Introduction
The most common histological form of non-Hodgkin’s
lymphoma is diffuse large B-cell lymphoma (DLBCL), which accounts
for ~30% of cases (1). The approved
chemotherapy regimen of rituximab, cyclophosphamide, doxorubicin,
vincristine and prednisolone (R-CHOP), has established an ~60%
three-year overall survival rate in patients, including those with
the poorest initial prognosis (2–4).
Nevertheless, those patients who experience relapse have a poor
disease outlook (5) when high-dose
chemotherapy with autologous stem cell transplantation is not
provided. Therefore, a detailed prognosis and prediction of the
likelihood of relapse may enable the treatment strategy to be
optimized for each patient.
The International Prognostic Index (IPI) is the
standard tool to determine the prognosis of patients with
aggressive non-Hodgkin’s lymphoma (6). The index is deduced from a number of
clinical factors, including patient age, performance status,
clinical stage, serum lactate dehydrogenase level and the number of
extranodal lesions. The original IPI remains useful for stratifying
patients with DLBCL who are assigned to rituximab therapy (4,7). The
revised IPI (R-IPI) (7) uses
identical parameters, but groups them differently in order to
identify three risk categories for patients with DLBCL. However,
the R-IPI is unable to identify those patients who have a chance of
survival of <60% at three years.
Predictors of prognosis for DLBCL are widely sought
after (8–10). These include molecular markers
identified by gene expression profiling (11), biological markers (12,13)
and clinical markers (4,6,7,14–25).
Among the clinical markers, and apart from the IPI, blood cell
counts and white blood cell differentials have been investigated
for their prognostic value in the treatment of DLBCL, since the
tests are simple to perform, inexpensive and easily accessible. In
previous studies, lymphopenia, a surrogate marker of immune
suppression, was found to correlate with patient survival in cases
of DLBCL (14–18). Furthermore, other studies identified
that the monocyte count, regarded as a surrogate indicator of the
tumor microenvironment, was a prognostic factor in DLBCL (19,20).
The ratio of lymphocytes to monocytes and the peripheral platelet
counts were also reported to be prognostic determinants for DLBCL
(21–25).
The present retrospective study was conducted to
evaluate peripheral blood cell counts and white blood cell
differentials in 30 patients with advanced DLBCL who had achieved
complete remission (CR) following 6–8 cycles of standard
chemotherapy using R-CHOP-like regimens. The primary objective of
the present study was to correlate blood cell counts as predictors
with the incidence of early disease relapse. The secondary
objective was to identify if these parameters would predict overall
patient survival.
Patients and methods
Patients
Patients who were admitted to the University of
Fukui Hospital (Yoshida, Japan) between 2006 and 2011 were included
in the present study. All patients were newly diagnosed with
advanced DLBCL (stages III and IV). Diagnosis was based on the
pathological findings in biopsy specimens and radiographic
determination using computed tomography (CT) and positron emission
tomography. Blood cell counts and white blood cell differentials
were determined prior to the initiation of chemotherapy. All
patients within the study received 6–8 cycles every 21 days of
either R-CHOP (375 mg/m2 rituximab on day 1, 50
mg/m2 doxorubicin on day 1, 750 mg/m2
cyclophosphamide on day 1, 1.4 mg/m2 vincristine on day
1 and 100 mg prednisolone on days 1–5) or R-tetrahydropyranyl
(THP)-COP (375 mg/m2 rituximab on day 1, 50
mg/m2 THP on day 1, 750 mg/m2
cyclophosphamide on day 1, 1.4 mg/m2 vincristine on day
1 and 100 mg prednisolone on days 1–5) with a substitution of
THP-doxorubicin for doxorubicin. Doxorubicin may produce severe
heart toxicity, therefore the THP-COP regime is a preferable choice
for elderly patients. Furthermore, the THP-COP regimen exhibits a
clinical efficacy that is comparable to the CHOP regimen. The
patient’s response to treatment and the incidence of relapse were
defined according to the International Workshop criteria for
non-Hodgkin’s lymphoma (26).
Following the completion of chemotherapy and the confirmation of
the achievement of CR, the patients returned periodically for
physical examinations, blood tests and CT scans to monitor their
disease status. This study was approved by the ethics committee of
the University of Fukui (Fukui, Japan).
Statistical analyses
Overall survival (OS) was calculated from the date
of diagnosis until mortality by any cause, or until the date last
known to be alive. The OS time was estimated by the Kaplan-Meier
method, and the differences were compared using a log-rank test.
Graph generation and the statistical analyses were performed using
Microsoft Excel 2007 software (Microsoft, Redmond, WA, USA) and
GraphPad Prism software (version 6.0; GraphPad Software, Inc., San
Diego, CA, USA).
