Introduction
Despite the declining incidence of gastric cancer in
certain parts of the world, it remains one of the leading causes of
cancer-related mortality worldwide (1). Gastric cancer is hypothesized to
develop in a multi-step process that includes the activation and
overexpression of oncogenes, such as K-sam and c-Met (2,3), as
well as the inactivation of tumor suppressor genes, such as APC
(4). Various genes are associated
with stomach cancer, such as TP53 (5), TGF-β (6) and Runx3 (7); however, their association with gastric
cancer is weak. Therefore, there is a requirement to identify novel
molecular markers to improve the prognosis of gastric cancer
patients.
The 14-3-3 protein family has seven distinct 14-3-3
genes, denoted β, ɛ, γ, ζ, σ, η and τ. Thus far, 14-3-3 proteins
have been identified to participate in the biological regulation of
cell behavior such as apoptosis, the cell cycle and malignant
transformation, by interacting with ligands (8,9). Of
the seven 14-3-3 genes, 14-3-3σ has been identified as an
epithelial-specific marker and is associated with G2/M checkpoint
control in the cell cycle (10);
14-3-3σ induced the activation of tumor protein p53, and bound to
cyclin-dependent kinase-2 (CDK2) and -4 (CDK4) (11). Thus, 14-3-3σ may be defined as a
negative regulator of the cell cycle. Protein expression levels of
14-3-3σ are significantly reduced or negligible in various types of
primary cancer of epithelial origin, including lung (12), prostate (13) and bladder carcinoma (14). This cancer-related inactivation of
the 14-3-3σ gene may be caused by hypermethylation of CpG islands
in the promoter region of 14-3-3σ (15). However, to date, few studies have
reported a correlation between 14-3-3σ expression levels and
clinicopathological features of gastric cancer. Therefore, to
investigate the clinical significance and prognostic value of
14-3-3σ in gastric cancer, the present study analyzed 14-3-3σ
expression in 60 gastric cancer samples and evaluated its
correlation with specific clinical outcomes of gastric cancer
patients.
Materials and methods
Patients and tissue samples
Sixty paraffin-embedded gastric cancer samples were
collected from The Central Hospital of Loudi Affiliated to the
University of South China (Loudi, China), between 2007 and 2008.
None of the patients had received preoperative anticancer
treatment. Clinicopathological features, including age, gender,
tumor differentiation degree, tumor volume, tumor invasion depth,
tumor node metastasis (TNM) stage and lymph node metastasis are
detailed in Table I. Informed
consent was obtained from each patient upon collection of the
samples. The present study was approved by the institutional
research medical ethics committee of The Central Hospital of Loudi
Affiliated to the University of South China.
 | Table ICorrelation between 14-3-3σ expression
rate and various clinicopathological features in 60 cases of
gastric cancer. |
Table I
Correlation between 14-3-3σ expression
rate and various clinicopathological features in 60 cases of
gastric cancer.
| | 14-3-3σ
expression | |
|---|
| |
| |
|---|
| Variable | Cases, n | Negative | Positive | P-value |
|---|
| Gender | | | | |
| Male | 31 | 15 | 16 | 0.064 |
| Female | 29 | 7 | 22 | |
| Age, years | | | | |
| ≥60 | 28 | 11 | 17 | 0.791 |
| <60 | 32 | 11 | 21 | |
| Tumor size, cm | | | | |
| ≥5 | 26 | 5 | 21 | 0.017a |
| <5 | 34 | 17 | 17 | |
| Differentiation
degree | | | | |
| Well/Moderately | 39 | 13 | 26 | 0.577 |
| Poorly | 21 | 9 | 12 | |
| Invasion depth | | | | |
| T1 + T2 | 41 | 18 | 23 | 0.149 |
| T3 + T4 | 19 | 4 | 15 | |
| TNM stage | | | | |
| I + II | 34 | 18 | 16 | 0.003a |
| III + IV | 26 | 4 | 22 | |
| Lymph node
metastasis | | | | |
| Yes | 37 | 17 | 20 | 0.097 |
| No | 23 | 5 | 18 | |
Tissue microarray and
immunohistochemistry
Tissue microarrays (TMAs) were constructed as
previously described (16).
