Introduction
MicroRNAs (miRNAs) are a conserved class of
endogenous non-coding small RNAs (length, 20–22 nt) that regulate
gene expression at the post-transcriptional level. This
predominantly occurs by binding to the 3′-untranslated region (UTR)
mRNA of target genes, resulting in mRNA degradation and, thus,
inhibition of translation (1–3).
Recent studies have indicated that miRNA expression may be
important in in the progression and outcome of various diseases
(4,5). Although studies investigating miRNA
expression profiles in esophageal carcinoma have been conducted
(6), there remains little
information available regarding specific miRNA expression patterns
and their roles in esophageal squamous cell carcinoma (ESCC).
The Notch signaling pathway is important in stem
cell maintenance and angiogenesis, as well as decisions regarding
cell fate in cancer (7). Notch
signaling is important for esophageal epithelial homeostasis, for
example Notch signaling regulates cell proliferation within the
squamous epithelia (8,9). Following activation of the Notch
receptor, the Notch intracellular domain (NICD) is cleaved,
released and translocated to the nucleus, where, in association
with recombination signal binding protein for immunoglobulin KJ, it
induces the expression of downstream target genes, including the
hairy and enhancer of split (HES)/hairy and enhancer of split
related with YRPW motif family of transcription factors (10).
The present study investigated the expression of
miR-29a in ESCC and the role of miR-29a in cell growth and
migration of the ESCC TE-1 cell line. Furthermore, the mechanisms
of miR-29a modulation during TE-1 cell growth were evaluated.
Materials and methods
Cell lines
Primary cultures of normal esophageal epithelial
cells (NEECs) were established from fresh biopsies of non-cancerous
esophageal tissues, in accordance with a previous study (11). The NEECs and ESCC cells were
cultured in keratinocyte serum-free medium (Gibco Life
Technologies, Carlsbad, CA, USA) supplemented with 40 μg/ml bovine
pituitary extract (Gibco Life Technologies), 1.0 ng/ml epidermal
growth factor (Invitrogen Life Technologies, Inc., Carlsbad, CA,
USA) 100 U/ml penicillin (Gibco Life Technologies) and 100 μg/ml
streptomycin (Gibco Life Technologies), at 37°C and an atmosphere
of 5% CO2. The ESCC TE-1 cell line was obtained from the
Cell Bank of Type Culture Collection of the Chinese Academy of
Sciences (Shanghai, China), and grown in RPMI-1640 medium
(Invitrogen Life Technologies, Inc.) supplemented with 10% fetal
bovine serum (FBS; Invitrogen Life Technologies), 100 μg/μl
streptomycin (Gibco Life Technologies) and 100 μg/μl penicillin
(Gibco Life Technologies) in a humidified incubator containing 5%
CO2 at 37°C.
Patient information and tissue
specimens
The present study included nine ESCC tissue samples,
which were histopathologically and clinically diagnosed at The
First Affiliated Hospital of Zhengzhou University (Zhengzhou,
China) in 2011, as well as nine adjacent non-cancerous esophageal
tissue samples. Written informed consent was obtained from each
patient and the present study was approved by the ethics committee
of Zhengzhou University.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA, including miRNA, was extracted using the
mirVana™ miRNA Isolation kit (Ambion Life Technologies, Carlsbad,
CA, USA), in accordance with the manufacturer’s instructions.
miR-29a was detected using the RT2 miRNA First Strand kit (SA
Biosciences, Frederick, MD, USA) and specific miR-29a and U6
primers (Qiagen, Shanghai, China) were used for RT-qPCR. The
relative expression of miR-29a was calculated using the comparative
2−ΔΔCt method. cDNA was synthesized using M-MLV reverse
transcriptase (Promega, Madison, WI, USA) following standard
protocols. Briefly, 3 μg total RNA and 2 μM oligo-dT primer
(Promega) were added to RNase-free H2O. The RNA primer
mixture was incubated at 65°C for 5 min then placed on ice for 3
min. Next, 0.5 mM dNTP mix (Promega), 10 μl M-MLV 5X reaction
buffer (Promega), 400 U M-MLV reverse transcriptase and RNase-free
H2O were added to the RNA primer mixture. The conditions
for reverse transcription were as follows: 30°C for 10 min, 42°C
for 1 h and 95°C for 10 min. The EzOmics SYBR qPCR kit was
purchased from Biomics USA Inc., (Palo Alto, CA, USA), which
included 5 μl cDNA template and 15 μl reaction mixture, containing
10 μl 2X SYBR Green mix, 0.5 μM forward primer, 0.5 μM reverse
primer and RNase-free H2O. A qRT-PCR detection system
(Applied Biosystems Life Technologies, Foster City, CA, USA) was
used to perform RT-PCR. The amplification procedure was as follows:
94°C for 5 min, followed by 30 cycles at 94°C for 30 sec, 58°C for
30 sec for Nfia and glyceraldehyde 3-phosphate dehydrogenase
(GAPDH), 55°C for 30 sec for Notch1 and Hes1, and 72°C for 10 min.
The primer sequences of the genes were synthesized by Sangon
Biotech (Shanghai) Co., Ltd., (Shanghai, China) and the sequences
were as follows: Forward, 5′-ACCAGCTCAAAAAACCTGTGGA-3′ and reverse,
5′-TGTTGTGAAACGAAACACCCC-3′ for Nfi; forward,
5′-CACTGTGGGCGGGTCC-3′ and reverse, 5′-GTTGTATTGGTTCGGCACCAT-3′ for
Notch1; forward, 5′-GTGTTAACGCCCTCACACG-3′ and reverse,
5′-TGGGAGGCAGACTAGCAGAG-3′ for (Hes1) and; forward,
5′-TTCAGCTCTGGGATGACCTT-3′ and reverse, 5′-TGCCACTCAGAAGACTGTGG-3′
for GAPDH. The mRNA expression of each gene was normalized to that
of GAPDH. The relative mRNA expression was calculated using the
comparative Ct method (2−ΔΔCt).