Results
Patients
The study population consisted of 30 patients with
advanced DLBCL (stages III and IV) who received 6–8 cycles of
R-CHOP or R-THP-COP therapy and achieved CR. The patient
characteristics, including the clinical stage, IPI,
lymphocyte-monocyte ratio and the numbers of lymphocytes, monocytes
and platelets, are summarized in Table
I. The follow-up period from diagnosis ranged between 11 and 84
months, with a median period of 43 months.
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Characteristic | Value |
|---|
| Patients, n | 30 |
| Median age at
diagnosis, years (range) | 72 (56–87) |
| Gender, n |
| Male | 19 |
| Female | 11 |
| Stage, n |
| III | 11 |
| IV | 19 |
| IPI, n |
| Low | 0 |
|
Low-intermediate | 9 |
|
High-intermediate | 6 |
| High | 15 |
| Revised IPI, n |
| Very good | 0 |
| Good | 9 |
| Poor | 21 |
| Median no. of
peripheral cells |
| Lymphocytes,
cells/μl (range) | 1111 (219–4398) |
| Monocytes, cells/μl
(range) | 519 (81–1080) |
| Platelets,
×103/μl (range) | 251 (86–509) |
Blood cell counts and white blood cell
differentials according to IPI scores
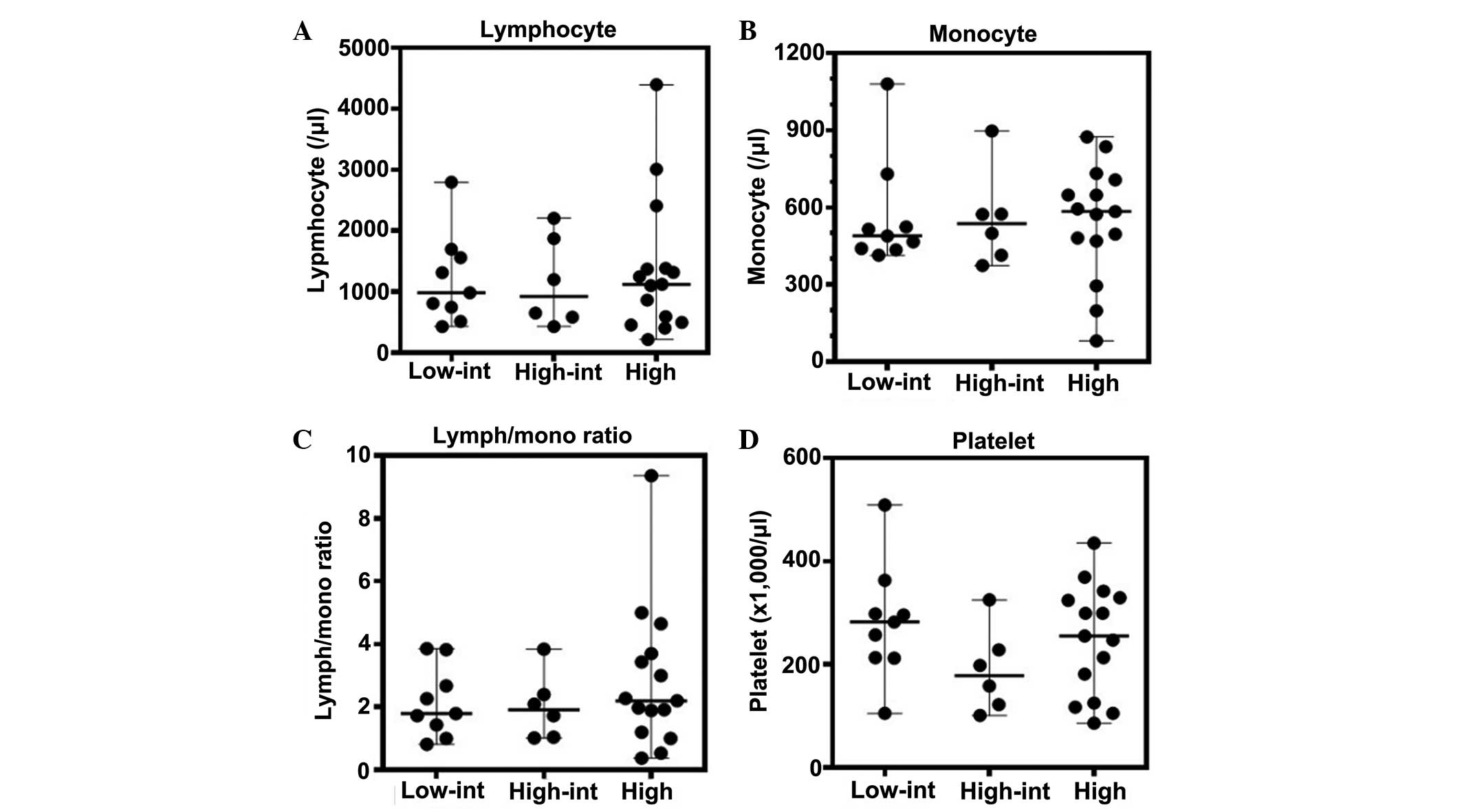
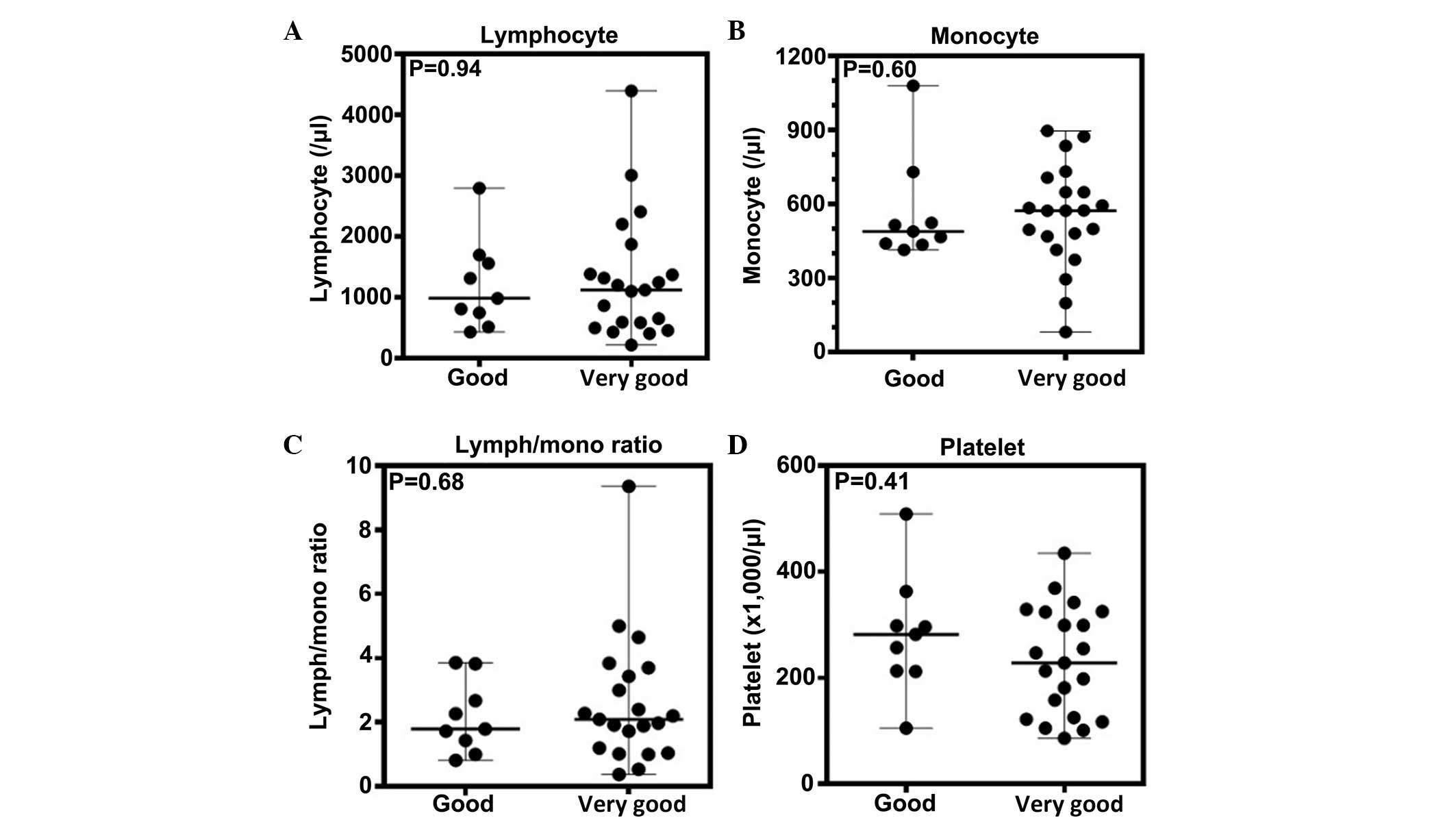
The lymphocyte, monocyte and platelet counts, and
the lymphocyte-monocyte ratio, all of which have been identified as
prognostic markers (14–25), were evaluated for any correlation
with the patient IPI scores. No association was identified between
each of these values and the IPI scores (Fig. 1). Moreover, these peripheral
parameters did not demonstrate any association with the R-IPI
scores (Fig. 2).