Briefly, paraffin-embedded donor tissue blocks and the
corresponding hematoxylin and eosin-stained slides were overlaid
for TMA sampling. Cylindrical tissue samples (diameter, 0.6 mm)
were punctured in triplicate from specific areas of the donor
tissue and re-embedded into a recipient paraffin block at the
designated location.
Routine techniques were utilized to cut 4-μm
sections from the deparaffinized TMAs. The slides were microwaved
in citrate buffer for 8 min for antigen retrieval, and goat
anti-human polyclonal 14-3-3σ (sc-7683; Santa Cruz Biotechnology,
Inc., Santa Cruz, CA, USA) was applied as the primary antibody in a
1:150 dilution. Goat anti-human monoclonal Ki-67 (GT209401), goat
anti-human polyclonal Bcl-2 (GM088701) and goat anti-human Bax
(A353302) were purchased from Gene Biotechnology (Shanghai, China).
Labeling was detected by horseradish peroxidase-conjugated mouse
anti-goat IgG and staining with 3,3′-diaminobenzidine (all
Maxim-Bio, Inc., Fuzhou, China). Finally, the slides were
counterstained with hematoxylin. 14-3-3σ, Bcl-2 and Bax were scored
according to the staining intensity (0, no staining; 1, weak
staining; 2, moderate staining; 3, strong staining) and the
percentage of positively stained tumor cells (0, 0% of tumor cells
stained; 1, 1–9% of tumor cells stained; 2, 10–50% of tumor cells
stained; 3, 51–75% of tumor cells stained; 4, >75% of tumor
cells stained). If the product of the staining intensity and the
percentage of positively stained tumor cells was ≥2, the staining
was considered to be positive (+). Ki-67 was scored according to
the number of positive gastric cancer cells. A negative control was
performed by replacing the primary antibody with the appropriate
serum controls.