The nuclear factor 1 A (Nfia) mRNAs were determined
using SYBR®-Green real-time PCR assay. The PCR primers
were as follows: Sense, 5′-ACCAGCTCAAAAAACCTGTGGA-3′ and
anti-sense, 5′-TGTTGTGAAACGAAACACCCC-3′ for Nfia; sense,
5′-CACTGTGGGCGGGTCC-3′ and anti-sense, 5′-GTTGTATTGGTTCGGCACCAT-3′
for Notch1; sense, 5′-GTGTTAACGCCCTCACACG-3′ and anti-sense,
5′-TGGGAGGCAGACTAGCAGAG-3′ for Hes1; and sense,
5′-TTCAGCTCTGGGATGACCTT-3′ and anti-sense,
5′-TGCCACTCAGAAGACTGTGG-3′ for GAPDH. GAPDH was used to normalize
the mRNA expression.
Lentiviral-mediated miR-29a
overexpression and Nfia knockdown in mesenchymal stem cells
The miR-29a precursor vector and scramble plasmid
were obtained from GeneCopoeia Inc., (cat no. RmiR6139; Guangzhou,
China) and contained the puromycin selection marker. The lentivirus
containing miR-29a precursor was generated using the Lenti-Pac™
Human Immunodeficiency Virus Expression Packaging kit (GeneCopoeia
Inc.). Briefly, the DNA-EndoFectin complex was formed by adding 2.5
μl lentiviral miR-29a precursor expression plasmid/scramble plasmid
to 5.0 μl EndoFectin™ Lenti transfection reagent diluted into
Opti-MEM®. Following a 20-min incubation at room
temperature, the DNA-EndoFectin complex was added to a petri dish
containing 293T cells that had been plated in Dulbecco’s modified
Eagle’s medium (DMEM; Gibco Life Technologies) supplemented with
10% FBS and incubated in 5% CO2 at 37°C overnight. The
culture medium was replaced with fresh DMEM supplemented with 5%
FBS and TiterBoost™ reagent (1:500; GeneCopoeia, Rockville, MD,
USA), and incubation was continued. The virus
pseudovirus-containing culture medium was collected 48 h
post-transfection, filtered and concentrated. For lentiviral
transduction, TE-1 cells were incubated for 2 h at 4°C, and
1×106 TE-1 cells were plated with 20 μl virus suspension
and cultured in an atmosphere of 5% CO2 at 37°C for 48
h. Following incubation, 10 μg/ml puromycin (EMD Millipore,
Billerica, MA, USA) was added to the cells, all of which were
subsequently preserved in a medium containing puromycin (final
concentration, 10 μg/ml). The TE-1 cells stably expressing the
exogenous genes, miR-29a precursor or scramble, were termed
miR-29a-TE-1 cells or scramble-TE-1 cells, respectively. The siRNA
vector against Nfia and the scramble plasmid were obtained from
GeneCopoeia, Inc.
MTT assay
Cells were seeded onto 96-well plates at a density
of 5×104 cells/well in 100 μl medium. All cells were
maintained in a humidified incubator at 37°C and an atmosphere of
5% CO2. MTT (20 μl; 5g/l) was added to each well of the
microplate and a microplate reader (Anthros 2010; Biochrom Ltd.,
Cambridge, UK) was used to measure the absorbance at 570 nm.
Following a 4-h incubation, the number of viable cells was
measured. Five wells were counted at each time point and the mean
was calculated.
Flow cytometry
The percentage of sub-G1 population (apoptotic)
cells and the cell cycle distribution were determined using flow
cytometry, based on the relative DNA content as previously
described (12). The data were
analyzed using ModFit LT software (version 3.1; Verity Software
House, Topsham, ME, USA).
Colony formation assays
Cells were plated on 60-mm plates
(0.5×103 cells/plate) and cultured for 10 days. The
colonies were stained with 1% crystal violet for 30 sec following
fixation with 10% formaldehyde for 5 min.
In vitro scratch assay
TE-1 cells (5×106 cells/well) were seeded
in a six-well plate and cultured overnight to reach a confluence of
90%. The following day, a scratch was made through the center of
each well using a 200-μl pipette tip, creating an obvious open
scratch or wound on the cells. The dislodged cells were removed by
washing three times with the complete culture media, and the
remaining cells were incubated under standard conditions. Migration
into the open area was identified 72 h post-scratching. In
addition, TE-1 cells were transfected with lentivirus containing a
miR-29a precursor or control for four days then treated with 20
ng/ml epidermal growth factor (EGF; Sigma-Aldrich, St. Louis, MO,
USA) for 16 hrs and seeded in six-well plates. After the cells had
reached 90% confluence, a scratch was made in the monolayer.
