Introduction
Melanotic neuroectodermal tumor of infancy (MNTI) is
a rare, pediatric tumor with 447 documented cases between 2012 and
the first report of the condition in 1918 (1). It is estimated that 92.8% of MNTIs
occur in the head and neck, most frequently in the maxilla
(68–80%), skull (10.8%), mandible (6%) and brain (4.3%) (2), without any evident gender difference.
The majority of cases occur within the first year of life, with 77%
discovered prior to the age of six months. In 1966, Borello and
Gorlin (3) identified elevated
levels of urine vanillylmandelic acid (VMA), a marker of neurogenic
tumors, in MNTI patients. Accordingly, they classified MNTI as a
neurogenic tumor; a definition accepted by the Word Health
Organization (4). Currently, MNTI
is treated primarily by surgical resection. However, the optimal
extent of surgical resection remains controversial, as the
post-operative recurrence rate is as high as 60%. The majority of
cases of MNTI grow rapidly and are invasive to a certain extent,
while a proportion undergo malignant transformation. Therefore,
extended resection is often adopted as a surgical approach in the
treatment of MNTI (5). However,
excessive tissue resection has the potential to adversely affect
infant growth and development. The present case study examines a
rare case of MNTI in the right mandible and its subsequent
treatment. In this study, the effects of the tumor on the
surrounding sclerotin, mandible, dental germ and inferior alveolar
nerve are described based on intra-operative observations and the
post-operative histopathology of the resected specimens. A
literature review discussing the optimal scope of surgical
resection is also presented.
Case report
Medical history and clinical
manifestations
A two-month-old infant female was admitted to The
Affiliated Women and Children’s Medical Center of Guangzhou Medical
University (Guangzhou, Guangdong, China) with a tumor on the lip
side of the right inferior gum, which had appeared one month
earlier. Initially, the tumor was consistent with the mucosal color
of the surrounding gums, however, it became cyanotic as it
increased in size. No fever or pain was reported by the infant’s
parents, however, the patient was taken to hospital due to concerns
that the tumor would interfere with eating and development. Upon
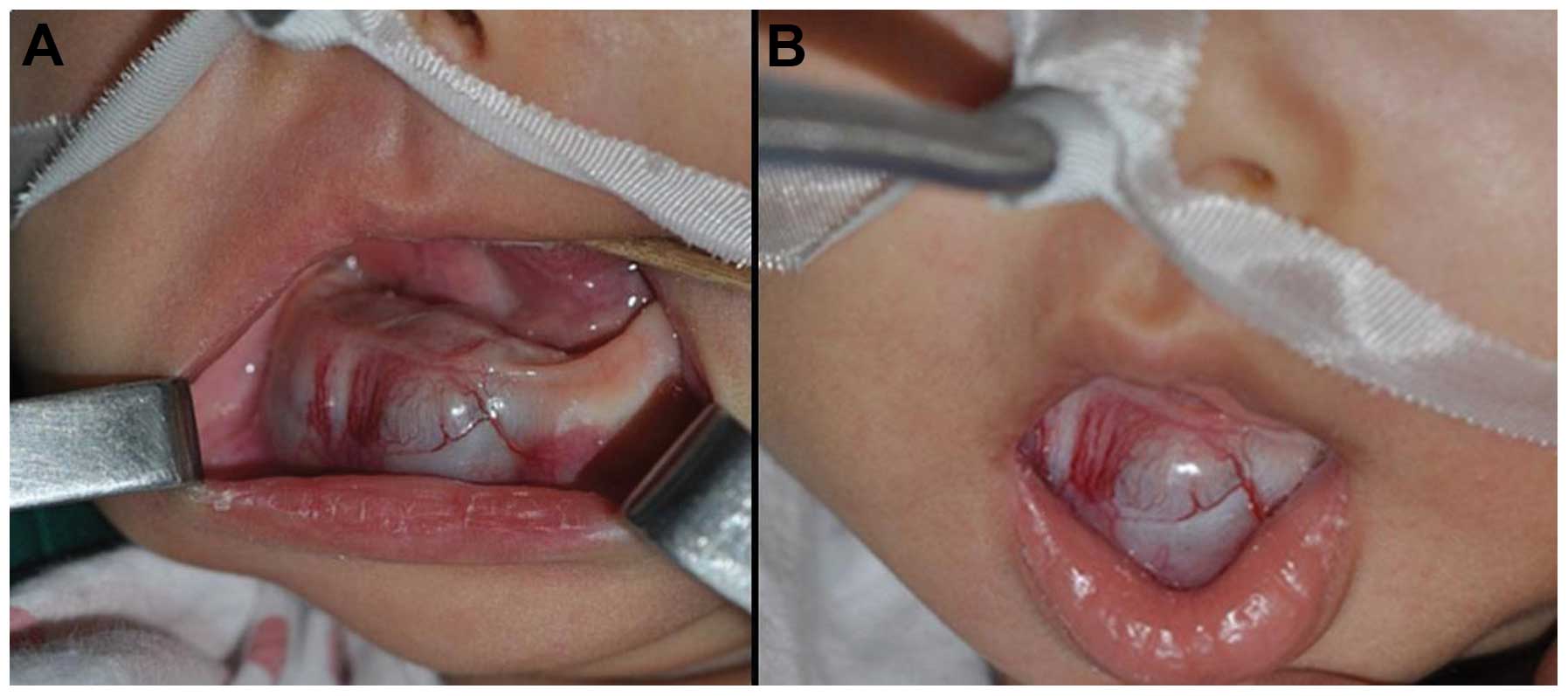
physical examination, a projecting mass measuring 2×2.5×2.5 cm was
detected on the right inferior and anterior alveolar ridge. There
was no tenderness of the tumor mass, and it lay firm on the gum