Statistical analysis
All statistical analyses were performed using SPSS
for Windows (version 13.0; SPSS, Inc., Chicago, IL, USA). The
correlation between 14-3-3σ expression rates and
clinicopathological features of gastric cancer cases was evaluated
using Fisher’s exact test. Multivariate analysis was performed
using Cox proportional-hazards regression. Overall survival curves
were generated according to the Kaplan-Meier method. P<0.05 was
considered to indicate a statistically significant difference.
Results
Expression of 14-3-3σ in gastric
cancer
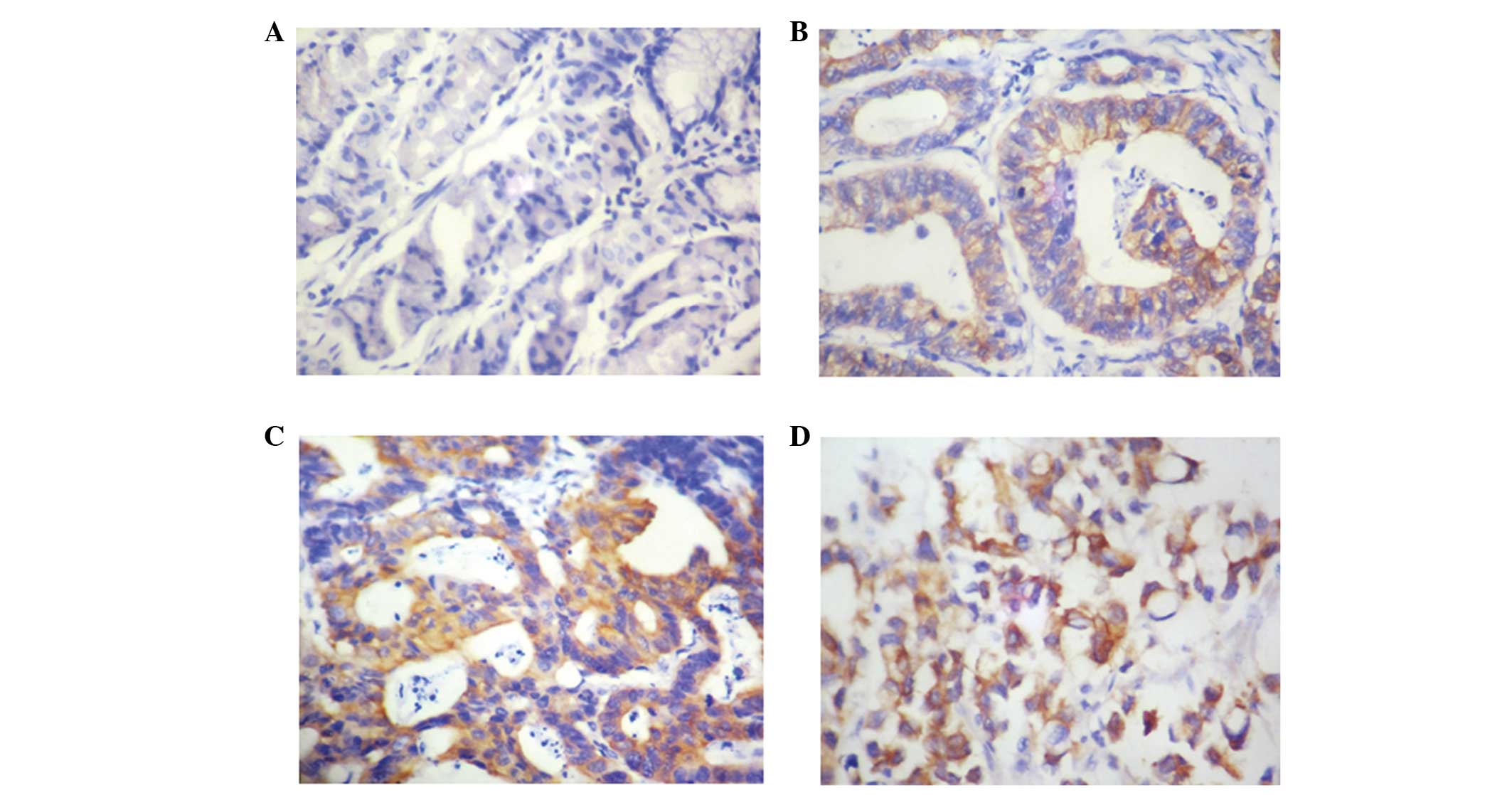
To investigate the expression of 14-3-3σ in gastric
cancer, immunohistochemical staining for 14-3-3σ was performed on a
TMA containing 60 pairs of gastric cancer samples and the
corresponding healthy gastric mucosa tissues. In the present study,
compared with weak or negative expression in normal gastric mucosal
tissues, 63.3% (38/60) of gastric cancer cases exhibited positive
14-3-3σ expression. As demonstrated in Fig. 1, 14-3-3σ staining was evident in the
cytoplasm of poorly, moderately and well-differentiated gastric
cancer cells.
Association between 14-3-3σ expression
and clinicopathological features of gastric cancer
Table I indicates
the expression rates of 14-3-3σ in gastric cancer with respect to
various clinicopathological features. The expression rates of
14-3-3σ were significantly higher in patients exhibiting larger
tumors (size, ≥5 cm; P=0.017) and an advanced TNM stage (P=0.003).
However, no significant difference was identified between 14-3-3σ
expression and the other clinicopathological features investigated,
such as age, gender, tumor differentiation degree, depth of tumor
invasion and lymph node metastasis (P>0.05).