Western blot analysis
TE-1 cells were washed with phosphate-buffered
saline (PBS; Gibco Life Technologies), then 200 μl/well cell lysis
buffer (Beyotime Institute of Biotechnology, Haimen, China) and 1
mM phenylmethanesulfonyl fluoride were added. Next, the cell lysate
was centrifuged twice at 2,000 × g for 20 min at 4°C and the
supernatant was transferred to a clean tube. Protein concentration
was measured using a BCA assay kit (Beyotime Institute of
Biotechnology). Proteins were separated by SDS-PAGE and transferred
to polyvinylidene difluoride membranes (Amersham Pharmacia Biotech
Inc., Piscataway, NJ, USA). The membranes were blocked with 5%
non-fat milk in PBS and Tween 20 (PBST; Beyotime Institute of
Biotechnology) for 1 h at room temperature and incubated with
polyclonal rabbit anti-goat Nfia (sc-30918; 1:1,000; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA), polyclonal rabbit
anti-goat Notch1 (sc-6014; 1:1,000; Santa Cruz Biotechnology,
Inc.), polyclonal rabbit anti-goat Hes1 (sc-13842; 1:1,000; Santa
Cruz Biotechnology, Inc.) or polyclonal rabbit anti-goat β-actin
(sc-1616; 1:1,000; Santa Cruz Biotechnology, Inc.), at 4°C
overnight. The membranes were washed with PBST three times for 5
min, then incubated with polyclonal horseradish
peroxidase-conjugated rabbit anti-goat antibody (sc-2768; Santa
Cruz Biotechnology, Inc.) for 1 h at room temperature. Enhanced
chemiluminescence was performed according to the manufacturer’s
instructions (Amersham Pharmacia Biotech Inc., Piscataway, NJ,
USA).
Vector construction and luciferase
assay
pGL3-Nfia was generated by amplifying a 197-bp 3′UTR
fragment of the Nfia gene containing the miR-29a binding site
predicted using the TargetScan version 6.0 (http://www.targetscan.org/) and subsequently cloning
it into the pmirGLO Dual-Luciferase miRNA Target Expression vector
(Promega) at the NheI and SalI (Takara Bio, Inc.,
Otsu, Japan) cleavage sites, immediately downstream of firefly
luciferase. The primer sequences used for amplification were as
follows: Sense, 5′-GCGCTAGCCAGCAAGCATTATGGTCAAACA-3′
and anti-sense, 5′-GCGTCGACGGAAGTCAGTGAGCAAGGGTAG-3′.
(the restriction enzyme sites are underlined). The TE-1 cells were
initially transfected with the miR-29a or scrambled virus for 4
days, and subsequently transfected with pmirGLO-Nfia using
Lipofectamine 2000 (Invitrogen Life Technologies, Inc.). Luciferase
activity was measured 24 h after transfection with pmirGLO-Nfia
using the Dual-Glo™ Luciferase assay system (Promega). The Renilla
luciferase activity served as the internal control.
Statistical analysis
Statistical evaluation of the data was conducted
using SPSS analysis software (version 13; SPSS Inc., Chicago, IL,
USA) and comparisons were performed using the Wilcoxon signed-rank
test or independent samples t-tests. P<0.05 was considered to
indicate a statistically significant difference.
Results
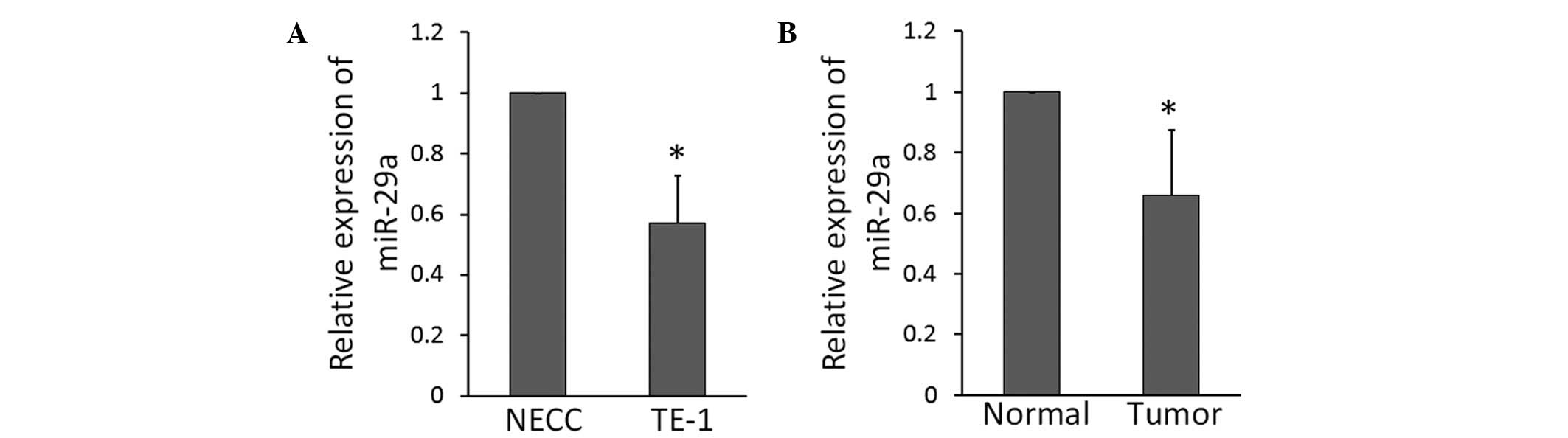
miR-29a expression is downregulated in
ESCC tissues and the ESCC TE-1 cell line
RT-qPCR analysis revealed that the expression of
miR-29a was significantly lower in the ESCC TE-1 cell line compared
with in NEECs (Fig. 1A). To
understand whether this miR-29a downregulation was clinically
correlated with ESCC progression, a comparative analysis of miR-29a
expression was conducted on paired primary cancerous tissue and
adjacent non-cancerous tissue from nine cases of ESCC. RT-qPCR
analysis revealed that the expression of miR-29a was significantly
lower in the tumor tissue compared with the adjacent non-cancerous
tissue (Fig. 1B).