(Fig. 1). Upon pinpricking the
right lower lip, the infant cried more noticeably compared with a
healthy child. No significant anomalies were detected in the oral
mucosa outside of the lesion site, and the deciduous teeth were not
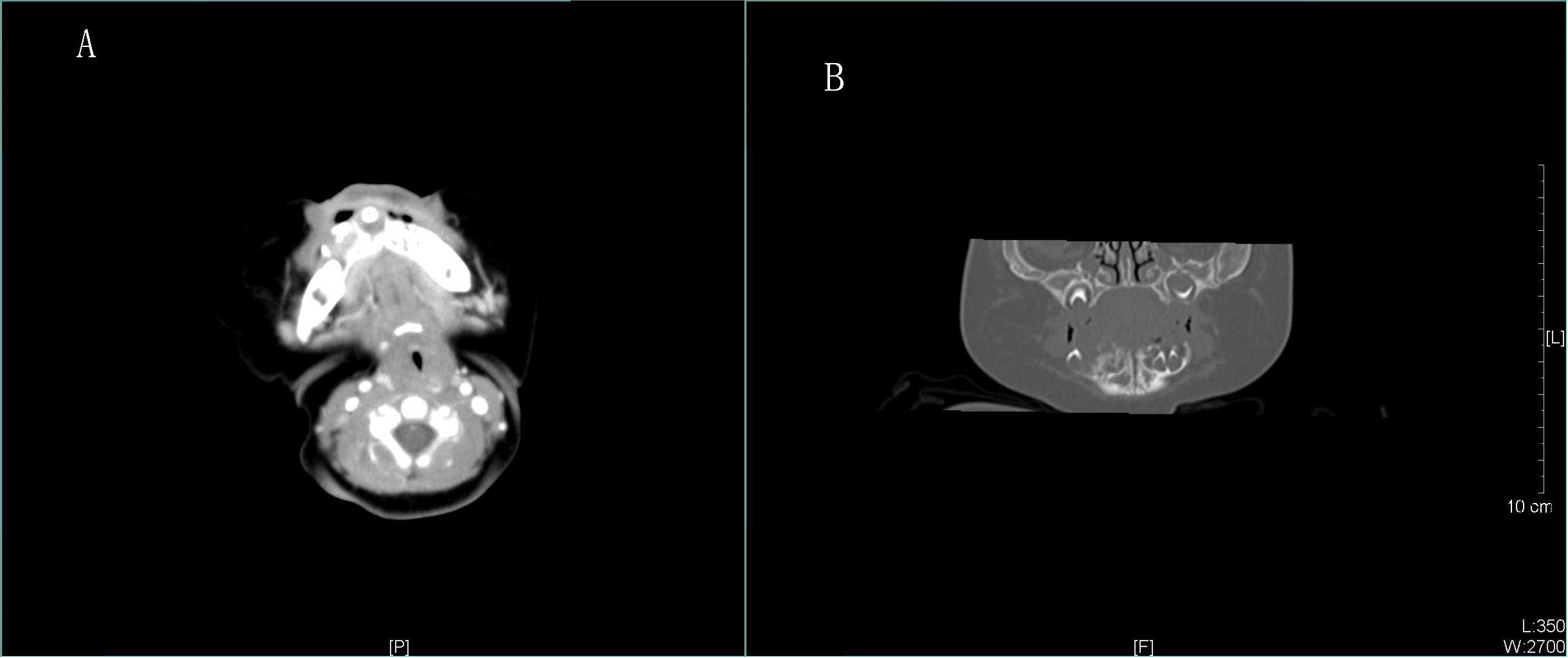
erupted. Computed tomography (CT) examination (Fig. 2) revealed low-density lesions of the
right mandible, with unclear boundaries. On magnetic resonance
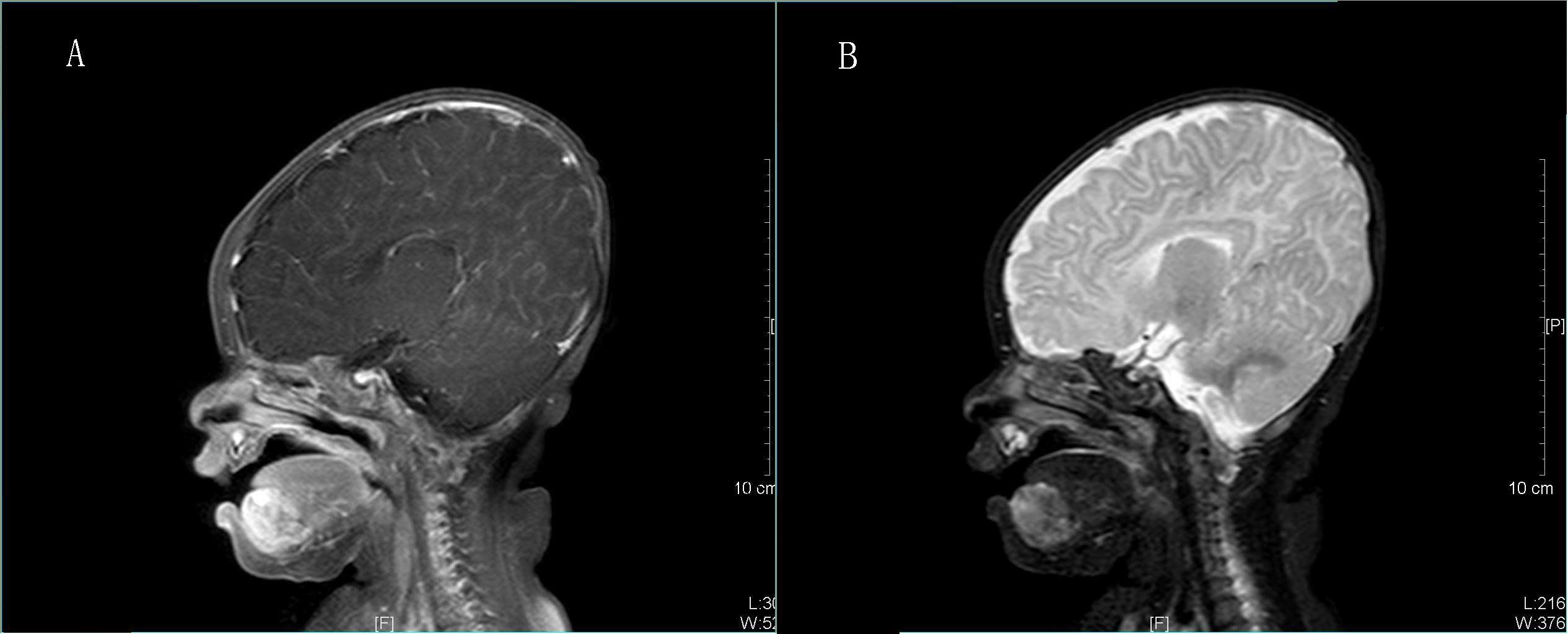
imaging (MRI), T1-weighted imaging (T1WI) revealed a heterogeneous,
but generally low-intensity, quasi-circular tumor (Fig. 3A). T2WI identified an uneven,
higher-intensity tumor, but no invasion into the mouth floor or the
vestibular sulcus mucosa (Fig. 3B).
Laboratory blood tests identified no clear abnormalities in
metabolism, biochemistry or coagulation functions. However, the
urine VMA level was elevated at 75.9 μmol/24 h (normal range,
24.98–70.2 μmol/24 h).
Surgical procedure
During the surgery, the periosteum of the mandible
and the inferior alveolar nerve were maintained, while tumors
surrounding the sclerotin and the relevant teeth were resected. The
vestibular sulcus was adopted, and the tumors were accessed
following incision of the mandibular mucosa of the vestibular
