Introduction
Intraoperative magnetic resonance imaging (iMRI) has
the advantage of real-time guidance and monitoring of the surgical
procedure. Furthermore, high-field iMRI can generate high-quality
anatomical and functional images. In recent years, with the use of
iMRI, frameless stereotactic brain biopsies have been introduced
into the clinic (1–3). Frame-based stereotactic brain biopsies
remain the gold-standard procedure for achieving accuracy, a
shorter surgical time, fewer complications and a higher positive
rate of biopsy (4,5). To the best of our knowledge, the
effects of high-field iMRI in patients with basal ganglia region
lesions who have undergone a frame-based stereotactic brain biopsy
have not yet been discussed in the literature. Furthermore, no
study with this design, combining MRI-positron emission tomography
(PET)-diffusion tensor imaging (DTI) fusion imaging with the
stereotactic biopsy of a basal ganglia lesion, has been performed
until now. In the present study, a deep lesion located in the area
of the left internal capsule is described. A needle biopsy was
successfully performed and a pathological diagnosis was explicitly
obtained. Written informed consent was obtained from the
patient.
Case report
A 68-year-old right-handed female presented with
slurred speech and a weakness of the right extremities five days
prior to admission to the Tianjin Medical University General
Hospital (Tianjin, China). These symptoms were not accompanied by a
headache, vomiting or fever. In addition, the patient’s medical
history contained no previous condition that could have contributed
to the symptoms. On the first day of hospitalization, tests
revealed a body temperature of 36.4°C (normal range, 36–37°C), an
oxygen saturation level of 97% (normal range, ≤94°C), a blood
pressure of 135/85 mmHg (normal range, 90–140/60–90 mmHg), a pulse
rate of 78 beats/min (normal range, 60–100 beats/min) and a
respiratory rate of 19 breaths/min (normal range, 12–20
breaths/min). Neurological examinations revealed slurred speech,
weakness of the right extremities (as demonstrated by a grade IV
muscle power score, measured by the manual muscle test) and the
presence of positive pathological reflexes of the right lower
extremity. An immediate MRI head scan identified left basal ganglia
lesions with a slightly long signal upon T1-weighted imaging (T1WI)
and a long signal upon T2WI. Subsequent to a
gadolinium-diethylenetriaminepentaacetic acid-enhanced scan, the
lesions appeared abnormal. The lumbar puncture revealed a normal
cerebrospinal fluid (CSF) pressure and normal outward appearance
during an unremarkable test. However, the results of the CSF
biochemical contents revealed levels of 1.5 g/l protein (normal
range, 0.15–0.45 g/l), 0.5 mmol/l glucose (normal range, 2.5–4.4
mmol/l) and 144 mmol/l sodium chloride (normal range, 120–130
mmol/l). With the exception of the lumbar puncture, the laboratory
examinations identified no notable results. An
18F-fluorodeoxyglucose (FDG)-PET scan of the brain,
performed two days prior to the surgery with the Discovery LS
PET/computed tomography (CT) scanner (GE Medical Systems, Waukesha,
WI, USA), revealed a focus of increased FDG uptake in the area of
the left basal ganglia.
