Introduction
Curcumin is a polyphenolic compound that is
extracted from ginger, turmeric and Curcuma rhizomes and
exerts a wide range of antitumor effects, including the induction
of tumor cell apoptosis, cell cycle arrest, and exhibits
anti-invasion and anti-angiogenic properties (1). Previous studies have revealed that the
growth, invasion and metastasis of tumors all depend on
angiogenesis (2–4). Vascular endothelial growth factor
(VEGF), comprising the superfamily of VEGFs, VEGF receptors
(VEGFRs), downstream signaling proteins and certain nuclear
transcription factors, can stimulate the proliferation of
endothelial cells, which indicates an association between VEGF and
the development, invasion and metastasis of tumors (5). Previous studies have also revealed
that VEGF is significant in hepatocellular carcinoma (HCC)
progression (6–10). Bevacizumab, a VEGF blocker, is a
humanized monoclonal antibody for VEGF that implements its
antitumor effects by inhibiting VEGF signaling pathways (10,11).
In the present study, a rat HCC model is employed to determine the
changes in mRNA levels of VEGF and VEGFR, and the expression of the
K-ras protein when curcumin is administered together with a
biological target, in order to provide experimental evidence for
design comprehensive therapy and improve clinical outcomes.
Materials and methods
Materials
The diethylnitrosamine (DENA) and curcumin were
purchased from Sigma-Aldrich (St. Louis, MO, USA). Bevacizumab (100
mg/4 ml) was purchased from People’s Liberation Army General
Hospital (Beijing, China). K-ras mouse monoclonal antibodies were
purchased from Santa Cruz (Dallas, TX, USA; sc-30), and GAPDH mouse
anti-rat monoclonal antibodies (TDY042) and the western blot
detection kit were purchased from Tian De Yue Biological Technology
Company (Beijing, China). The 30 male SD rats, which weighed
between 150 and 180 g, were provided by the Experimental Animal
Center of Henan Province (Henan, China).
Establishment of the rat hepatoma
model
According to the method described by Futakuchi et
al (12), the 30 SD rats were
randomly divided into five groups, with six rats in each group,
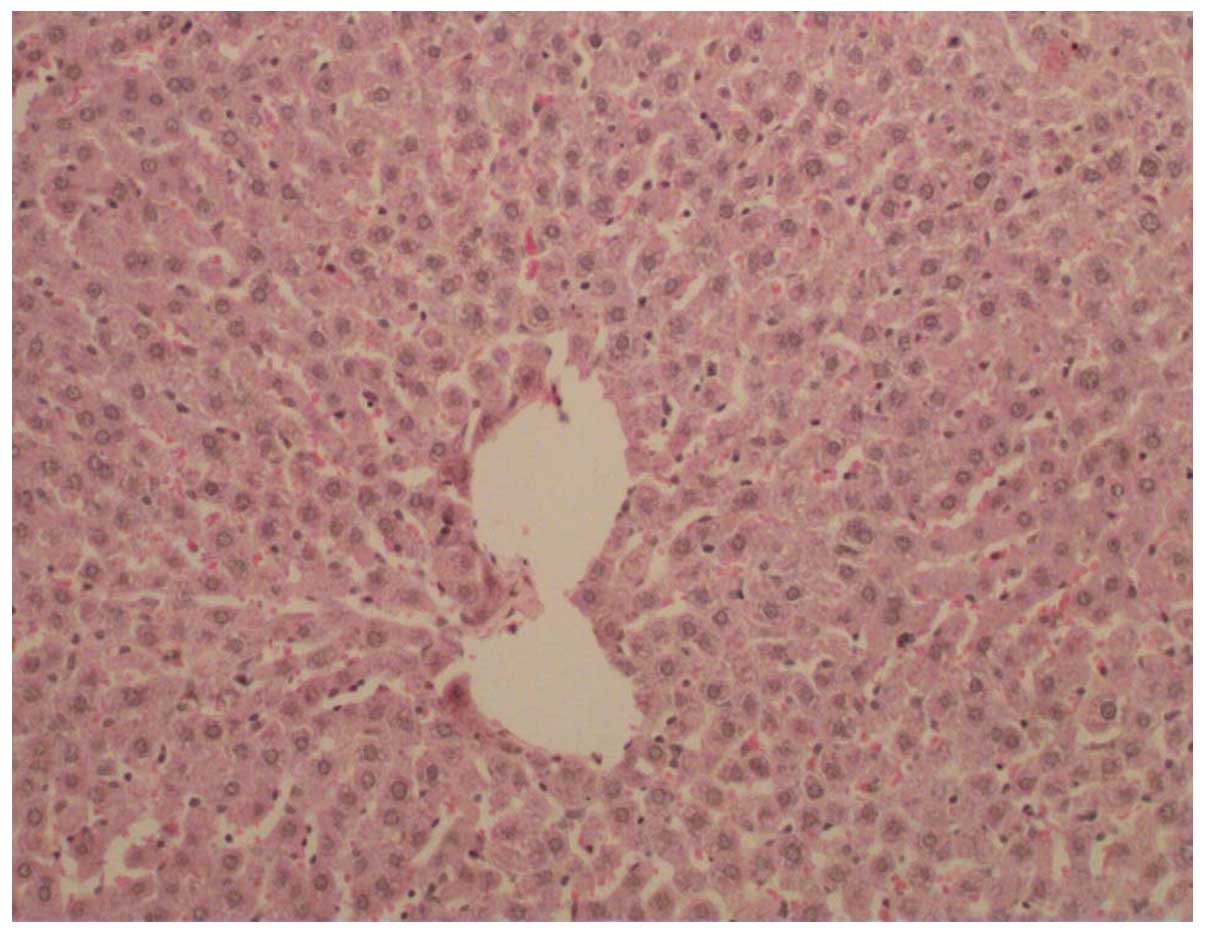
which were termed the control (Fig.
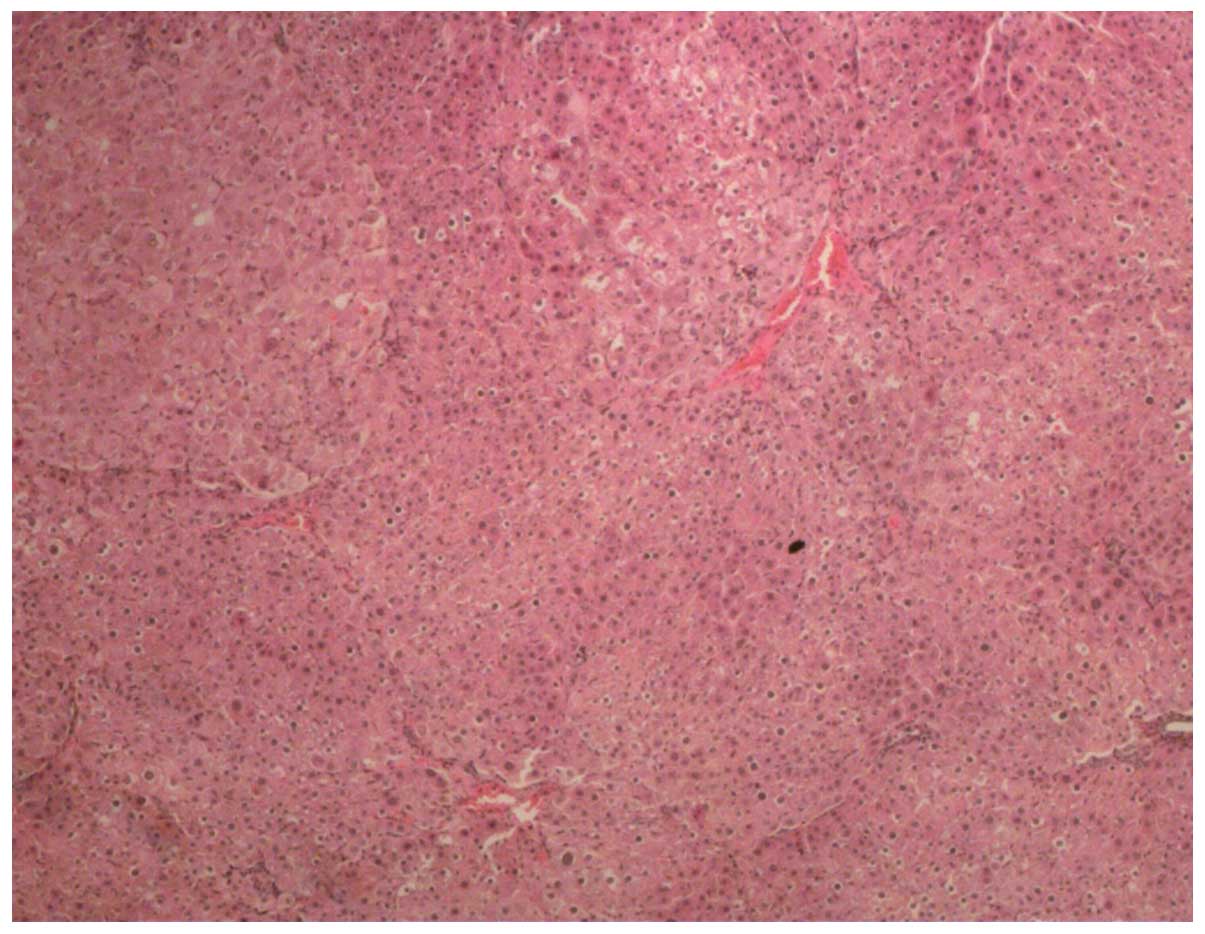
1), model (Fig. 2), curcumin,
VEGF blocker and curcumin + VEGF blocker groups. All the groups,
with the exception of the control group, were intragastrically
administered once per week with 70 mg/kg of DENA for eight weeks.