sulcus. The periosteum of the mandible was disrupted at the lesion
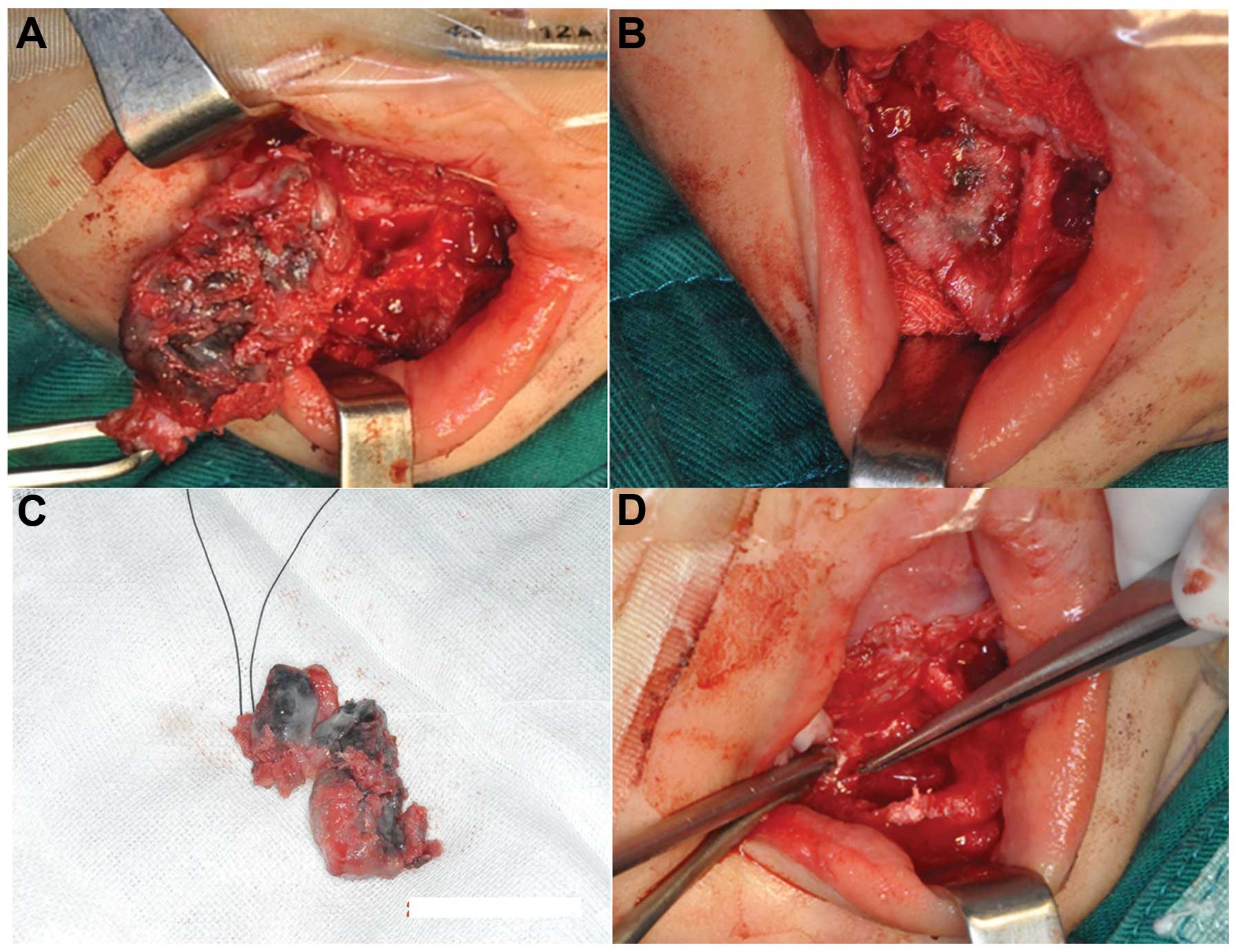
sites. A solid black tumor in the mental foramen, with intact
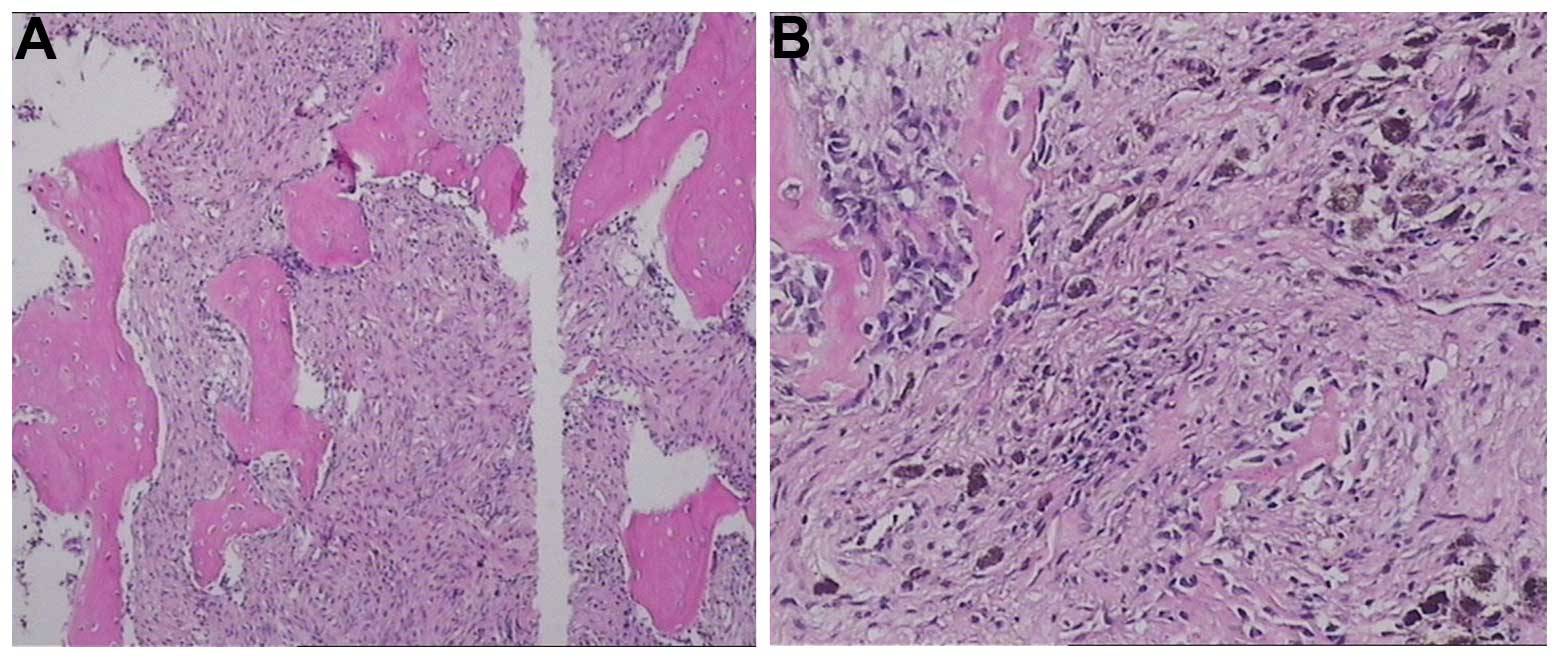
envelope (Fig. 4A), exhibited
infiltrative growth into the surrounding cancellous bone (Figs. 4B and 6), and contained the dental germ of tooth
81 and 82. The tumor and its dental germ were excised for
subsequent pathological examination. The remaining cavity of the
mandible exhibited a honeycomb structure, and invasive black flecks
were observed in the sclerotin (Fig.
4B). The sclerotin around the tumors was resected for
pathological examination, and areas invaded by black flecks were
removed using an abrasive drill until the remaining bone was
smooth. Due to contact of the inferior alveolar neural tube with
the tumor, the sclerotin around the mental nerve was removed, but
the mental nerve itself was retained. Following tumor resection
(Fig. 4D), the tumor envelop was
found to be intact, with black and white stripes visible on the
side of the section. Resection of the tumor resulted in a reduction
in the thickness of the mandible cortex; in order to prevent
fracture, a titanium plate was inserted to strengthen the lower
edge of the mandible.