An accurate could not be made based upon clinical
presentation and imaging studies alone. Therefore, a high-field
iMRI-guided stereotactic brain biopsy was performed five days after
admission. The pre-operative and intraoperative contrast-enhanced
MRI and DTI data were acquired with a 1.5T high-field iMRI scanner
(Signa HDi; GE Healthcare, Pittsburgh, PA, USA). The high-field
iMRI data collection, surgical planning and stereotactic brain
biopsy were performed on the same day. The procedure was performed
under local anesthetic using a Leksell G coordinate frame (Elekta,
Stockholm, Sweden). The patient was placed on an MRI-compatible
operating table in the MRI scanner room, which was transferred
between the MRI scanner room and the surgical room through an MRI
screening door. Following the scan, the digital imaging data and
pre-operative PET/CT data were transferred to the navigation
planning workstation software, iPlan 3.0 (BrainLAB, Feldkirchen,
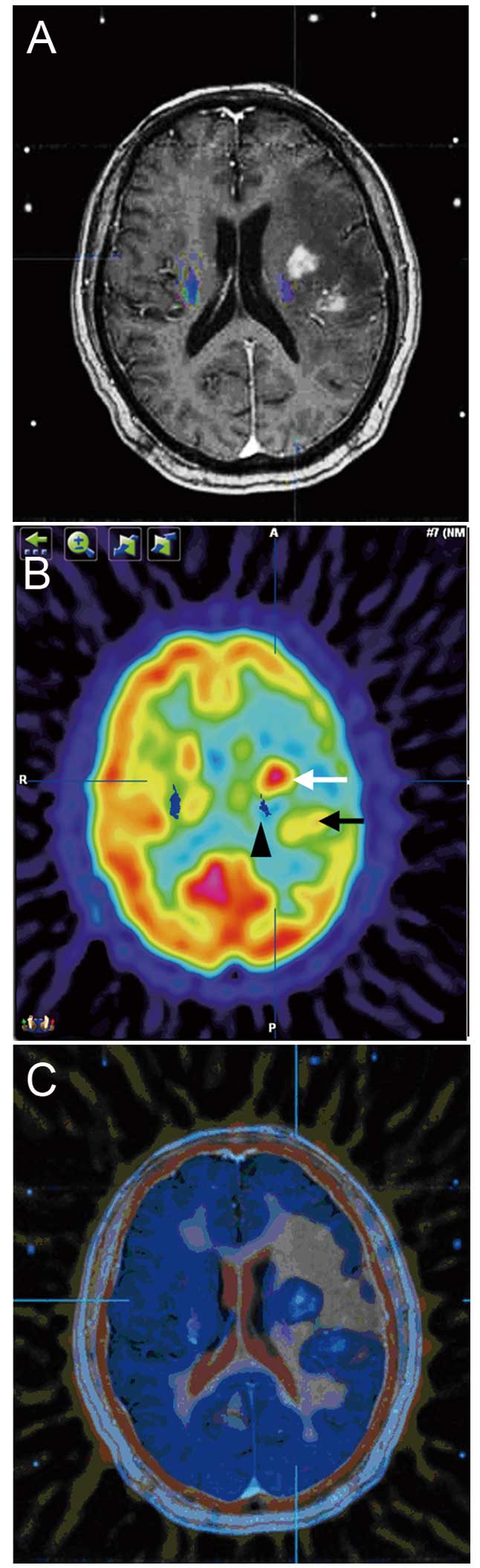
Germany), for automatic multi-image fusion (Fig. 1A–C). Subsequent to confirmation of
the accuracy of the multi-image fusion, the navigation planning
workstation software was used to reconstruct the corticospinal
tracts (CSTs) for fibre tracking, and to define the lesion range
according to the FDG-PET and contrast-enhanced MRI fusion images.