The model group was continuously intragastrically administered with
DENA. The curcumin group was continuously intragastrically
administered with DENA and curcumin solution, which consisted of
curcumin dissolved in a 0.5% sodium carboxymethyl cellulose
solution to yield a 2% concentration of curcumin suspension that
was administered at 20 mg/kg of body weight every second week for
six weeks. The VEGF blocker group was intragastrically administered
with DENA and 0.5% sodium carboxymethyl cellulose between weeks
9–18, and with 0.003 ml/g bevacizumab by intraperitoneal injection
every second week for six weeks. The curcumin + VEGF blocker group
was administered with DENA and curcumin from the beginning of week
nine, followed by intraperitoneal injection of bevacizumab from
week 10, as with the VEGF blocker group. The control group was
administered with saline for the first eight weeks, followed by
saline plus 0.5% sodium carboxymethyl cellulose by gavage between
weeks 9–18. All animals were sacrificed at week 18, the liver
tissue was dissected from the control group and a small
hepatocellular carcinoma was dissected from each rat in the other
four groups. A portion of the tissue was placed in 4%
paraformaldehyde for fixture, and the remainder was frozen in
liquid nitrogen.
Western blot analysis of K-ras
protein
All samples were segmented and lysed in protein
lysis buffer overnight at 4°C and the protein concentrations were
then quantified using the coomassie blue method. An equal amount of
each protein sample was subjected to polyacrylamide gel
electrophoresis, transferred onto a polyvinylidene difluoride
membrane, blocked with milk for 4 h and incubated with monoclonal
mouse anti-rat K-ras primary antibody (1:200; sc-30; Santa Cruz)
overnight at 4°C. The samples were subsequently incubated with
polyclonal goat anti-mouse secondary antibodies (S001; Tian De Yue
Biological Technology Company, Beijing, China) for 2 h at room
temperature and chemiluminescence was detected using an
electrochemiluminescence western blot detection kit (Pierce
Biotechnology, Inc., Rockford, IL, USA). The western blot band
density was analyzed using ImageJ software version 1.57 (National
Institutes of Health, Bethesda, MD, USA).
Total RNA extraction and reverse
transcriptase-polymerase chain reaction (RT-PCR)
All the primers were designed according to the cDNA
sequences in GenBank (National Center for Biotechnology
Information, Bethesda, MD, USA), and were synthesized at the
Beijing Sainuobo Biotechnology Center (Beijing, China). The PCR
amplification for VEGF and VEGFR was performed initially at 95°C
for 10 min, and then at 95°C for 15 sec followed by 60°C for 60
sec, for 45 cycles. The primer sequences used in PCR were as
follows: VEGFR forward, 5′-ACGGACAGTGGTATGGTTCTTGCC-3′ and reverse,
5′-GGTAGCCGCTTGTCTGGTTTGAG-3′ (amplified fragment length, 145 bp);
VEGF forward, 5′-CCACTGAGGAGTCCAACATCACCAT-3′ and reverse,
5′-CGGGATTTCTTGCGCTTTCGT-3′ (amplified fragment length, 190 bp);
and internal control β-actin (ACTB) forward,
5′-ACTTAGTTGCGTTACACCCTT-3′ and reverse, 5′-GTCACCTTCACCGTTCCA-3′
(amplified fragment length, 156 bp). All the mRNA levels were
normalized against ACTB and were expressed as relative values using
the 2−ΔΔCT method: ΔΔCT = (Cttarget gene −
CtACTB)HCC − (Cttarget gene −
CtACTB)control liver tissue.
Statistical analysis
All the data are presented as the mean ± standard
error of the mean. SPSS version 13.0 statistical software (SPSS,
Inc., Chicago, IL, USA) was used for analysis of variance. The
correlation between the VEGFR mRNA level and K-ras protein
expression was analyzed using Pearson correlation. P<0.05 was
considered to indicate a statistically significant difference.