Pathological examination
Tumor tissue
The primary tumor volume was 3×2×1.2 cm, and two
teeth were found on its surface without an obvious envelop. The
side of the section side was gray and black, with clear boundaries
visible (Fig. 4C). No noticeable
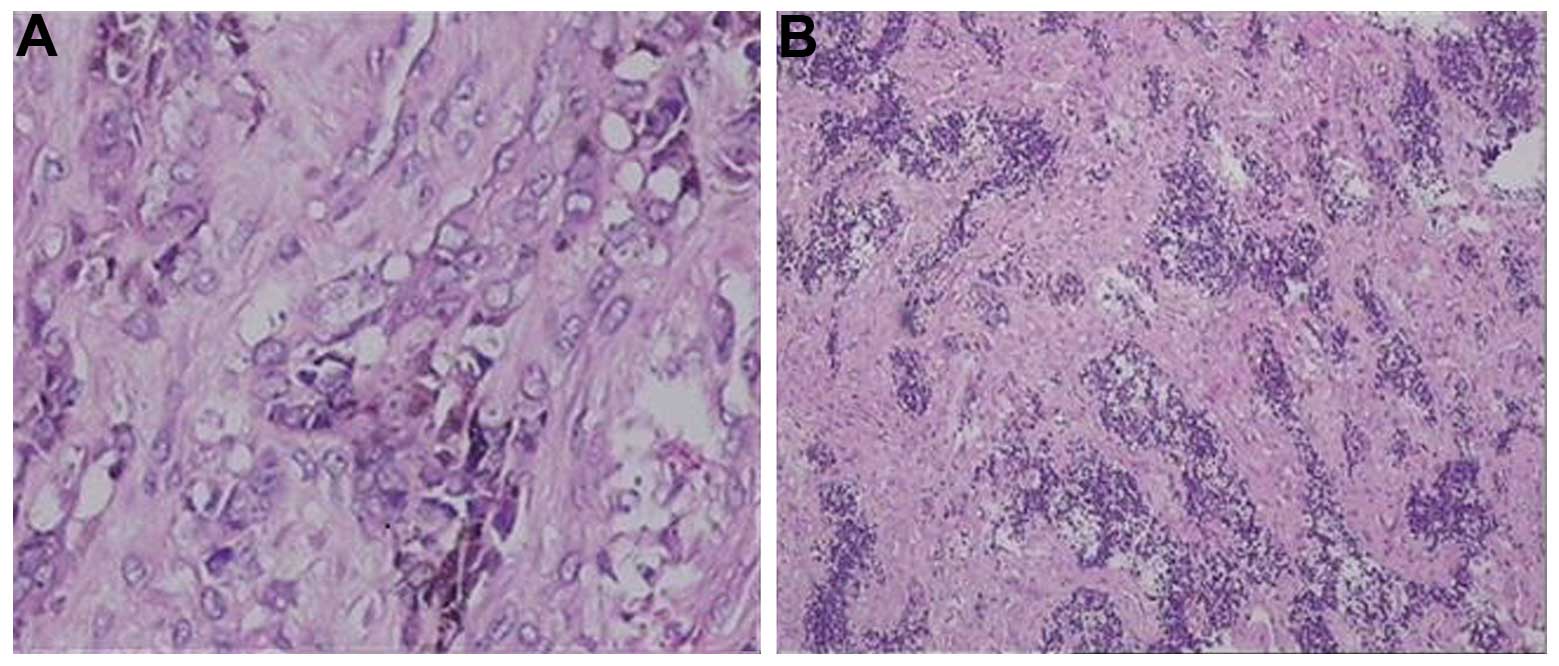
tumor invasion was identified in the teeth. Microscopic examination
revealed nested, small round cells and larger pigmented cells in
the fibrous connective tissue, without evident pathological nuclear
fission, necrosis or perineural invasion (Fig. 5). Smaller round cells were
vimentin/neuron-specific enolase (NSE)/melanoma-associated antigen
45 (HMB45)/synaptophysin(+), and larger cells were cytokeratin
(CK)/epithelial membrane antigen(+), with no significant glial
fibrillary acidic protein (GFAP)/S-100 staining. Scattered cells
exhibited desmin immunoreactivity, and ~2% of the cells were
Ki-67(+).
Sclerotin around the tumors
The mandibular tissue was disrupted by the tumor
(Fig. 4C). Certain tumor cells
within the sclerotin were pigmented, but without a noticeable
allotype (Fig. 6).