The software was then used to generate the three-dimensional (3D)
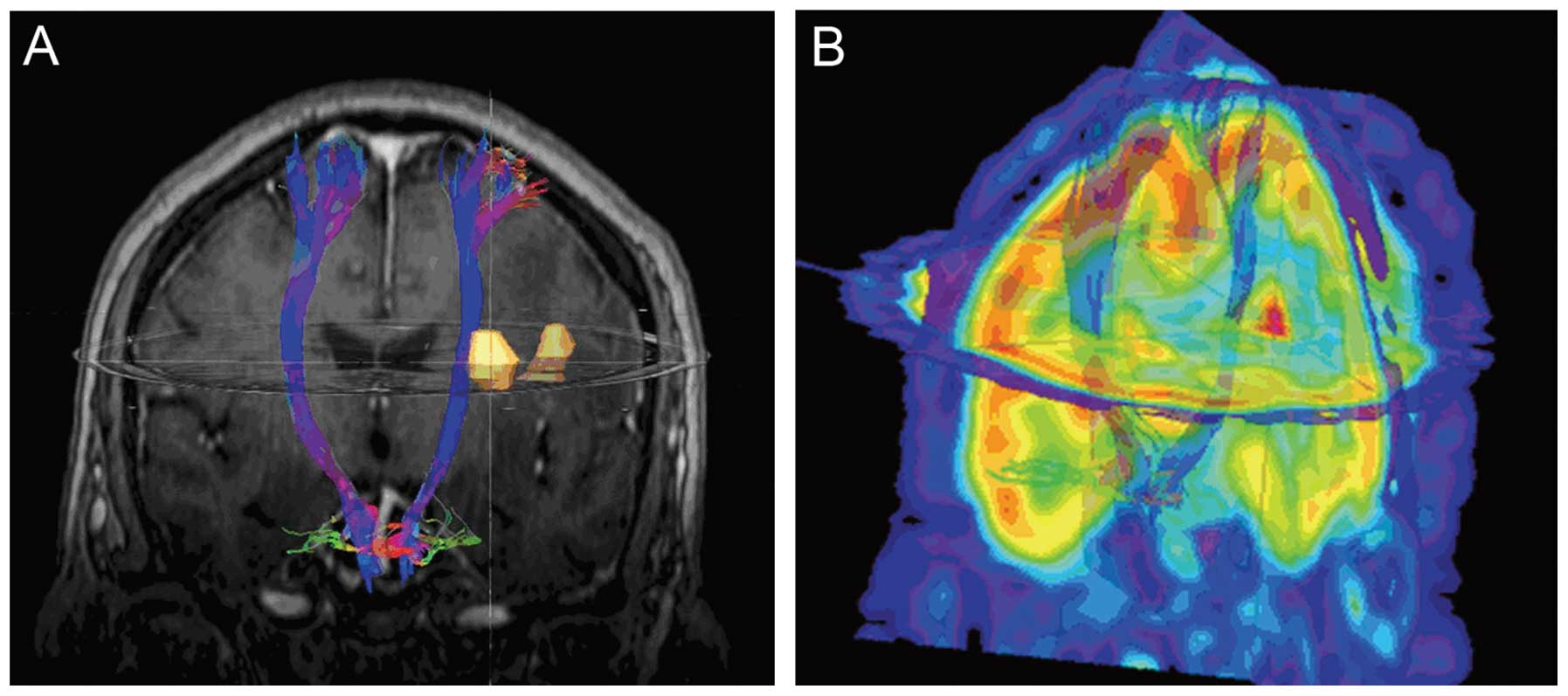
objects, which displayed the anatomical association between the CST
and the brain lesions (Fig. 2A and
B).
In addition, the contrast-enhanced MRI and
pre-operative PET/CT data were transferred into the stereotaxic
planning system software, Leksell SurgiPlan 10.1 (Elekta), for
image fusion. Using the Leksell stereotaxic planning system, the
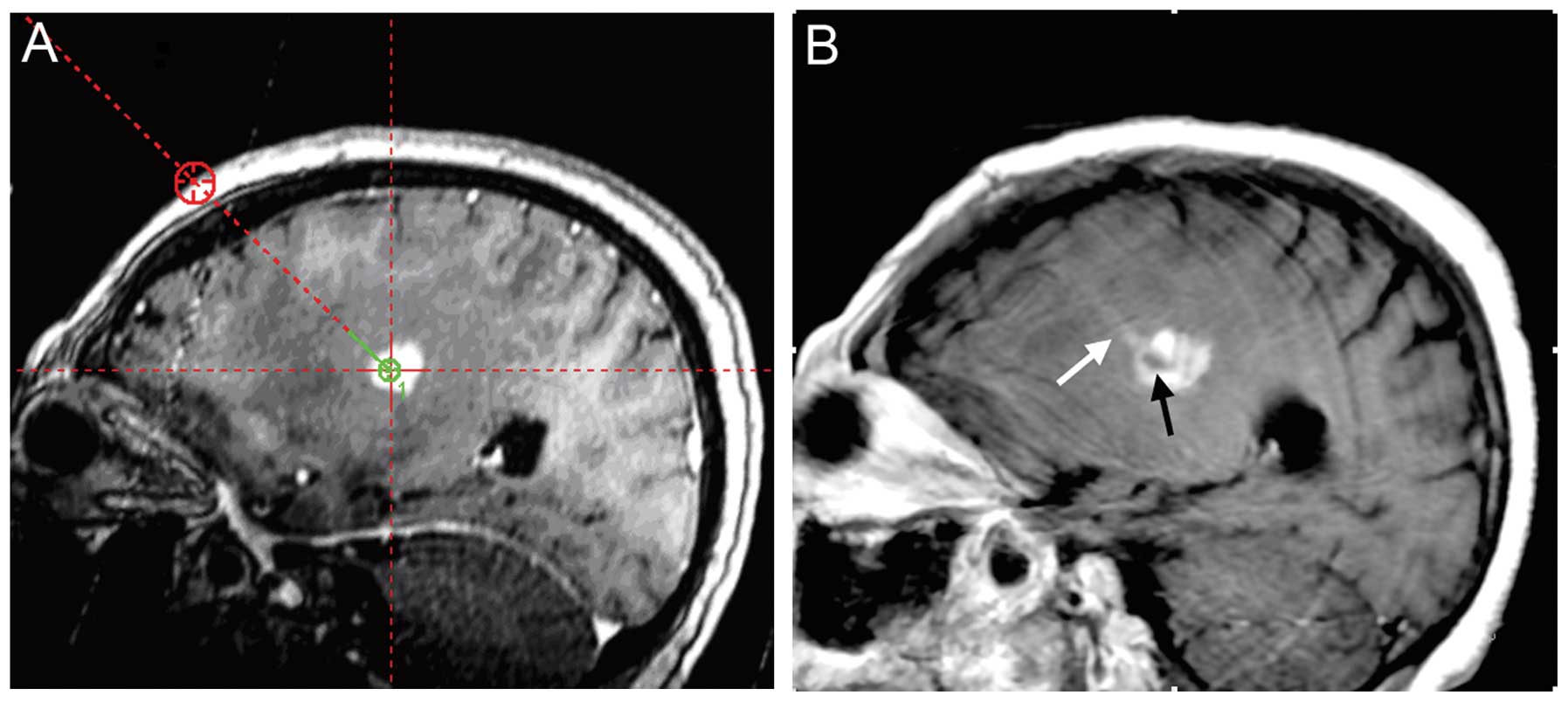
ideal target site, a reasonable needle track (Fig. 3A) and the final target and
trajectory coordinates of the stereotactic FDG-PET and
contrast-enhanced MRI fusion images were identified.
Simultaneously, the patient was transferred into the surgical room
where a burr-hole was drilled and the stereotaxic biopsy was
performed using a Sedan side-cutting biopsy needle (Elekta).
The 1.5T iMRI was used to effectively monitor the
intracranial condition during the brain biopsy procedure. The
immediate post-operative MRI identified no surgical bleeding or
other complications, and comparison of the pre-operative planning
imaging was performed to ensure that the target biopsy trajectory
had been achieved (Fig. 3B). Upon
post-operative examination, the original symptoms of the patient
were not aggravated and no new neurological deficits were apparent.
Histopathological examination of the biopsy specimen revealed
sheets of large diffuse lymphoblastic cells with ovoid vesicular
nuclei and prominent nucleoli with indistinct cell borders. In
addition, immunohistochemistry revealed large neoplastic cells
which were positive for CD19, CD20 and B-cell lymphoma 2.
Therefore, the histopathological diagnosis of B-cell non-Hodgkin’s
lymphoma was made. The patient received systemic chemotherapy:
cyclophosphamide 800 mg/m2 intravenously (IV) on days 1
and 2, doxorubicin 50 mg/m2 IV on day 1, vincristine 1.4
mg/m2 IV on days 1 and 8, methotrexate 6720
mg/m2 IV on day 10. At the one-year follow-up, the
patient was alive and free of disease.