Ethics statement
The present study was approved by the Xinxiang
University Animal Care and Use Committee (Xinxiang, Henan, China)
and the ethics committee of Xinxiang Medical University. The mice
were maintained, bred, animal modeled, sacrificed and utilized in
accordance with Xinxiang University Animal Care and Use
Committee.
Results
The mRNA levels of VEGF and VEGFR in HCC
groups
Compared with the control group, the mRNA levels of
VEGF and VEGFR were revealed to be significantly increased in the
model, curcumin and VEGF blocker groups (P<0.05), but not in the
curcumin + VEGF blocker group (P>0.05). The VEGF mRNA levels in
the curcumin, VEGF blocker and curcumin + VEGF blocker groups were
all lower compared with the model group (P<0.05). No significant
difference was identified between the VEGF mRNA levels in the VEGF
blocker group and those in the curcumin group (P>0.05), whereas
those in the curcumin + VEGF blocker group were lower than those in
the curcumin group (P<0.05). In addition, the VEGF mRNA levels
in the curcumin + VEGF blocker group were significantly lower
compared with the VEGF blocker group (P<0.05). The relative
VEGFR mRNA levels in the curcumin group and curcumin + VEGF blocker
group were significantly lower compared with the model group
(P<0.05), however, no significant difference was identified
between the VEGF blocker group and model group (P>0.05), or
between those in the VEGF blocker and curcumin + VEGF blocker group
and in the curcumin group (P>0.05). The VEGFR mRNA levels in the
curcumin + VEGF blocker group were significantly lower compared
with the VEGF blocker group (P<0.05) (Table I).
 | Table I2−ΔΔct of expression of
VEGF and VEGFR mRNA in each group (n=6). |
Table I
2−ΔΔct of expression of
VEGF and VEGFR mRNA in each group (n=6).
| Group | VEGF/ACTB | VEGFR/ACTB |
|---|
| Control | 0.85±0.17bcd | 0.78±0.13bcd |
| Model | 4.96±0.88acde | 4.45±1.18ade |
| Curcumin | 1.69±0.28abe | 2.28±0.43abd |
| VEGF blocker | 1.93±0.40abe | 4.08±1.21ace |
| Curcumin + VEGF
blocker | 1.12±0.08bcd | 1.93±0.38bcd |
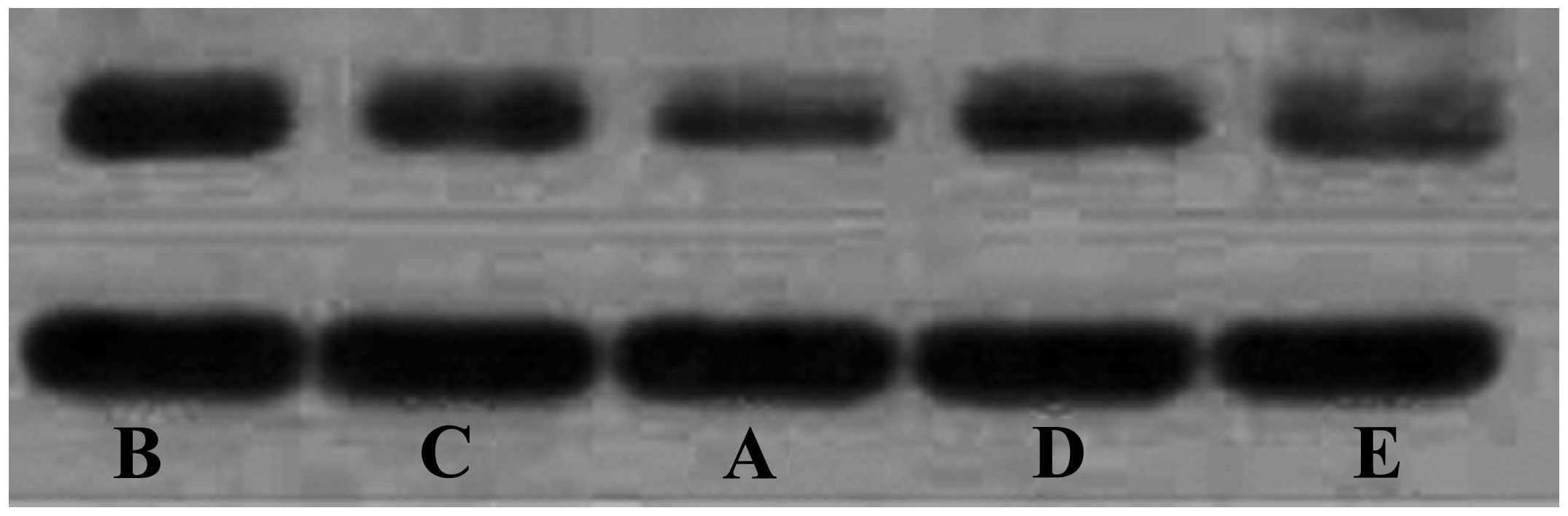
K-ras protein expressions in HCC
groups
The protein expression of K-ras was significantly
lower in the curcumin, VEGF blocker and curcumin + VEGF blocker
groups compared with the model group (P<0.05). The protein
expression of K-ras in the VEGF blocker and curcumin groups were
similar (P>0.05), and the two levels were significantly higher
compared with curcumin + VEGF blocker group (P<0.05) (Fig. 3; Table
II).