Association between 14-3-3σ and Ki-67
expression
To explore the influence of 14-3-3σ on the
proliferation of gastric cancer cells, the present study analyzed
the correlation between 14-3-3σ and Ki-67 expression. The
percentage of cells exhibiting positive Ki-67 expression was
significantly higher (48.1±5.1) in the 14-3-3σ positive expression
group compared with the 14-3-3σ negative expression group
(17.6±4.8) (Table II). These
findings indicate that 14-3-3σ may participate in tumor cell
proliferation.
 | Table IICorrelation between 14-3-3σ, Bcl-2 and
Ki-67 expression. |
Table II
Correlation between 14-3-3σ, Bcl-2 and
Ki-67 expression.
| | Bcl-2 staining | |
|---|
| |
| |
|---|
| 14-3-3σ staining | Cases, n | Positive | Negative | Ki-67, % (±SD) |
|---|
| Positive | 38 | 25a | 13 | 48.1±5.1a |
| Negative | 22 | 8 | 14 | 17.6±4.8 |
Association between 14-3-3σ and Bcl-2/Bax
expression
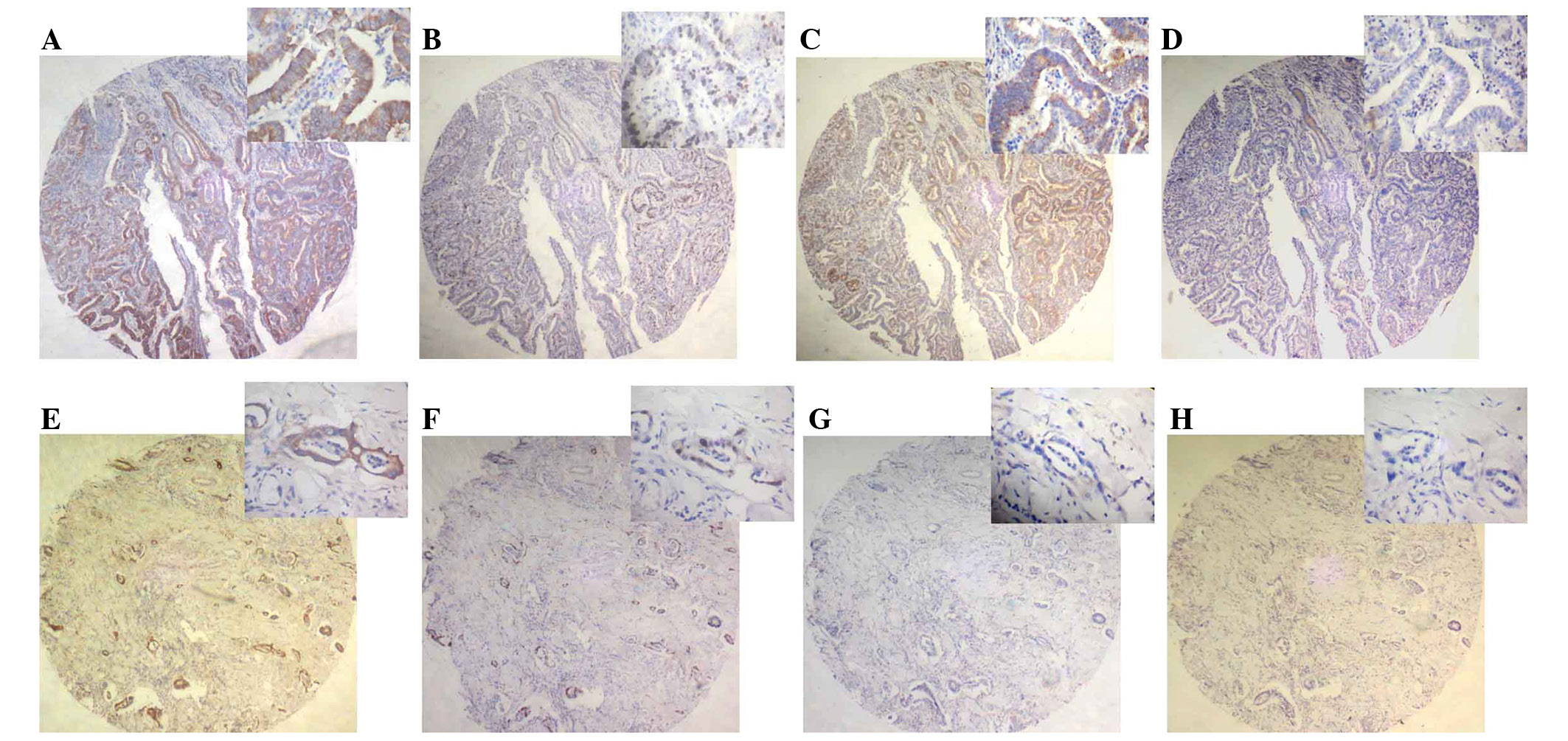
To identify the effect of 14-3-3σ on the apoptosis
of gastric cancer cells, the present study investigated the
correlation between 14-3-3σ and Bcl-2/Bax expression. Bcl-2
staining was identified in 25/38 cases of positive 14-3-3σ
expression (Table II). As
demonstrated in Fig. 2, Bcl-2
expression was predominantly present within the cytoplasm of
gastric cancer cells. Furthermore, 14/22 cases of negative 14-3-3σ
expression did not exhibit Bcl-2 staining (Table II). However, with the exception of
six positive expression cases, no Bax immunoreactivity was detected
in these samples.
Association between 14-3-3σ expression
and overall survival in gastric cancer
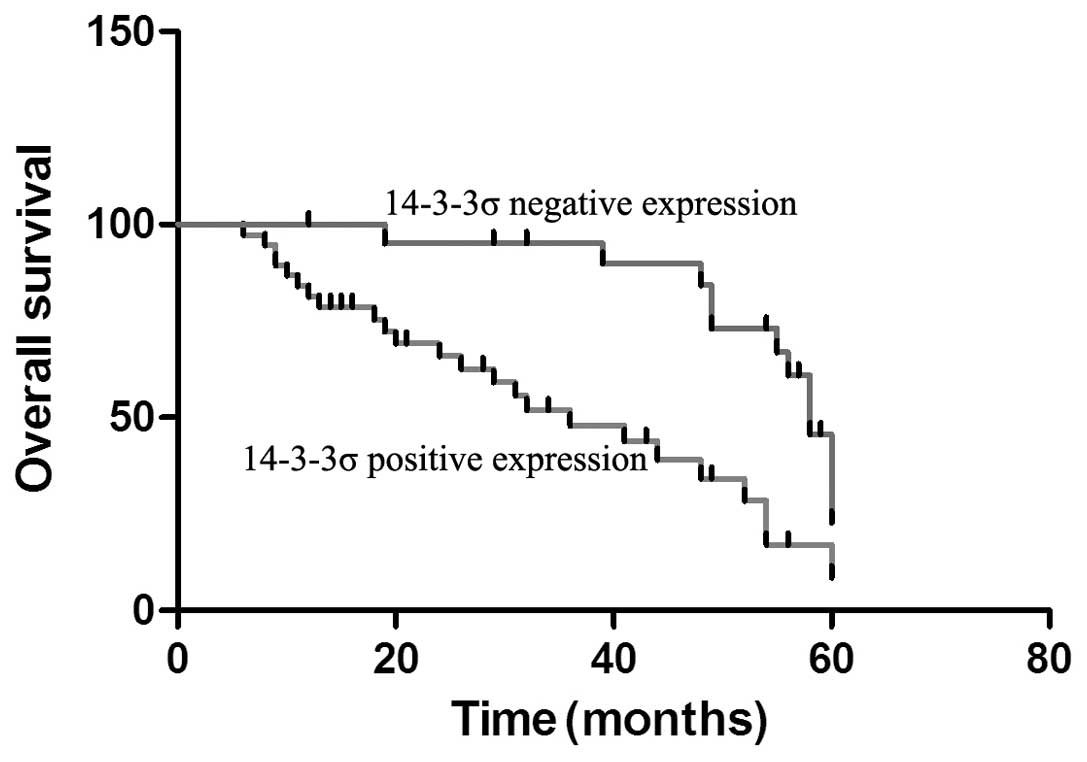
To better elucidate the effect of 14-3-3σ expression