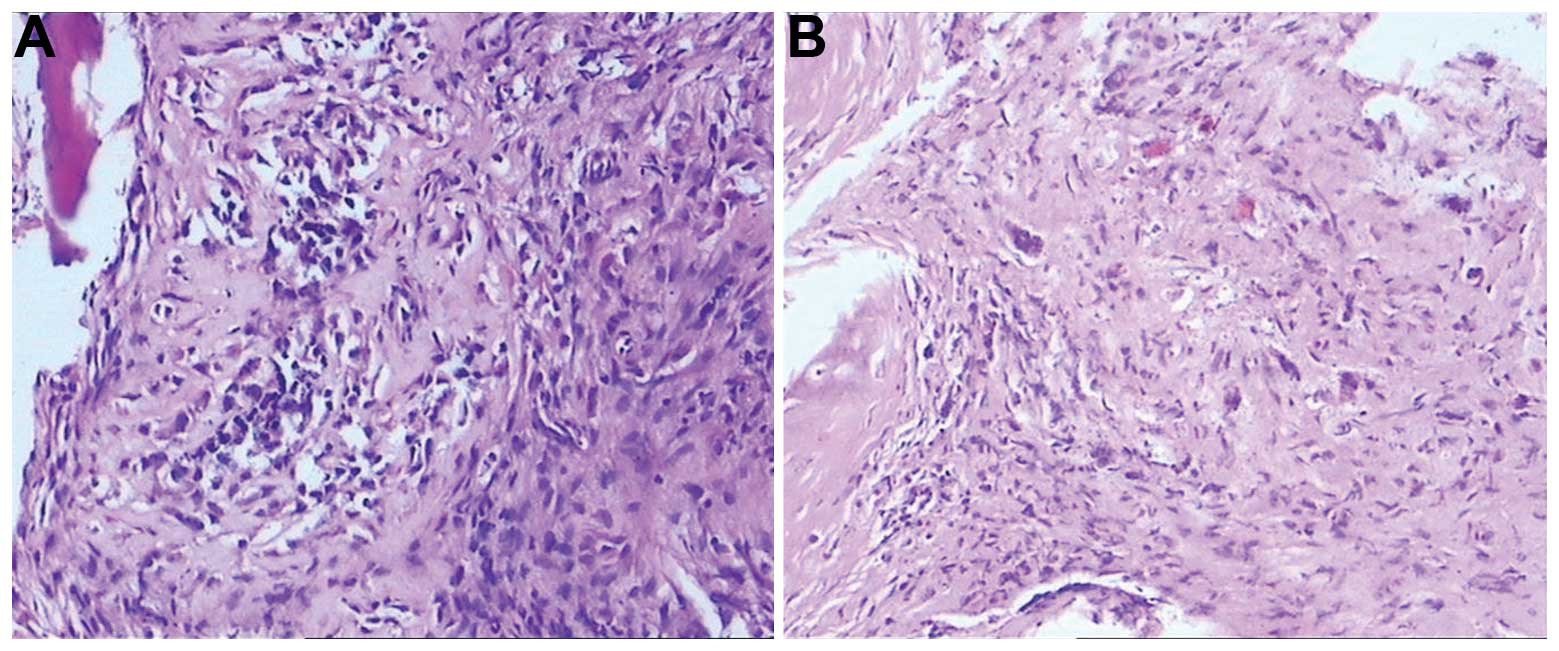
Sclerotin around the mental
foramen
Fewer pigmented epithelial tumor cells were present
within the bone tissue (Fig.
7).
Post-operative conditions
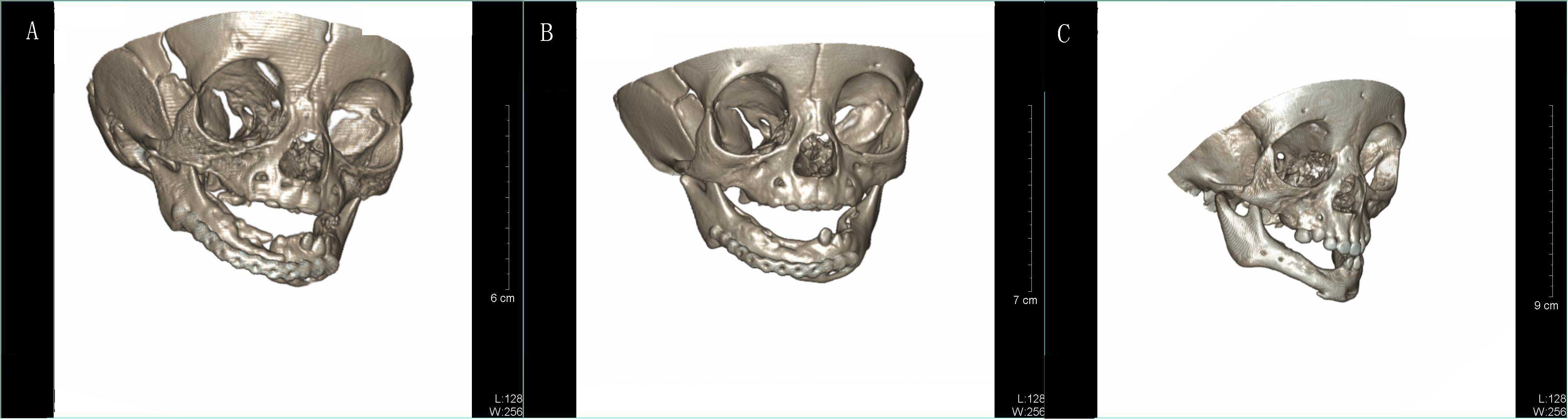
In order to detect any recurrence of the MNTI and
observe the development of the mandible, CT images were acquired
immediately after surgery, and at four months and one year
post-surgery (Fig. 8). The titanium
plate implanted during the surgery was removed four months later.
Observational results revealed no tumor recurrence and good
development of the remaining mandible.
Discussion
MNTI most often occurs in the first year of life,
although rare adult cases have been reported (6). There is a general agreement that the
neural crest is the origin of MNTI for the following reasons: i)
The tumor cells are similar to neuroblasts with respect to
histological evaluation; ii) neurosecretory granules can be
observed under an electron microscope, and iii) the catecholamine
metabolite VMA level increases in the urine. Furthermore, the
levels of VMA gradually return to normal following tumor resection
(7). MNTI that originates from the
bone is associated with osteolytic bone destruction, expansive bone
destruction, cystic bone destruction, hyperosteogeny and
osteosclerosis. Typical CT images reveal low-density masses with
irregular edges. However, MNTI occasionally manifests as
higher-density lesions with clearer boundaries between surrounding
tissues. The appearance of MNTI on CT provides important
information for surgical design. MRI can identify hypodense masses,
with focal areas of hyperdensity in T1WI images, and isointense
masses on T2WI images (8). Upon
pathological examination, MNTI is usually composed of large
pigmented epithelioid cells and small neuroblastoma-like cells
(9). It is known that tumor cells
exhibit heterogeneous immunoreactivity. There is usually a high
expression level of CK and HMB-45 in the large pigmented
epithelioid cells, but a low expression level of S-100 protein. In
the smaller neuroblastoma-like cells, cluster of differentiation
(CD)56 and synaptophysin are expressed (10). In the majority of cases, NSE can be
detected in the two cell types. Certain MNTI cells may also express
Ki-67/CD99 positivity, which indicates a faster growth rate
(11).