 | Table IIThe relative expression of K-ras
protein in each group (n=6). |
Table II
The relative expression of K-ras
protein in each group (n=6).
| Group | K-ras/GADPH |
|---|
| Control | 0.54±0.07bcd |
| Model | 0.85±0.12acde |
| Curcumin | 0.67±0.09abe |
| VEGF blocker | 0.67±0.06abe |
| Curcumin + VEGF
blocker | 0.56±0.07abcd |
Correlation between VEGF/VEGFR mRNA and
K-ras protein
Pearson correlation analysis revealed that the VEGFR
mRNA and K-ras protein in all HCCs exhibit a significant positive
correlation (r=0.835; P=0.0001).
Discussion
HCC has a high incidence worldwide and is the third
most prevalent cause of cancer-related mortality (13). HCC is highly vascular and the
progression, invasion and metastasis of HCC all depend on
angiogenesis. VEGF is a vascular endothelial, cell-specific,
heparin-binding growth factor that is capable of inducing
angiogenesis. VEGF is one of the predominant factors that induces
angiogenesis in tumors by means of paracrine and autocrine
signaling; this results in tumor growth and metastasis. The
induction of VEGF in HCC in the present study was consistent with
that observed in previous studies (5,10).
At present, inhibiting tumor angiogenesis and
blocking the corresponding signaling pathway has become the focus
of numerous studies investigating basic medical and clinical
research in cancer treatment (14,15).
As VEGF is significant in HCC progression, it may be of therapeutic
benefit in the downregulation of VEGF/VEGFR/K-ras signaling
pathways. The oncogene Ras is a member of the oncogene family,
consisting of H-ras, K-ras and N-ras (16). Numerous studies have revealed that a
loss of control over oncogenes and tumor suppressor genes is
closely associated with tumor progression, invasion and metastasis
(17–19).
In recent years, studies have revealed that a
variety of other risk factors, including chronic alcoholism and
hepatitis B viral infection, may induce the increased expression of
growth factors, including VEGF and PDGF-β, and their receptors in
the cytoplasm and membrane of hepatocytes (20–22).
The binding of induced growth factors with the corresponding
receptors leads to the activation of receptor tyrosine kinase
signaling pathways and hyperphosphorylation at the carboxyl
terminus of receptor tyrosine kinase, which results in the
hyperactivation of Ras protein kinases (23–25).
Activated Ras, through its downstream signaling pathway, inhibits
apoptosis and promotes cell proliferation and survival, thus
eventually leading to abnormal cell proliferation and tumor
formation (26).
Bevacizumab is a recombinant humanized anti-VEGF
monoclonal antibody that was developed by Roche (Basel,
Switzerland). The half-life in humans is 17–21 days, and
humanization can extend its half-life. Bevacizumab, which consists
of 7% rat structure and 93% human IgG fragments, targets VEGF and
competitively binds the VEGF receptor, resulting in a blockage of
VEGF-mediated downstream signaling pathways and the inhibition of
VEGF-induced vascular endothelial cell proliferation and tumor
angiogenesis. These effects block the blood, oxygen and other
nutrients that are necessary for tumor growth, thus exerting its
antitumor effect via the limitation of tumor growth (12). Therefore, the inhibition of
angiogenesis in tumors and blockade of the corresponding signaling
pathways has become the focus of much basic medical and clinical
research investigating cancer treatments.