Discussion
Stereotactic biopsy, which relies on pre-operative
imaging, is recognized as a safe and effective procedure (3,5,6). Due
to accurate target localization and reliable immobilization, the
frame-based stereotactic biopsy is accepted as a gold-standard
technique (4,5). However, limitations of this approach
exist; most notably the shifts in the integrity of intracranial
structures. Brain shift may occur subsequent to the opening of the
dura, biopsy needle insertion or cerebrospinal fluid loss, and can
cause the target lesion to deviate away from the default planning
target (3,7). In addition to the risk of brain shift,
brain stereotactic surgery is rarely performed as open surgery.
Therefore, in the present study, samples could not be taken under
direct vision. As a result, the surrounding functionally-active
normal tissue and cerebral vessels may be damaged, which could lead
to intracranial bleeding and neurological deficits. These factors,
compounded by lesion heterogeneity (8,9),
reduce the positive biopsy rate, despite high-quality pre-operative
imaging and the accurate frame-based stereotactic system.
During surgery, iMRI guidance for frame-based
stereotactic brain biopsies has the ability to effectively solve
the aforementioned issues, as the technique provides near real-time
intraoperative imaging and allows surgeons to visualize the biopsy
target and puncture track. iMRI scanning is performed according to
the requirements of the surgeon and image data can be promptly
updated in order to make comparisons with pre-operative images. In
the event that the stereotactic biopsy track is not consistent with
the pre-operatively planned track, surgeons may alter the surgical
planning, adjust the puncture direction and take samples.
Furthermore, the global status of the brain can be monitored in
case of intraoperative complications, including intracranial
hemorrhage and cerebral edema (10). In the present study, whilst the
biopsy samples were being examined by the pathologist to confirm
the presence of the diagnostic tissue, T2WI and turbo
fluid-attenuated inversion recovery scans were performed to exclude
the presence of biopsy-induced intraoperative bleeding.
The high-field iMRI system has advantages over the
low-field system in that it provides more precise image data
concerning the anatomical status of the neurovascular tissues. In
addition, high-field iMRI affords advanced functional capabilities
during the course of surgery, such as magnetic resonance
angiography, venography and stimulation, diffusion-weighted and
diffusion-tensor imaging (DTI), and perfusion and functional MRI
(fMRI) (11,12). DTI is a novel fMRI technique that
uses the diffusion energy (Brownian motion) of water molecules to
map white matter tracts within the brain (13). Corresponding imaging software
enables the white matter tracts to be visualized in 3D. The
intraoperative visualization of white matter tracts has enabled the
safe and effective resection of tumors in close proximity to the
functional areas such as the eloquent cortex or optic radiation
(14–16). In addition, DTI has proven reliable
for the prediction of the clinical outcomes of patients with
intracerebral hemorrhage (17). To
the best of our knowledge, the present study was the first to apply
DTI to the iMRI-guided stereotactic biopsy of basal ganglia region
lesions. The DTI scan was used to trace the integrity of the
subcortical white matter, which in this case was the CST, in order
to avoid secondary damage during tissue extraction. The deep brain
lesions were located in the area of the left basal ganglia and the
left CST of the posterior limb of the internal capsule was
compressed significantly (Fig. 1B).
This principally resulted in a weakness of the patient’s right
extremities. Therefore, a Sedan side-cutting biopsy needle was
inserted into the center of the overlap between the high-FDG uptake
and contrast-enhanced lesion areas. The lower-right tissue was
avoided in order to prevent secondary damage to the CST (Fig. 1C). Following the stereotactic brain
biopsy, the CST was preserved in post-operative DTI. This may
explain why the original neurological symptoms of the patient were
not aggravated and why further neurological dysfunction did not
develop.
Using high-field iMRI, PET and MRI fusion images,
guided stereotactic brain biopsies achieve an accurate histological
diagnosis and avoid complications. DTI is an effective technique
for the visualization of the CSTs. Therefore, this particular
imaging technique may have a significant role in frame-based
stereotactic biopsies of basal ganglia region lesions.