on the prognosis of gastric cancer, Kaplan-Meier survival curves
were used to investigate the overall survival time of gastric
cancer patients. Negative 14-3-3σ expression patients exhibited
improved prognoses compared with positive 14-3-3σ expression
patients (Fig. 3). Additionally,
univariate and multivariate analyses were employed to assess the
role of 14-3-3σ expression and specific clinicopathological
features on the prognosis of gastric cancer patients. Univariate
analysis demonstrated that lymph node metastasis, TNM stage, tumor
invasion depth and 14-3-3σ expression were significantly associated
with the overall survival of gastric cancer patients. Furthermore,
multivariate analyses identified that lymph node metastasis, TNM
stage and 14-3-3σ expression were correlated with poor overall
survival of gastric cancer patients (Table III). Of the clinicopathological
features investigated in the present study, lymph node metastasis
was the most independent prognostic indicator of gastric cancer
(P=0.013). Therefore, 14-3-3σ expression may act as a prognostic
biomarker for gastric cancer.
 | Table IIIUnivariate and multivariate analyses
of overall survival in gastric cancer patients. |
Table III
Univariate and multivariate analyses
of overall survival in gastric cancer patients.
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|---|
| Variable | Hazard ratio | 95% CI | P-value | Hazard ratio | 95% CI | P-value |
|---|
| Gender | 1.561 | 0.832–3.914 | 0.372 | | | |
| Age, year | 1.126 | 0.982–1.145 | 0.541 | | | |
| Invasion depth | 5.530 | 2.479–13.885 | 0.001a | 2.726 | 0.789–6.543 | 0.054 |
| Differentiation
degree | 0.986 | 0.323–2.879 | 0.788 | | | |
| Tumor size | 1.013 | 0.346–3.190 | 0.977 | | | |