Overexpression of miR-29a reduces cell
proliferation and inhibits the migration of ESCC cells
To investigate the biological role of miR-29a in
ESCC progression, the ESCC TE-1 cell line was transfected with
lentivirus containing the miR-29a precursor or scramble control.
The overexpression of miR-29a reduced cell proliferation and
resulted in an accumulation of G0/G1 phase cells, indicating that
miR-29a induces G0/G1 arrest in TE-1 cells (Fig. 2A, B, C). Furthermore, the ability of
miR-29a to regulate cell proliferation was indicated by a colony
formation assay, which demonstrated that miR-29a significantly
decreased the colony formation ability of TE-1 cells (Fig. 2D). Long intervals are required to
measure cell migration or to observe healing of scratches in cancer
cell monolayers (13); after 72 h,
overexpression of miR-29a significantly inhibited the ability of
TE-1 cells to heal following scratching (Fig. 2E). To investigate the effect of
miR-29a on cell migration over a shorter interval (16 h), thereby
minimizing the confounding effect of cell proliferation, TE-1 cell
migration was stimulated with EGF (Sigma-Aldrich). The motility of
TE-1 cells overexpressing miR-29a was significantly slower compared
with that of the control (Fig.
2F).
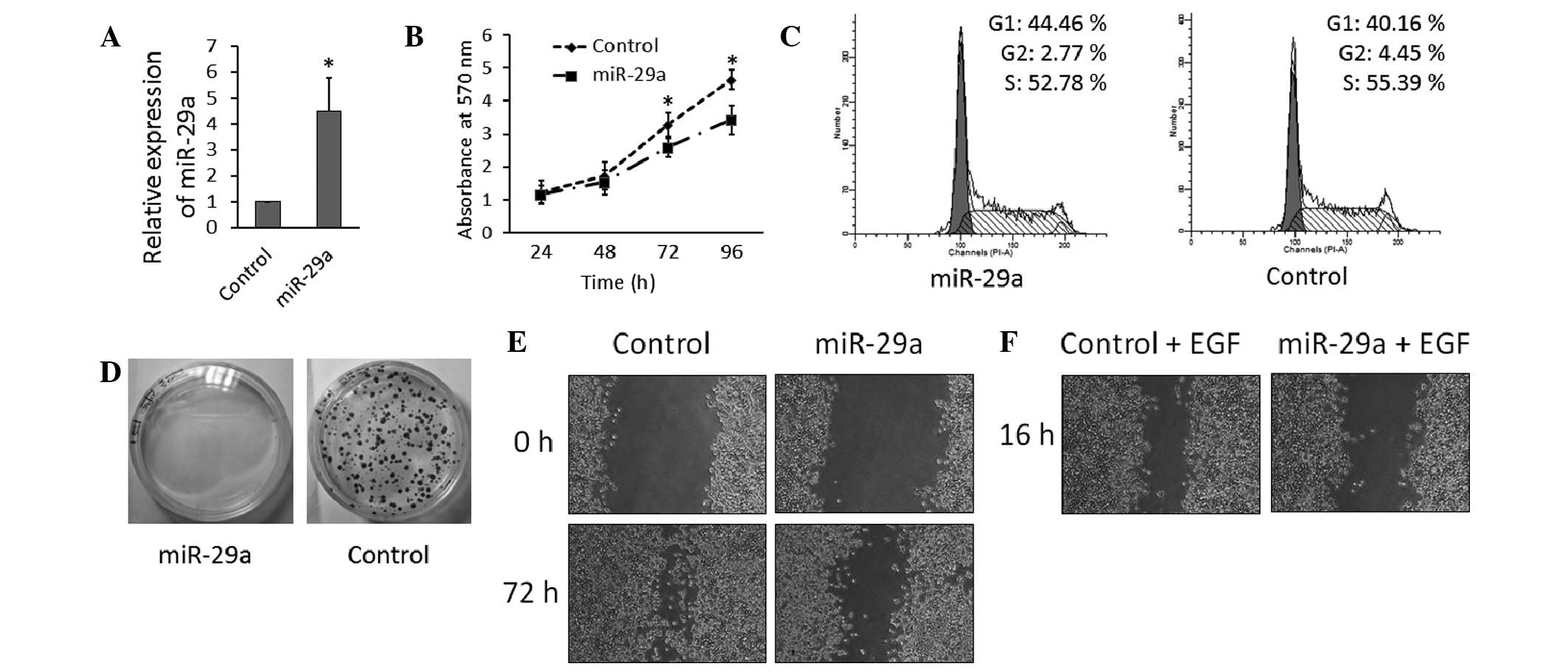 | Figure 2Overexpression of miR-29a in ESCC
cells reduces cell proliferation and inhibits cell migration. (A)
Expression levels of miR-29a were significantly increased in TE-1
cells following virus transfection, as analyzed by reverse
transcription-quantitative polymerase chain reaction.