On clinical examination, MNTI must be distinguished
from developmental cysts, odontogenic lesions (including odontoma,
enameloblastoma, odontogenic myxoma and odontogenic keratocysts),
non-odontogenic and non-cancerous lesions (including eosinophilic
granuloma and fibrous dysplasia), and non-odontogenic benign tumors
(including rhabdomyosarcoma, Hodgkin’s lymphoma, Langerhans cell
syndrome and Ewing’s sarcoma). A differential diagnosis can be made
according to the typical clinical and imaging manifestations of
MNTI, as well as the histopathological hallmarks, such as the
epithelioid and neuroblastoma-like biphasic differentiation of
tumor cells and the existence of pigment (2). However, biopsy results may be
equivocal and therefore have the potential to make the differential
diagnosis challenging. The epithelioid cells of MNTI bear
resemblance to melanocytes, which are indicative of malignant
melanoma, although malignant melanoma of the oral mucous membrane
is rare in children. Therefore, a diagnosis of malignant melanoma
of the oral cavity mucous membrane in children requires a more
in-depth investigation. The typical immunohistochemical staining
patterns of melanoma are CK(−)/HMB45(+)/S100(+), as opposed to the
MNTI pattern of CK(+)/HMB45(+)/S100(−). If the tumor specimen
consists mainly of neuroblastoma-like cells, with suspected
malignancy upon clinical evaluation, it should be classified as a
‘small round cell’ tumor arising in infancy, such as neuroblastoma
or rhabdomyosarcoma (12).
Neuroblastoma cells can form rosettes, with retinal ganglial cell
differentiation in certain cases. In rhabdomyosarcoma, certain
cells exhibit red pigmentation in the cytoplasm, and the
immunohistochemical expression pattern is desmin(+)/muscle
regulatory protein MyoDl(+)/NSE(−). Conversely, the typical MNTI
pattern is NSE(+)/MyoD1(−). Other small round cell tumors (such as
those appearing in Ewing’s sarcoma, peripheral primitive
neuroectodermal tumors and desmoplastic small round cell tumors)
are rare prior to the age of five (13).
There is no typical biological behavior of MNTI.
Although it is locally fast-growing and is considered benign,
recent studies have indicated that the local recurrence rate
following conservative resection is 10–60%, with 6.5% of cases also
showing distant metastasis (14).
Recurrence may occur due to invasion of the tumor edge into the
bone, difficulty in complete resection due to a tumor with no
envelope (15) or multicenter
growth. In previous studies, high recurrence rates and no
recurrence despite incomplete resection have been reported
(16). The lack of recurrence
without complete resection may stem from a compaction effect that
triggers an immune response to destroy the remaining tumor cells.
Other studies have proposed that peripheral cells depend on a group
of stimulating cells in the tumor center, therefore, when the
central stimulating cells are removed, peripheral tumor cells also
die (17).
The primary treatment for MNTI is surgical
resection, although there are examples of MNTI treatment using
chemotherapy alone. However, it is generally agreed that
chemotherapy is indicated for patients not amenable to surgical
treatment, or for use as an adjuvant therapy prior to and following
surgery (18). The optimal scope of
surgical resection is a matter of debate. Radical resection may
reduce the risk of relapse for a fast growing tumor, and extended
resection is often applied to reduce the risk of malignant
transformation (19). However, the
effects of radical resection on post-operative growth and
development should be taken into consideration to minimize any loss
of tissue function. In cases where extensive resection will not
cause severe defects, it should include the removal of adjacent
tissues to reduce the likelihood of malignant transformation and
metastasis. When the complete removal of lesions may incur severe
defects and dysfunction in the surrounding tissues, more
conservative scaling can be an effective treatment option given
that further treatment can be administered during follow-up
(20). MNTI occurs in the head and
neck in ~92.8% of cases, and as a result, the tumor has the
potential to invade areas important for nerve distribution and bone
development. Therefore, the surgeon must evaluate the benefits of
maintaining the important nerves and periosteum during the surgery
against the probability of tumor recurrence.
In the present study, the associations between MNTI
and the surrounding sclerotin, inferior alveolar nerve and relevant
teeth were examined during the surgery and by post-operative
histopathology. During tumor removal, the periosteum of the
mandible was preserved to minimize deficits in mandibular
development, and teeth were identified in the tumor that was
removed. Following tumor removal, honeycomb sclerotin was visible
around the tumors, which contained a large number of black spots
(Fig. 4). Pathological examination
of the sclerotin specimens revealed the presence of tumor cells
(Fig. 5), indicating that the
tumors were invasive. The tumor cells could therefore not be
completely removed by a single excision, increasing the likelihood
of recurrence. In order to further remove the tumor cells, abrasive
drilling was applied to grind the affected sclerotin. During the
grinding process, it was discovered that the affected sclerotin was
associated with the inferior alveolar neural tube, and subsequent
pathological examination was consistent with a neural crest origin
(Fig. 7). The importance of the
inferior alveolar nerve meant that is was retained, whilst the
sclerotin around it was removed (Fig.