The present study reveals that compared with the
control group, the mRNA levels of VEGF and VEGFR, and the
expression of the K-ras protein in the HCC model groups were all
significantly increased. In addition, compared with the model
group, the mRNA level of VEGF in the VEGF blocker group was
significantly reduced, whereas the mRNA level of VEGFR was not
reduced; additionally, the expression of the K-ras protein in the
HCC tissue was also reduced. All results indicate the significance
of VEGF signaling pathways in the progression of HCC, demonstrating
that VEGF is a promising target for tumor-targeted therapy.
Curcumin is a turmeric polyphenol extracted from the
rhizome of ginger plants; it exerts several important effects,
including anti-oxidant and anti-inflammatory effects, lowering of
blood pressure and anti-atherosclerotic and antitumor effects
(27,28). Previous studies have revealed that
curcumin may prevent a variety of cancers, including esophageal,
breast and colon cancers (29–31).
Curcumin has strong effects on the inhibition of tumor cell growth,
characterized by the induction of apoptosis, and the inhibition of
angiogenesis in tumor tissue via the promotion of cytochrome C
release. Curcumin also regulates the Akt, NF-κB, AP-1 and JNK
signaling pathways (32,33). Furthermore, a number of studies have
reported that curcumin may inhibit the abnormal proliferation of
liver cancer cells in a time- and concentration-dependent manner
(34).
Although the antitumor effect of curcumin has been
studied for years, it remains unclear whether or not the VEGF
signaling pathway is of significance. One mechanism underlying the
antitumor effect of curcumin is the inhibition of tumor
angiogenesis (35). However,
several questions remain, including whether this effect is due to
blocking the VEGF signaling pathway and if so, whether there is any
competition or synergistic effects when curcumin is used in
combination with bevacizumab. These questions were addressed in the
present study.
The results in the current study reveal that when
treated with curcumin, rat HCC tissues exhibit lower VEGF and VEGFR
mRNA levels compared with the model group. The curcumin group also
exhibited lower K-ras protein expression compared with the HCC
model group. These observations indicate that curcumin may inhibit
HCC progression, invasion and metastasis by decreasing the
expression of VEGF, VEGFR and K-ras. Following treatment with
bevacizumab in combination with curcumin, the VEGF mRNA level was
lower compared with the curcumin and VEGF blocker groups, but was
not significantly different from the level observed in the control
group. Furthermore, the VEGFR mRNA level was lower compared with
the VEGF blocker group, but was not significantly different from
the curcumin group. The K-ras protein expression in the HCC tissue
of the curcumin + VEGF blocker group was also lower compared with
the curcumin and VEGF blocker groups. In addition, the relative
expression of VEGFR in each group exhibited a significant positive
correlation with the K-ras protein expression. These observations
indicate that the combination of the two compounds has a
synergistic effect, which provides a theoretical and experimental
basis for the comprehensive clinical evaluation in targeted
therapy.
In conclusion, these results indicate that curcumin
is capable of reducing the expression of VEGF, VEGFR and K-ras,
which results in the repression of HCC tumor growth, invasion and
metastasis, and that curcumin interacts with the same signaling
pathway as bevacizumab. The results from the combination of
curcumin with bevacizumab indicate that the two have a synergistic
effect rather than a competitive effect, which provides
confirmation for the potential benefits of the combined use of
anti-HCC drugs that target the same signaling pathway.
Acknowledgements
This study was supported by funding projects for the
Natural Science Foundation in Henan Province (grant no. 14A310003)
and the Scientific Research Fund of Xinxiang Medical University
(grant no.2013ZD105).
References
|
1
|
Esatbeyoglu T, Huebbe P, Ernst IM, Chin D,
Wagner AE and Rimbach G: Curcumin - from molecule to biological
function. Angew Chem Int Ed Engl. 51:5308–5332. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bauer AL, Jackson TL, Jiang Y and Rohlf T:
Receptor cross-talk in angiogenesis: mapping environmental cues to
cell phenotype using a stochastic, Boolean signaling network model.