*P<0.05. (B) Overexpression of miR-29a inhibits TE-1
cell proliferation, as determined by MTT assay. (C) Overexpression
of miR-29a inhibits TE-1 cell proliferation, as determined by three
independent flow cytometry experiments (data are presented as the
mean ± standard deviation). (D) Upregulation of miR-29a inhibits
cell growth, as determined by colony formation assays. (E) Effect
of miR-29a on cell migration in a long-interval scratch assay. TE-1
cells were transfected with lentivirus containing miR-29a precursor
or control for four days, seeded in six-well plates and grown to
confluence. A scratch was made in the cell monolayer and images
were captured at 72 h (magnification, ×40). (F) Effect of miR-29a
on cell migration in a short-interval scratch assay. TE-1 cells
were transfected with lentivirus containing miR-29a precursor or
control for four days, treated with EGF (20 ng/ml), seeded in
six-well plates and grown to confluence. A scratch was made in the
cell monolayer and images were captured 16 h after EGF stimulation
(magnification, ×40). miR, microRNA; ESCC, esophageal squamous cell
carcinoma; EGF, epidermal growth factor. |
Overexpression of miR-29a upregulates
Hes1 and downregulates Nfia
RT-qPCR and western blot analysis were used to
determine the expression of Notch1 and Hes1 in TE-1 cells. The mRNA
(Fig. 3A) and protein (Fig. 3B) expression levels of Notch1 did
not significantly change in miR-29a-overexpressing-TE-1 cells;
however, Hes1, located downstream of the Notch signaling pathway,
was significantly upregulated and Nfia, which reduces Hes1
expression by repressing Hes1 promoter transcriptional activity,
was downregulated by miR-29a overexpression.
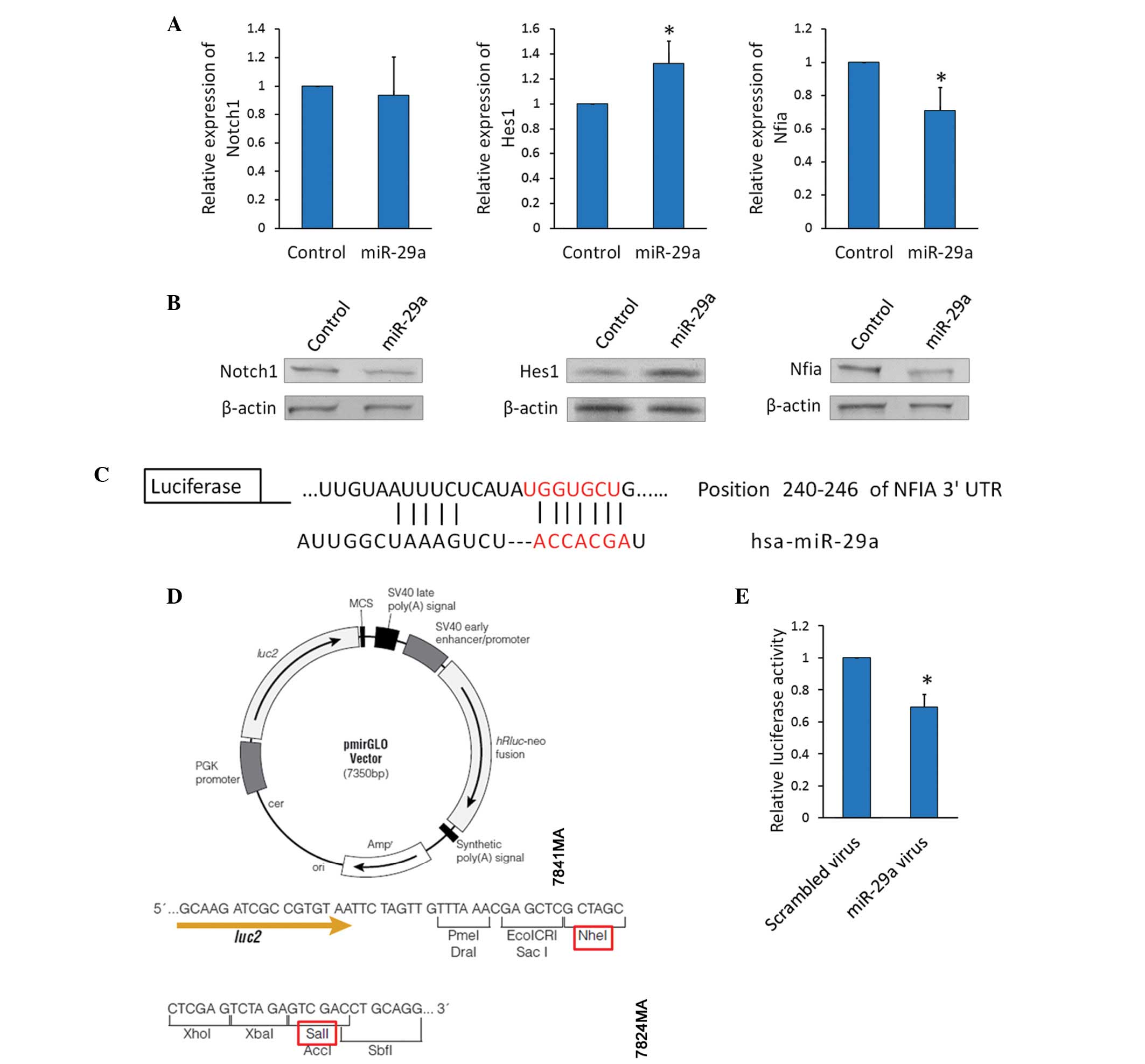 | Figure 3Overexpression of miR-29a upregulates
Hes1 and downregulates Nfia. (A) Notch1, Hes1, and Nfia mRNA
expression levels were detected using reverse
transcription-quantitative polymerase chain reaction in TE-1 cells
transfected with the miR-29a precursor or control
virus.*P<0.05. (B) Notch1, Hes1, and Nfia protein
expression levels were detected using western blot analysis in TE-1
cells transfected with the miR-29a precursor virus or control
virus. (C) TargetScan prediction of the miR-29a binding site within
Nfia mRNA. (D) Map of the pmirGLO luciferase reporter vector. The
red rectangles indicate the restrictive endonucleases used for
cloning. (E) Luciferase activity assay: TE-1 cells were transfected
with the miR-29a precursor or scrambled virus for four days,
transfected with the reporter vectors for 24 h, and harvested.