| TNM stage | 0.104 | 0.032–0.501 | 0.003a | 0.195 | 0.040–0.765 | 0.026a |
| Lymph node
metastasis | 6.114 | 3.062–18.767 | 0.002a | 3.378 | 1.483–9.323 | 0.013a |
| 14-3-3σ
expression | 5.983 | 2.690–17.654 | 0.002a | 3.896 | 1.719–9.891 | 0.028a |
Discussion
14-3-3σ is vital at the G2/M checkpoint, as it
sequesters the Cdc2/cyclin B1 complex; therefore, 14-3-3σ may be
involved in the development of cancer. However, it should be
considered that 14-3-3σ exhibits different functions in the
carcinogenesis of different human organs. For example,
downregulation of 14-3-3σ expression has been observed in colon
(17), liver (18), breast (19) and ovarian cancer (20), and loss of 14-3-3σ expression has
been associated with poor prognosis in epithelial ovarian carcinoma
(20). By contrast, 14-3-3σ
expression is upregulated in head and neck squamous cell carcinoma
(21) and pancreatic cancer
(22). This data supports a dual
role for 14-3-3σ in the development of cancer.
In the present study, 14-3-3σ expression was
significantly higher in gastric cancer compared with corresponding
healthy gastric tissue. Immunohistochemical assays demonstrated
that cases of gastric cancer exhibiting high 14-3-3σ expression
levels also exhibited larger tumor volumes, indicating that 14-3-3σ
may be involved in gastric cancer proliferation. Furthermore,
14-3-3σ expression was correlated with TNM stage. This finding was
consistent with those of a previous study conducted by Perathoner
et al (23), which revealed
a significant correlation between 14-3-3σ overexpression and tumor
stage in colorectal carcinoma. These characteristics of 14-3-3σ
expression may influence the prognosis of gastric cancer patients.