J Theor Biol. 264:838–846. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jiang JT, Zhang LF, Zhou B, Zhang SQ, Li
SM, Zhang W, Zhang J, Qiao Z, Kong RR, Ma YF and Chen S:
Relationships of uPA and VEGF expression in esophageal cancer and
microvascular density with tumorous invasion and metastasis. Asian
Pac J Cancer Prev. 13:3379–3383. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sun P, Yu H, Zhang WQ, Hu M and Lv R:
Lentivirus-mediated siRNA targeting VEGF inhibits gastric cancer
growth in vivo. Oncol Rep. 28:1687–1692. 2012.PubMed/NCBI
|
|
5
|
Meng QW, Li Y, Hu BS, Shao PJ, Zhan MX, Yu
XY and Lu LG: Prognostic significance of serum level of vascular
endothelial growth factor receptor-2 in hepatocellular carcinoma
patients after transcatheter arterial chemoembolization. Zhonghua
Yi Xue Za Zhi. 93:341–4. 2013.(In Chinese). PubMed/NCBI
|
|
6
|
Zhao X, Li J, Zhuo J and Cai L:
Reexpression of ARHI inhibits tumor growth and angiogenesis and
impairs the mTOR/VEGF pathway in hepatocellular carcinoma. Biochem
Biophys Res Commun. 403:417–421. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang R, Zhao N, Li S, Fang JH, Chen MX,
Yang J, Jia WH, Yuan Y and Zhuang SM: MicroRNA-195 suppresses
angiogenesis and metastasis of hepatocellular carcinoma by
inhibiting the expression of VEGF, VAV2, and CDC42. Hepatology.
58:642–653. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Miuma S, Ichikawa T, Arima K, Takeshita S,
Muraoka T, Matsuzaki T, Ootani M, Shibata H, Akiyama M, Ozawa E, et
al: Branched-chain amino acid deficiency stabilizes insulin-induced
vascular endothelial growth factor mRNA in hepatocellular carcinoma
cells. J Cell Biochem. 113:3113–3121. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Brauer MJ, Zhuang G, Schmidt M, Yao J, Wu
X, Kaminker JS, Jurinka SS, Kolumam G, Chung AS, Jubb A, et al:
Identification and analysis of in vivo VEGF downstream markers link
VEGF pathway activity with efficacy of anti-VEGF therapies. Clin
Cancer Res. 19:3681–3692. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Inoue S, Hartman A, Branch CD, Bucana CD,
Bekele BN, Stephens LC, Chada S and Ramesh R: mda-7 in combination
with bevacizumab treatment produces a synergistic and complete
inhibitory affect on lung tumor xenograft. Mol Ther. 15:287–294.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hsu CH, Kang YK, Yang TS, Shun CT, Shao
YY, Su WC, Sandoval-Tan J, Chiou TJ, Jin K, Hsu C and Cheng AL:
Bevacizumab with erlotinib as first-line therapy in Asian patients
with advanced hepatocellular carcinoma: a multicenter phase II
study. Oncology. 85:44–52. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Futakuchi M, Hirose M, Ogiso T, Kato K,
Sano M, Ogawa K and Shirai T: Establishment of an in vivo highly
metastatic rat hepatocellular carcinoma model. Jpn J Cancer Res.
90:1196–1202. 1999. View Article : Google Scholar
|
|
13
|
Stroescu C, Dragnea A, Ivanov B, Pechianu
C, Herlea V, Sgarbura O, Popescu A and Popescu I: Expression of
p53, Bcl-2, VEGF, Ki67 and PCNA and prognostic significance in
hepatocellular carcinoma. J Gastrointestin Liver Dis. 17:411–417.
2008.PubMed/NCBI
|
|
14
|
Zhao K, Song X, Huang Y, Yao J, Zhou M, Li
Z, You Q, Guo Q and Lu N: Wogonin inhibits LPS-induced tumor
angiogenesis via suppressing PI3K/Akt/NF-κB signaling. Eur J
Pharmacol. 737:57–69. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yoshiji H, Kuriyama S, Yoshii J, Ikenaka
Y, Noguchi R, Yanase K, Namisaki T, Kitade M, Yamazaki M, Tsujinoue
H, et al: Involvement of the vascular endothelial growth factor
receptor-1 in murine hepatocellular carcinoma development. J
Hepatol. 41:97–103. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee J, Choi KJ, Lim MJ, Hong F, Choi TG,
Tak E, Lee S, Kim YJ, Chang SG, Cho JM, et al: Proto-oncogenic
H-Ras, K-Ras, and N-Ras are involved in muscle differentiation via
phosphatidylinositol 3-kinase. Cell Res. 20:919–934. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee JE, Cranna NJ, Chahal AS and Quinn LM:
Genetic systems to investigate regulation of oncogenes and tumour
suppressor genes in Drosophila. Cells. 1:1182–1196. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mateus AR, Simões-Correia J, Figueiredo J,
Heindl S, Alves CC, Suriano G, Luber B and Seruca R: E-cadherin
mutations and cell motility: a genotype-phenotype correlation. Exp
Cell Res. 315:1393–1402. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rak J and Yu JL: Oncogenes and tumor
angiogenesis: the question of vascular “supply” and vascular
“demand”. Semin Cancer Biol. 14:93–104. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cornellà H, Alsinet C and Villanueva A:
Molecular pathogenesis of hepatocellular carcinoma. Alcohol Clin
Exp Res. 35:821–825. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Heberlein A, Muschler M, Lenz B, Frieling
H, Büchl C, Gröschl M, Riera R, Kornhuber J, Bleich S and
Hillemacher T: Serum levels of vascular endothelial growth factor A
increase during alcohol withdrawal. Addict Biol. 15:362–364. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang JC, Teng CF, Wu HC, Tsai HW, Chuang
HC, Tsai TF, Hsu YH, Huang W, Wu LW and Su IJ: Enhanced expression
of vascular endothelial growth factor-A in ground glass hepatocytes
and its implication in hepatitis B virus hepatocarcinogenesis.