Protein extracts were prepared and assayed for firefly and Renilla
luciferase activity, and firefly luciferase activity was normalized
to Renilla luciferase activity. Data are presented as the mean ±
standard deviation from three independent experiments.
*P<0.05. miR, microRNA; Hes1, hairy and enhancer of
split 1; Nfia, nuclear factor 1 A; UTR, untranslated region. |
Investigations into the mechanism for the
downregulation of Nfia expression by miR-29a resulted in the
identification of a potential binding site for miR-29a at position
240–246 of the Nfia 3′UTR mRNA (Fig.
3C). It was hypothesized that miR-29a represses Nfia expression
via this site; thus, reporter vectors, containing luciferase
complementary DNA followed by the Nfia 3′UTR, were constructed
(Fig. 3D). TE-1 cells were
transfected with the miR-29a precursor or scrambled virus for 4
days, and the resultant miR-29a-overexpressing TE-1 cells were then
transfected with the reporter vectors. Luciferase activity was
significantly decreased in the miR-29a-overexpressing reporter
vector (Fig. 3E). This demonstrates
that miR-29a directly inhibited Nfia expression by binding to its
mRNA.
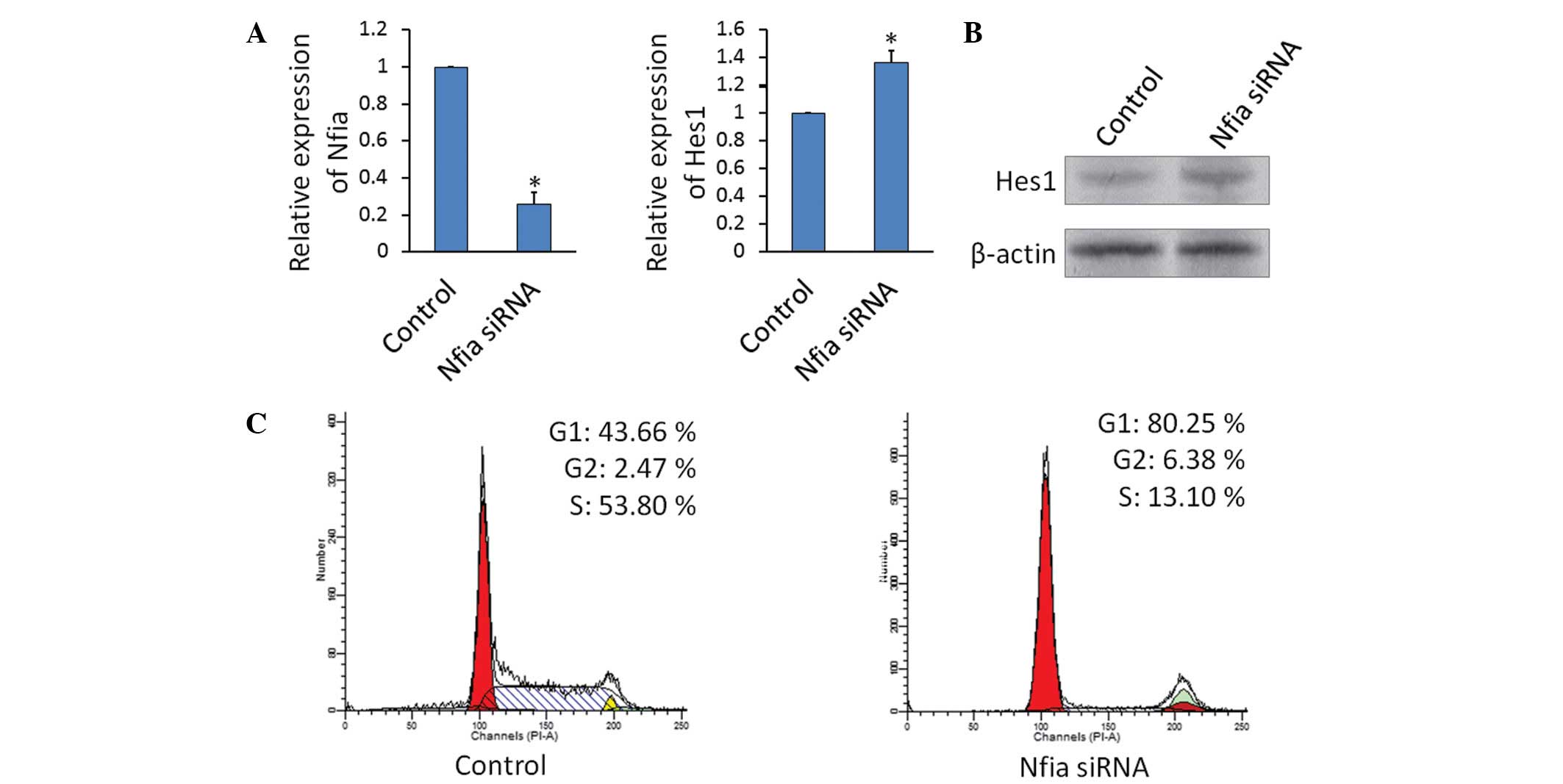
Knockdown of Nfia increases Hes1
expression and inhibits the growth of TE-1 cells
TE-1 cells were transfected with
lentivirus-containing siRNA against Nfia or the scramble RNA
control. The knockdown of Nfia significantly increased the gene
(Fig 4A) and protein (Fig 4B) expression levels of Hes1 in TE-1
cells. Furthermore, the knockdown of Nfia reduced cell
proliferation and resulted in an accumulation of cells in the G0/G1
phase (Fig. 4C).
