
status exhibits no correlation with patient age or gender, tumor
differentiation degree, tumor T stage or lymph node metastasis in
LSCC. This lack of correlation indicates that MGMT gene methylation
is not limited to a particular stage or sub-type of LSCC, but is
involved in the entire development process. In addition, the
present study examined the effect of Aza application on the
migration and invasion ability of HEp-2 cells using wound-healing
and Matrigel® invasion assays. Aza application inhibited
the migration and invasion ability of HEp-2 cells, indicating that
DNA demethylation may inhibit the invasion ability of HEp-2 cells.
Recently, Aza was demonstrated to synergize with progesterone
therapy to inhibit endometrial cancer cell growth and invasion
(29). Thus, additional studies are
required to fully elucidate the potential of epigenetic agents in
cancer therapy.
In conclusion, the present study demonstrates that
frequent epigenetic alterations regulate MGMT gene expression level
in LSCC. The data provides a foundation for further investigations
into the role of the MGMT gene in laryngeal carcinoma and its
potential as a biomarker in the early diagnosis, treatment and
prognosis of laryngeal carcinoma.
References
|
1
|
Marioni G, Marchese-Ragona R, Cartei G,
Marchese F and Staffieri A: Current opinion in diagnosis and
treatment of laryngeal carcinoma. Cancer Treat Rev. 32:504–515.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bugni JM, Meira LB and Samson LD:
Alkylation-induced colon tumorigenesis in mice deficient in the
Mgmt and Msh6 proteins. Oncogene. 28:734–741. 2009. View Article : Google Scholar
|
|
3
|
Meng CF, Zhu XJ, Peng G and Dai DQ: Role
of histone modifications and DNA methylation in the regulation of
O6-methylguanine-DNA methyltransferase gene expression in human
stomach cancer cells. Cancer Invest. 28:331–339. 2010. View Article : Google Scholar
|
|
4
|
McCabe MT, Brandes JC and Vertino PM:
Cancer DNA methylation: molecular mechanisms and clinical
implications. Clin Cancer Res. 15:3927–3937. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim YJ, Yoon HY, Kim SK, et al: EFEMP1 as
a novel DNA methylation marker for prostate cancer: array-based DNA
methylation and expression profiling. Clin Cancer Res.
17:4523–4530. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chang X, Li Z, Ma J, et al: DNA
methylation of NDRG2 in gastric cancer and its clinical
significance. Dig Dis Sci. 58:715–723. 2013. View Article : Google Scholar
|
|
7
|
Wozniak RJ, Klimecki WT, Lau SS, Feinstein
Y and Futscher BW: 5-Aza-2′-deoxycytidine-mediated reductions in
G9A histone methyltransferase and histone H3 K9 di-methylation
levels are linked to tumor suppressor gene reactivation. Oncogene.
26:77–90. 2007. View Article : Google Scholar
|
|
8
|
Sharma S, Kelly TK and Jones PA:
Epigenetics in cancer. Carcinogenesis. 31:27–36. 2010. View Article : Google Scholar :
|
|
9
|
O’Sullivan B and Shah J: New TNM staging
criteria for head and neck tumors. Semin Surg Oncol. 21:30–42.
2003. View Article : Google Scholar
|
|
10
|
Samudio-Ruiz SL and Hudson LG: Increased
DNA methyltransferase activity and DNA methylation following
epidermal growth factor stimulation in ovarian cancer cells.
Epigenetics. 7:216–224. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kondo Y, Shen L, Cheng AS, et al: Gene
silencing in cancer by histone H3 lysine 27 trimethylation
independent of promoter DNA methylation. Nat Genet. 40:741–750.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Socha MJ, Said N, Dai Y, et al: Aberrant
promoter methylation of SPARC in ovarian cancer. Neoplasia.
11:126–135. 2009.PubMed/NCBI
|
|
13
|
Wu LP, Wang X, Li L, et al: Histone
deacetylase inhibitor depsipeptide activates silenced genes through
decreasing both CpG and H3K9 methylation on the promoter. Mol Cell
Biol. 28:3219–3235. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Grewal SI and Moazed D: Heterochromatin
and epigenetic control of gene expression. Science. 301:798–802.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chang X, Zhang S, Ma J, et al: Association
of NDRG1 gene promoter methylation with reduced NDRG1 expression in
gastric cancer cells and tissue specimens. Cell Biochem Biophys.
66:93–101. 2013. View Article : Google Scholar
|
|
16
|
Agathanggelou A, Cooper WN and Latif F:
Role of the Ras-association domain family 1 tumor suppressor gene
in human cancers. Cancer Res. 65:3497–3508. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fahrner JA, Eguchi S, Herman JG and Baylin
SB: Dependence of histone modifications and gene expression on DNA
hypermethylation in cancer. Cancer Res. 62:7213–7218.
2002.PubMed/NCBI
|
|
18
|
Liu J, Zhu X, Xu X and Dai D: DNA promoter
and histone H3 methylation downregulate NGX6 in gastric cancer
cells. Med Oncol. 31:8172014. View Article : Google Scholar
|
|
19
|
Dong W, Wang L and Shen R: MYO5B is
epigenetically silenced and associated with MET signaling in human
gastric cancer. Dig Dis Sci. 58:2038–2045. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chakraborty AK, de Sousa JF, Chakraborty
D, et al: GnT-V expression and metastatic phenotypes in
macrophage-melanoma fusion hybrids is down-regulated by 5-Aza-dC:
evidence for methylation sensitive, extragenic regulation of GnT-V
transcription. Gene. 374:166–173. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Peng DF, Razvi M, Chen H, et al: DNA
hypermethylation regulates the expression of members of the
Mu-class glutathione S-transferases and glutathione peroxidases in
Barrett’s adenocarcinoma. Gut. 58:5–15. 2009. View Article : Google Scholar
|
|
22
|
Fuks F, Hurd PJ, Deplus R and Kouzarides
T: The DNA methyltransferases associate with HP1 and the SUV39H1
histone methyltransferase. Nucleic Acids Res. 31:2305–2312. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lin W and Dent SY: Functions of
histone-modifying enzymes in development. Curr Opin Genet Dev.
16:137–142. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kondo Y: Epigenetic cross-talk between DNA
methylation and histone modifications in human cancers. Yonsei Med
J. 50:455–463. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Schneider BG, Peng DF, Camargo MC, et al:
Promoter DNA hypermethylation in gastric biopsies from subjects at
high and low risk for gastric cancer. Int J Cancer. 127:2588–2597.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tost J: DNA methylation: an introduction
to the biology and the disease-associated changes of a promising
biomarker. Methods Mol Biol. 507:3–20. 2009. View Article : Google Scholar
|
|
27
|
Tong JD, Jiao NL, Wang YX, Zhang YW and
Han F: Downregulation of fibulin-3 gene by promoter methylation in
colorectal cancer predicts adverse prognosis. Neoplasma.
58:441–448. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhi Y, Chen J, Zhang S, et al:
Down-regulation of CXCL12 by DNA hypermethylation and its
involvement in gastric cancer metastatic progression. Dig Dis Sci.
57:650–659. 2012. View Article : Google Scholar
|
|
29
|
Hu Q, Yu L, Chen R, Wang YL, et al:
5-aza-2′-deoxycytidine improves the sensitivity of endometrial
cancer cells to progesterone therapy. Int J Gynecol Cancer.
22:951–959. 2012. View Article : Google Scholar : PubMed/NCBI
|