With regard to the prognostic impact of 14-3-3σ in human cancer,
positive 14-3-3σ overexpression was associated with a significantly
decreased survival time compared with negative 14-3-3σ expression
in colorectal carcinoma (23).
Consistent with this, the present study performed Kaplan-Meier
survival analysis and multivariate Cox proportional-hazards
regression analysis, which revealed that a high expression of
14-3-3σ was significantly associated with a poorer prognosis and
shortened overall survival time.
In the present study, 14-3-3σ expression was
correlated with tumor volume. Therefore, the association between
14-3-3σ, and proliferation and apoptosis in gastric cancer was
investigated. The results of the present study demonstrated a
positive correlation between 14-3-3σ and Ki-67, indicating that
14-3-3σ may be associated with the proliferation of gastric cancer
cells. 14-3-3σ exerts anti-apoptotic effects by interacting with
Bax, a pro-apoptotic protein (24).
To investigate the role of 14-3-3σ in the apoptosis of gastric
cancer cells, the correlation between 14-3-3σ and Bcl-2/Bax was
explored. The positive rate of Bcl-2 expression in the
14-3-3σ-positive cases was significantly higher compared with the
rate of Bcl-2 expression in the 14-3-3σ-negative cases.
Furthermore, no correlation was identified between 14-3-3σ and Bax
expression.
In conclusion, the present study investigated the
expression pattern of 14-3-3σ in gastric cancer and its correlation
with specific clinicopathological features. The results of the
present study indicate that 14-3-3σ may be involved in cell
proliferation and apoptosis in gastric cancer. Furthermore, 14-3-3σ
overexpression is associated with an unfavorable prognosis.
Therefore, 14-3-3σ may be a promising target for the treatment of
gastric cancer and, critically, investigations into the
14-3-3σ-associated molecular mechanisms of gastric cancer cell
proliferation and apoptosis should be conducted.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (grant nos. 81302245
and 81201831).
References
|
1
|
Correa P: Human gastric carcinogenesis: a
multistep and multifactorial process - First American Cancer
Society Award Lecture on Cancer Epidemiology and Prevention. Cancer
Res. 52:6735–6740. 1992.PubMed/NCBI
|
|
2
|
Hara T, Ooi A, Kobayashi M, et al:
Amplification of c-myc, K-sam, and c-met in gastric cancers:
detection by fluorescence in situ hybridization. Lab Invest.
78:1143–1153. 1998.PubMed/NCBI
|
|
3
|
Yu J, Miehlke S, Ebert MP, et al:
Frequency of TPR-MET rearrangement in patients with gastric
carcinoma and in first-degree relatives. Cancer. 88:1801–1805.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nakatsuru S, Yanagisawa A, Ichii S, et al:
Somatic mutation of the APC gene in gastric cancer: frequent
mutations in very well differentiated adenocarcinoma and
signet-ring cell carcinoma. Hum Mol Genet. 1:559–563. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim JH, Takahashi T, Chiba I, et al:
Occurrence of p53 gene abnormalities in gastric carcinoma tumors
and cell lines. J Natl Cancer Inst. 83:938–943. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Park K, Kim SJ, Bang YJ, et al: Genetic
changes in the transforming growth factor beta (TGF-β) type II
receptor gene in human gastric cancer cells: correlation with
sensitivity to growth inhibition by TGF-β. Proc Natl Acad Sci USA.
91:8772–8776. 1994. View Article : Google Scholar
|
|
7
|
Xu HW, Ren F, Yu YM and Cai CZ: Runx3
expression in lymph nodes with metastasis is associated with the
outcome of gastric cancer patients. Oncol Lett. 2:1275–1279.
2011.
|
|
8
|
Lalle M, Leptourgidou F, Camerini S, et
al: Interkingdom complementation reveals structural conservation
and functional divergence of 14-3-3 proteins. PLoS One.