Discussion
miRNAs are small, endogenous, non-coding RNA
molecules that regulate the expression of protein-coding genes.
miRNAs appear to affect numerous biological processes and diseases
(14,15). Although the mechanisms of various
miRNAs remain poorly understood, and the existence of
specific miRNAs remains controversial, recent studies have provided
significant insights the miR-29 family, including its biology and
relevance to cancer (16,17). Mature miR-29s in humans include
hsa-miR-29a, -29b, and -29c, which are highly conserved in humans,
mice and rats (17). All mature
miR-29s share identical sequences at nucleotide positions 2–7, the
seed region that is key in determining which protein-coding genes
an miRNA targets (17).
The downregulation of miR-29 family members has been
correlated with various types of cancer, including leukemia
(18,19), melanoma (20), and liver (21), colon (22), cervical (23) and lung (24,25)
cancer; thus, miR-29s may serve as tumor suppressors. In the
present study, miR-29a was initially demonstrated to be
downregulated in ESCC tissue and ESCC TE-1 cells. It has been
reported that the dysfunction of miR-29a results in abnormal cell
growth (16,26). In the current study, in order to
investigate the role of miR-29a in ESCC, an assay of the cell cycle
of TE-1 cells was conducted following pre-miR-29a
transfection-induced miR-29a overexpression. Overexpression of
miR-29a markedly arrested the cell cycle in the G0/G1 transition,
indicating that miR-29a predominantly regulates ESCC cell
proliferation through the modulation of cell cycle progression. In
numerous studies, the downregulation of miR-29 has been shown to
correlate with the motility and migration of carcinoma cells
(27–30). In the present study, the
overexpression of miR-29a reduced cell migration in TE-1 cells.
These results indicate that miR-29a downregulation results in
uncontrolled cell cycle progression in ESCC cells and is involved
in ESCC tumorigenesis.
The Notch signaling pathway is a highly conserved
cell signaling system, present in the majority of multicellular
organisms. The Notch signaling pathway is involved in cell fate
decisions during normal development and during the development of
various types of cancer (7). The
Notch signaling pathway is present in all metazoans and includes
four different Notch receptors, termed NOTCH1, NOTCH2, NOTCH3 and
NOTCH4. When the Notch signaling pathway is activated, the
intracellular domain is released and enters the cell nucleus to
modify gene expression, including that of Hes-1. In the present
study, it was identified that miR-29a overexpression did not affect
Notch1 gene expression levels but did increase the expression
levels of its downstream gene, Hes1. Unlike the majority of
signaling pathways, Notch signaling can be oncogenic or
tumor-suppressive, depending on the cellular context (7). In ESCC cells, Ohashi et al
(31) reported that downregulation
of the Notch signaling pathway resulted in the attenuation of
squamous cell differentiation and the enhancement of an invasive
subset of ESCCs, indicating that Notch may act as tumor suppressor
in ESCCs. Thus, the present study proposes that the overexpression
of miR-29a reduces cell growth and migration by activating the
Notch signaling pathway in TE-1 cells.
Furthermore, the present study investigated the
mechanisms by which miR-29a modulates Hes1 gene expression levels
and identified that the transcription factor Nfia may be key in
this progression. Nfia belongs to the nuclear factor I (NFI) family
of site-specific DNA-binding proteins, which are important in
various fields, including animal physiology, biochemistry and
pathology. NFI proteins have been associated with changes in the
growth state of cells and with a number of oncogenic processes and
disease states. Previous studies demonstrated that Nfia reduces the
expression of Hes1 by repressing transcriptional activity under the
control of the Hes1 promoter (32).
In the present study, miR-29a overexpression decreased Nfia gene
and protein expression levels in TE-1 cells. In addition to the
gene prediction analysis conducted by TargetScan, this observation
clarified that Nfia is the direct target gene of miR-29a. In order
to verify the role of Nfia in TE-1 cells, Nfia was knocked down;
this resulted in increased Hes1 gene and protein expression levels
and inhibited the TE-1 cell growth. Thus, the results of the
present study demonstrated that the overexpression of miR-29a
downregulated Nfia, which in turn increased the Hes1 expression in
TE-1 cells. Therefore, the present study proposes that Notch
pathway-targeted therapy using miR-29a may be a promising treatment
for ESCC.
In conclusion, the present study proposes that
miR-29a is an important miRNA that negatively regulates the Notch
signaling pathway by targeting Nfia and modulating Hes1 expression.
The study demonstrated that miR-29a was poorly expressed in ESCC
and involved in ESCC tumorigenesis. Furthermore, data from the
present study indicates that overexpression of miR-29a inhibits the
growth of TE-1 cells, supporting the therapeutic potential of this
novel miRNA in ESCC.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81171250) and
Zhengzhou University 211 project, Phase II - The Basic and Clinical
Research of Stem Cells.
References
|
1
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bartel DP: MicroRNAs: genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dykxhoorn DM: MicroRNAs and metastasis:
little RNAs go a long way. Cancer Res. 70:6401–6406. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rebane A and Akdis CA: MicroRNAs:
Essential players in the regulation of inflammation. J Allergy Clin
Immunol. 132:15–26. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Szabo G and Bala S: MicroRNAs in liver
disease. Nat Rev Gastroenterol Hepatol. 10:542–552. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wu BL, Xu LY, Du ZP, et al: MiRNA profile
in esophageal squamous cell carcinoma: downregulation of miR-143
and miR-145. World J Gastroenterol. 17:79–88. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
South AP, Cho RJ and Aster JC: The
double-edged sword of Notch signaling in cancer. Semin Cell Dev
Biol. 23:458–464. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ohashi S, Natsuizaka M, Yashiro-Ohtani Y,
et al: NOTCH1 and NOTCH3 coordinate esophageal squamous
differentiation through a CSL-dependent transcriptional network.