Hepatology. 49:1962–1971. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Aravalli RN, Steer CJ and Cressman EN:
Molecular mechanisms of hepatocellular carcinoma. Hepatology.
48:2047–2063. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Berasain C, Nicou A, Garcia-Irigoyen O,
Latasa MU, Urtasun R, Elizalde M, Salis F, Perugorría MJ, Prieto J,
Recio JA, Corrales FJ and Avila MA: Epidermal growth factor
receptor signaling in hepatocellular carcinoma: inflammatory
activation and a new intracellular regulatory mechanism. Dig Dis.
30:524–531. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Overmeyer JH and Maltese WA: Death
pathways triggered by activated Ras in cancer cells. Front Biosci
(Landmark Ed). 16:1693–1713. 2011. View
Article : Google Scholar
|
|
26
|
Ehrkamp A, Herrmann C, Stoll R and Heumann
R: Ras and rheb signaling in survival and cell death. Cancers
(Basel). 5:639–661. 2013. View Article : Google Scholar
|
|
27
|
Chen X, Lin YN, Fang DH, Zhang HQ and
Huang WJ: Effect of crucumin on vascular endothelial function in
atherosclerotic rabbits. Zhongguo Zhong Yao Za Zhi. 38:3343–3347.
2013.(In Chinese).
|
|
28
|
Ma J, Fang B, Zeng F, Pang H, Zhang J, Shi
Y, Wu X, Cheng L, Ma C, Xia J and Wang Z: Curcumin inhibits cell
growth and invasion through up-regulation of miR-7 in pancreatic
cancer cells. Toxicol Lett. 231:82–91. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Almanaa TN, Geusz ME and Jamasbi RJ: A new
method for identifying stem-like cells in esophageal cancer cell
lines. J Cancer. 4:536–548. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Johnson JJ and Mukhtar H: Curcumin for
chemoprevention of colon cancer. Cancer Lett. 255:170–181. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen WC, Lai YA, Lin YC, Ma JW, Huang LF,
Yang NS, Ho CT, Kuo SC and Way TD: Curcumin suppresses
doxorubicin-induced epithelial-mesenchymal transition via the
inhibition of TGF-β and PI3K/AKT signaling pathways in
triple-negative breast cancer cells. J Agric Food Chem.
61:11817–11824. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sun ZJ, Chen G, Zhang W, Hu X, Liu Y, Zhou
Q, Zhu LX and Zhao YF: Curcumin dually inhibits both mammalian
target of rapamycin and nuclear factor-κB pathways through a
crossed phosphatidylinositol 3-kinase/Akt/IκB kinase complex
signaling axis in adenoid cystic carcinoma. Mol Pharmacol.
79:106–118. 2011. View Article : Google Scholar
|
|
33
|
Pantazis P, Varman A, Simpson-Durand C,
Thorpe J, Ramalingam S, Subramaniam D, Houchen C, Ihnat M, Anant S
and Ramanujam RP: Curcumin and turmeric attenuate arsenic-induced
angiogenesis in ovo. Altern Ther Health Med. 16:12–14.
2010.PubMed/NCBI
|
|
34
|
Wang M, Ruan Y, Chen Q, Li S, Wang Q and
Cai J: Curcumin induced HepG2 cell apoptosis-associated
mitochondrial membrane potential and intracellular free Ca(2+)
concentration. Eur J Pharmacol. 650:41–47. 2011. View Article : Google Scholar
|
|
35
|
Ranjan AP, Mukerjee A, Helson L, Gupta R
and Vishwanatha JK: Efficacy of liposomal curcumin in a human
pancreatic tumor xenograft model: inhibition of tumor growth and
angiogenesis. Anticancer Res. 33:3603–3609. 2013.PubMed/NCBI
|