11:e780902013. View Article : Google Scholar
|
|
9
|
Rosenquist M, Alsterfjord M, Larsson C and
Sommarin M: Data mining the Arabidopsis genome reveals fifteen
14-3-3 genes. Expression is demonstrated for two out of five novel
genes. Plant Physiol. 127:142–149. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chan TA, Hermeking H, Lengauer C, et al:
14-3-3σ is required to prevent mitotic catastrophe after DNA
damage. Nature. 401:616–620. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Laronga C, Yang HY, Neal C and Lee MH:
Association of the cyclin-dependent kinases and 14-3-3 sigma
negatively regulates cell cycle progression. J Biol Chem.
275:23106–23112. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xiao T, Mi W, Li M, et al: Analyzed the
molecular interaction network of tumor suppressor gene 14-3-3 sigma
in lung cancer cell based on stable isotope labeling by amino acids
in cell culture technology. Zhonghua Yu Fang Yi Xue Za Zhi.
47:752–756. 2013.(In Chinese). PubMed/NCBI
|
|
13
|
Evren S, Dermen A, Lockwood G, et al:
mTOR-RAPTOR and 14-3-3σ immunohistochemical expression in high
grade prostatic intraepithelial neoplasia and prostatic
adenocarcinomas: a tissue microarray study. J Clin Pathol.
64:683–688. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang Z, Zhang G and Kong C: High
expression of Cdc25B and low expression of 14-3-3σ is associated
with the development and poor prognosis in urothelial carcinoma of
bladder. Tumour Biol. 35:2503–2512
|
|
15
|
Liu S, Howell P, Ren S, et al: The 14-3-3σ
gene promoter is methylated in both human melanocytes and melanoma.
BMC Cancer. 27:1622009. View Article : Google Scholar
|
|
16
|
Xie D, Sham JS, Zeng WF, et al:
Heterogeneous expression and association of β-catenin, p16 and
c-myc in multistage colorectal tumorigenesis and progression
detected by tissue microarray. Int J Cancer. 107:896–902. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ide M, Nakajima T, Asao T and Kuwano H:
Inactivation of 14-3-3σ by hypermethylation is a rare event in
colorectal cancers and its expression may correlate with cell cycle
maintenance at the invasion front. Cancer Lett. 207:241–249. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Iwata N, Yamamoto H, Sasaki S, et al:
Frequent hypermethylation of CpG islands and loss of expression of
the 14-3-3 σ gene in human hepatocellular carcinoma. Oncogene.
19:5298–5302. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nakamura Y, Oshima K, Naoi Y, et al:
14-3-3σ expression is associated with poor pathological complete
response to neoadjuvant chemotherapy in human breast cancers.
Breast Cancer Res Treat. 134:229–236. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mhawech-Fauceglia P, Herrmann FR, Anderws
C, et al: 14-3-3σ expression and prognostic value in patients with
epithelial ovarian carcinoma: a high throughput tissue microarray
analysis. Eur J Surg Oncol. 35:763–767. 2009. View Article : Google Scholar
|
|
21
|
Erovic BM, Pelzmann M, Grasl MCh, et al:
Mcl-1, vascular endothelial growth factor-R2, and 14-3-3σ
expression might predict primary response against radiotherapy and
chemotherapy in patients with locally advanced squamous cell
carcinomas of the head and neck. Clin Cancer Res. 11:8632–8636.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li Z, Dong Z, Myer D, et al: Role of
14-3-3σ in poor prognosis and in radiation and drug resistance of
human pancreatic cancers. BMC Cancer. 10:5982010. View Article : Google Scholar
|
|
23
|
Perathoner A, Pirkebner D, Brandacher G,
et al: 14-3-3σ expression is an independent prognostic parameter
for poor survival in colorectal carcinoma patients. Clin Cancer
Res. 11:3274–3279. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Guweidhi A, Kleeff J, Giese N, et al:
Enhanced expression of 14-3-3sigma in pancreatic cancer and its
role in cell cycle regulation and apoptosis. Carcinogenesis.
25:1575–1585. 2004. View Article : Google Scholar : PubMed/NCBI
|