Gastroenterology. 139:2113–2123. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Stange DE and Clevers H: Concise review:
the yin and yang of intestinal (cancer) stem cells and their
progenitors. Stem Cells. 31:2287–2295. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Louvi A and Artavanis-Tsakonas S: Notch
signalling in vertebrate neural development. Nat Rev Neurosci.
7:93–102. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yu C, Chen K, Zheng H, et al:
Overexpression of astrocyte elevated gene-1 (AEG-1) is associated
with esophageal squamous cell carcinoma (ESCC) progression and
pathogenesis. Carcinogenesis. 30:894–901. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Le XF, McWatters A, Wiener J, Wu JY, Mills
GB and Bast RC Jr: Anti-HER2 antibody and heregulin suppress growth
of HER2-overexpressing human breast cancer cells through different
mechanisms. Clin Cancer Res. 6:260–270. 2000.PubMed/NCBI
|
|
13
|
Le XF, Almeida MI, Mao W, et al:
Modulation of MicroRNA-194 and cell migration by HER2-targeting
trastuzumab in breast cancer. PLoS One. 7:e411702012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gargalionis AN and Basdra EK: Insights in
microRNAs biology. Curr Top Med Chem. 13:1493–1502. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Suzuki H, Maruyama R, Yamamoto E and Kai
M: DNA methylation and microRNA dysregulation in cancer. Mol Oncol.
6:567–578. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schmitt MJ, Margue C, Behrmann I and Kreis
S: MiRNA-29: a microRNA family with tumor-suppressing and
immune-modulating properties. Curr Mol Med. 13:572–585. 2013.
View Article : Google Scholar
|
|
17
|
Kriegel AJ, Liu Y, Fang Y, Ding X and
Liang M: The miR-29 family: genomics, cell biology, and relevance
to renal and cardiovascular injury. Physiol Genomics. 44:237–244.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Garzon R, Garofalo M, Martelli MP, et al:
Distinctive microRNA signature of acute myeloid leukemia bearing
cytoplasmic mutated nucleophosmin. Proc Natl Acad Sci USA.
105:3945–3950. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Garzon R, Heaphy CE, Havelange V, et al:
MicroRNA 29b functions in acute myeloid leukemia. Blood.
114:5331–5341. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nguyen T, Kuo C, Nicholl MB, et al:
Downregulation of microRNA-29c is associated with hypermethylation
of tumor-related genes and disease outcome in cutaneous melanoma.
Epigenetics. 6:388–394. 2011. View Article : Google Scholar :
|
|
21
|
Xiong Y, Fang JH, Yun JP, et al: Effects
of microRNA-29 on apoptosis, tumorigenicity, and prognosis of
hepatocellular carcinoma. Hepatology. 51:836–845. 2010.
|
|
22
|
Cummins JM, He Y, Leary RJ, et al: The
colorectal microRNAome. Proc Natl Acad Sci U S A. 103:3687–3692.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li Y, Wang F, Xu J, et al: Progressive
miRNA expression profiles in cervical carcinogenesis and
identification of HPV-related target genes for miR-29. J Pathol.
224:484–495. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yanaihara N, Caplen N, Bowman E, et al:
Unique microRNA molecular profiles in lung cancer diagnosis and
prognosis. Cancer Cell. 9:189–198. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wu Z, Huang X, Huang X, Zou Q and Guo Y:
The inhibitory role of Mir-29 in growth of breast cancer cells. J
Exp Clin Cancer Res. 32:982013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhu XC, Dong QZ, Zhang XF, et al:
microRNA-29a suppresses cell proliferation by targeting SPARC in
hepatocellular carcinoma. Int J Mol Med. 30:1321–1326.
2012.PubMed/NCBI
|
|
27
|
Calin GA, Ferracin M, Cimmino A, et al: A
MicroRNA signature associated with prognosis and progression in
chronic lymphocytic leukemia. N Engl J Med. 353:1793–1801. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pekarsky Y, Santanam U, Cimmino A, et al:
Tcl1 expression in chronic lymphocytic leukemia is regulated by
miR-29 and miR-181. Cancer Res. 66:11590–11593. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sengupta S, Den Boon JA, Chen IH, et al:
MicroRNA 29c is down-regulated in nasopharyngeal carcinomas,
up-regulating mRNAs encoding extracellular matrix proteins. Proc
Nat Acad Sci USA. 105:5874–5878. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li Y, Kong D, Ahmad A, Bao B, Dyson G and
Sarkar FH: Epigenetic deregulation of miR-29a and miR-1256 by
isoflavone contributes to the inhibition of prostate cancer cell
growth and invasion. Epigenetics. 7:940–949. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ohashi S, Natsuizaka M, Naganuma S, et al:
A NOTCH3-mediated squamous cell differentiation program limits
expansion of EMT-competent cells that express the ZEB transcription
factors. Cancer Res. 71:6836–6847. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Piper M, Barry G, Hawkins J, et al: NFIA
controls telencephalic progenitor cell differentiation through
repression of the Notch effector Hes1. J Neurosci. 30:9127–9139.
2010. View Article : Google Scholar : PubMed/NCBI
|