4). The remaining rudimentary sclerotin of the mandible was
thin and so a titanium plate was used for structural reinforcement
(Fig. 8A). Since the mandible
periosteum and inferior alveolar nerve were retained, the patient
was monitored for tumor recurrence. During the follow-up, it was
found that preservation of the inferior alveolar nerve and the
mandibular periosteum did not cause relapse; rather, the mandible
with preserved periosteum developed well (Fig. 8B and C) and the inferior alveolar
nerve was functional.
The treatment of MNTI relies primarily on surgical
resection. During the surgery, the affected sclerotin around the
tumors should be completely removed. Important nerves can be
preserved where appropriate to maintain function, and as much of
the periosteum should be retained to reduce any negative effect on
bone development. As these benign tumors may display an invasive
capacity, patients should be closely monitored for any signs of
recurrence for up to one year post-surgery.
References
|
1
|
Manojlović S, Virag M, Lukšić I and Müller
D: Melanotic neuroectodermal tumour of infancy: Report of two cases
and review of the literature. J Craniomaxillofac Surg. 40:103–107.
2012. View Article : Google Scholar
|
|
2
|
Kruse-Lösler B, Gaertner C, Bürger H,
Seper L, Joos U and Kleinheinz J: Melanotic neuroectodermal tumor
of infancy: systematic review of the literature and presentation of
a case. Oral Surg Oral Med Oral Pathol Oral Radiol Endod.
102:204–216. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Borello ED and Gorlin RJ: Melanotic
neuroectodermal tumor of infancy - a neoplasm of neural crest
origin. Report of a case associated with high urinary excretion of
vanilmandelic acid. Cancer. 19:196–206. 1966. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Madrid C, Aziza J, Hlali A, Bouferrache K
and Abarca M: Melanotic neuroectodermal tumour of infancy: a case
report and review of the aetiopathogenic hypotheses. Med Oral Patol
Oral Cir Bucal. 15:e739–e742. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Béogo R, Nikiéma Z, Traoré SS and
Bouletreau P: Maxillary melanotic neuroectodermal tumor of infancy
management: is conservative surgery the best approach? J Craniofac
Surg. 24:e338–e340. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jain P, Garg RK and Kapoor A: Melanotic
neuroectodermal tumor of infancy in oral cavity at unusual age.
Fetal Pediatr Pathol. 29:344–352. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Camuzard O, Rosello O, Maschi C, Castillo
L, Deville A, Boyer C, Chevallier A and Bailleux S: Melanotic
neuroectodermal tumor of infancy: case report and review of the
literature. Rev Laryngol Otol Rhinol (Bord). 132:173–176. 2011.
|
|
8
|
Haque S, McCarville MB, Sebire N and
McHugh K: Melanotic neuroectodermal tumour of infancy: CT and MR
findings. Pediatr Radiol. 42:699–705. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dehner LP, Sibley RK, Sauk JJ Jr, Vickers
RA, Nesbit ME, Leonard AS, Waite DE, Neeley JE and Ophoven J:
Malignant melanotic neuroectodermal tumor of infancy: a clinical,
pathological and ultrastructural and tissue culture study. Cancer.
43:1389–1410. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Metwaly H, Cheng J, Maruyama S, Ohshiro K,
Suzuki I, Hoshina Y and Saku T: Establishment and characterization
of new cell lines derived from melanotic neuroectodermal tumor of
infancy arising in the mandible. Pathol Int. 55:331–342. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Barrett AW, Morgan M, Ramsay AD, Farthing
PM, Newman L and Speight PM: A clinicopathologic and
immunohistochemical analysis of melanotic neuroectodermal tumor of
infancy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod.
93:688–698. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Oruckaptan HH, Soylemezoglu F, Kutluk T
and Akalan N: Benign melanocytic tumor in infancy: discussion on a
rare case and review of the literature. Pediatr Neurosurg.
32:240–247. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nagase M, Ueda K, Fukushima M and Nakajima
T: Recurrent melanotic neuroectodermal tumour of infancy. Case
report and survey of 16 cases. J Maxillofac Surg. 11:131–136. 1983.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shaia WT, Dinardo LJ, Underhill TE and
Cesca CE: Recurrent melanotic neuroectodermal tumor of infancy. Am
J Otolaryngol. 23:249–252. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nagase M, Ueda K, Fukushima M and Nakajima
T: Recurrent melanotic neuroectodemaltumor of infancy: case report
and survey of 16 cases. J Maxillofac Surg. 11:131–136. 1983.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Piperi EP, Rake SA, Tosios KI,
Vasilopoulou EE, Rake AP, Sandler NA, Issacson T, Sklavounou A and
Koutlas IG: Mandibular melanotic neuroectodermal tumor of infancy
treated conservatively with enucleation. J Craniofac Surg.
21:685–688. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hellström KE and Hellström I: Immunity to
neumblastomas and melanomas. Annu Rev Med. 23:19–38. 1972.
View Article : Google Scholar
|
|
18
|
Woessmann W, Neugebauer M, Gossen R,
Blütters-Sawatzki R and Reiter A: Successful chemotherapy for
melanotic neuroectodermal tumor of infancy in a baby. Med Pediatr
Oncol. 40:198–199. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Béogo R, Nikiéma Z, Traoré SS and
Bouletreau P: Maxillary melanotic neuroectodermal tumor of infancy
management: is conservative surgery the best approach? J Craniofac
Surg. 24:e338–e340. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Antunes AC, Freitas RM, Oliveira PP and
Rebouças RG: Melanotic neuroectodermal tumor of infancy: case
report. Arq Neuropsiquiatr. 63:670–672. 2005. View Article : Google Scholar : PubMed/NCBI
|