References
|
1
|
Moriarty TM, Quinones-Hinojosa A, Larson
PS, et al: Frameless stereotactic neurosurgery using intraoperative
magnetic resonance imaging: stereotactic brain biopsy.
Neurosurgery. 47:1138–1146. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hall WA, Liu H, Martin AJ, et al: Brain
biopsy sampling by using prospective stereotaxis and a trajectory
guide. J Neurosurg. 94:67–71. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Quinn J, Spiro D and Schulder M:
Stereotactic brain biopsy with a low-field intraoperative magnetic
resonance imager. Neurosurgery. 68(1 Suppl Operative): 217–224.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Smith JS, Quiñones-Hinojosa A, Barbaro NM
and McDermott MW: Frame-based stereotactic biopsy remains an
important diagnostic tool with distinct advantages over frameless
stereotactic biopsy. J Neurooncol. 73:173–179. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Owen CM and Linskey ME: Frame-based
stereotaxy in a frameless era: current capabilities, relative role,
and the positive- and negative predictive values of blood through
the needle. J Neurooncol. 93:139–149. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ersahin M, Karaaslan N, Gurbuz MS, et al:
The safety and diagnostic value of frame-based and CT-guided
stereotactic brain biopsy technique. Turk Neurosurg. 21:582–590.
2011.PubMed/NCBI
|
|
7
|
Hata N, Nabavi A, Wells WM III, et al:
Three-dimensional optical flow method for measurement of volumetric
brain deformation from intraoperative MR images. J Comput Assist
Tomogr. 24:531–538. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wong TZ, van der Westhuizen GJ and Coleman
RE: Positron emission tomography imaging of brain tumors.
Neuroimaging Clin N Am. 12:615–626. 2002. View Article : Google Scholar
|
|
9
|
Pirotte B, Goldman S, Salzberg S, et al:
Combined positron emission tomography and magnetic resonance
imaging for the planning of stereotactic brain biopsies in
children: experience in 9 cases. Pediatr Neurosurg. 38:146–155.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Albayrak B, Samdani AF and Black PM:
Intra-operative magnetic resonance imaging in neurosurgery. Acta
Neurochir (Wien). 146:543–557. 2004. View Article : Google Scholar
|
|
11
|
Hall WA and Truwit CL: Intraoperative
MR-guided neurosurgery. J Magn Reson Imaging. 27:368–375. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hall WA, Liu H, Martin AJ, et al: Safety,
efficacy, and functionality of high-field strength interventional
magnetic resonance imaging for neurosurgery. Neurosurgery.
46:632–642. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tummala RP, Chu RM, Liu H, et al:
Application of diffusion tensor imaging to
magnetic-resonance-guided brain tumor resection. Pediatr Neurosurg.
39:39–43. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nimsky C, Ganslandt O, Merhof D, et al:
Intraoperative visualization of the pyramidal tract by
diffusion-tensor-imaging-based fiber tracking. Neuroimage.
30:1219–1229. 2006. View Article : Google Scholar
|
|
15
|
Sun GC, Chen XL, Zhao Y, et al:
Intraoperative high-field magnetic resonance imaging combined with
fiber tract neuronavigation-guided resection of cerebral lesions
involving optic radiation. Neurosurgery. 69:1070–1084.
2011.PubMed/NCBI
|
|
16
|
Bello L, Gambini A, Castellano A, et al:
Motor and language DTI fiber tracking combined with intraoperative
subcortical mapping for surgical removal of gliomas. Neuroimage.
39:369–382. 2008. View Article : Google Scholar
|
|
17
|
Yoshioka H, Horikoshi T, Aoki S, et al:
Diffusion tensor tractography predicts motor functional outcome in
patients with spontaneous intracerebral hemorrhage. Neurosurgery.
62:97–103. 2008. View Article : Google Scholar : PubMed/NCBI
|