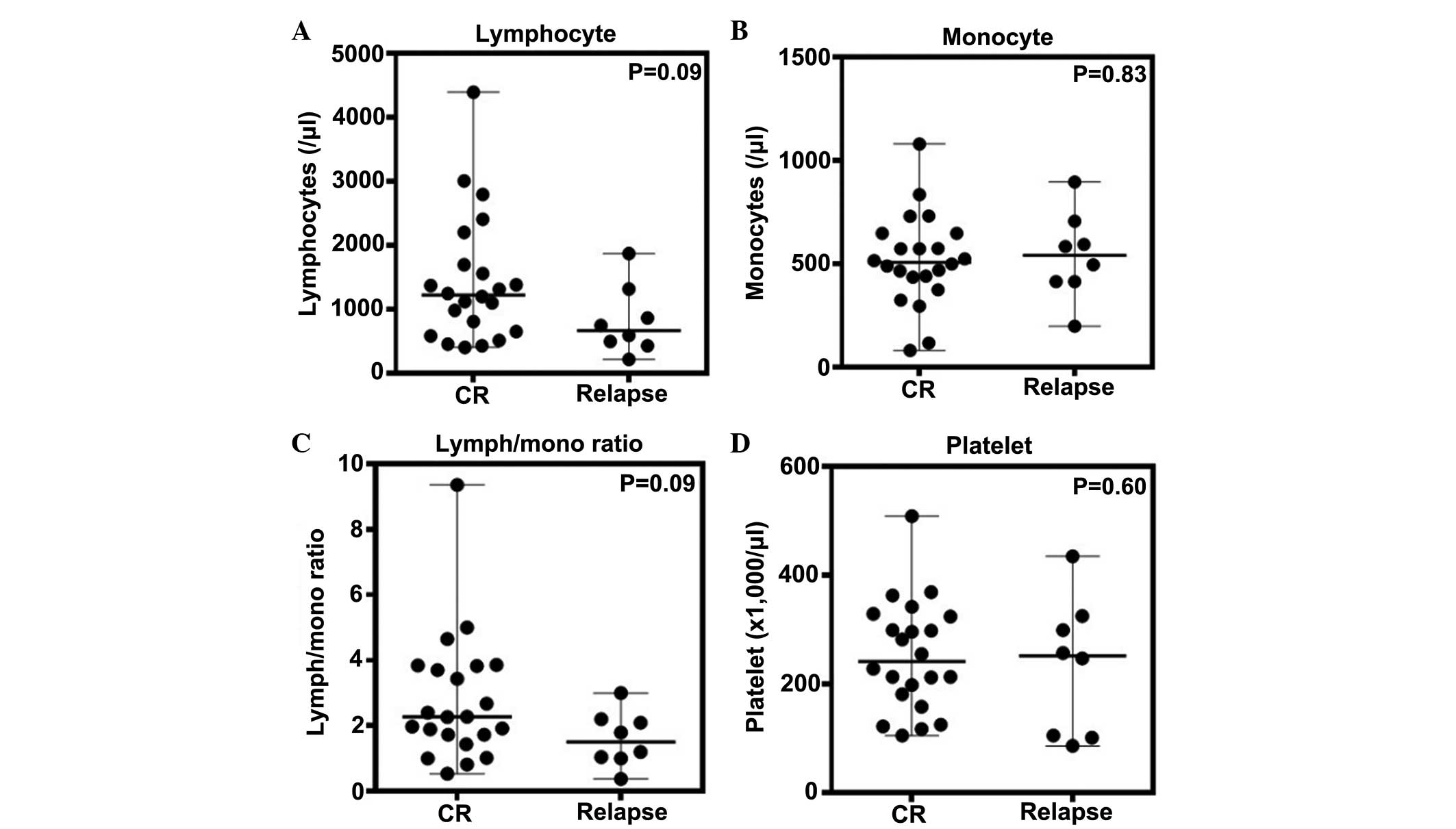
Correlation between blood cell counts and
early disease relapse
Of the 30 patients evaluated, eight relapsed within
two years of diagnosis, despite achieving CR by the first-line
treatment. The blood cell counts were evaluated in terms of a
correlation with the occurrence of early relapse; this being
relapse within two years from initial diagnosis (Fig. 3). The absolute lymphocyte count
appeared to be higher in the patients who maintained CR (median,
1,248/μl) than in the patients who experienced early relapse
(median, 747/μl), however, no significant difference was identified
(Fig. 3A). In addition, no
significant differences were identified between the two patient
groups with regard to absolute monocyte count (median, 515/μl for
CR group and 540/μl for relapse group), lymphocyte/monocyte ratio
(median, 2.3 for CR group and 1.5 for relapse group) and platelet
count (median, 239,000/μl for CR group and 251,000/μl for relapse
group) (Fig. 3B–D). Thus, these
results suggest that the peripheral blood cell counts do not
predict the incidence of early disease relapse.
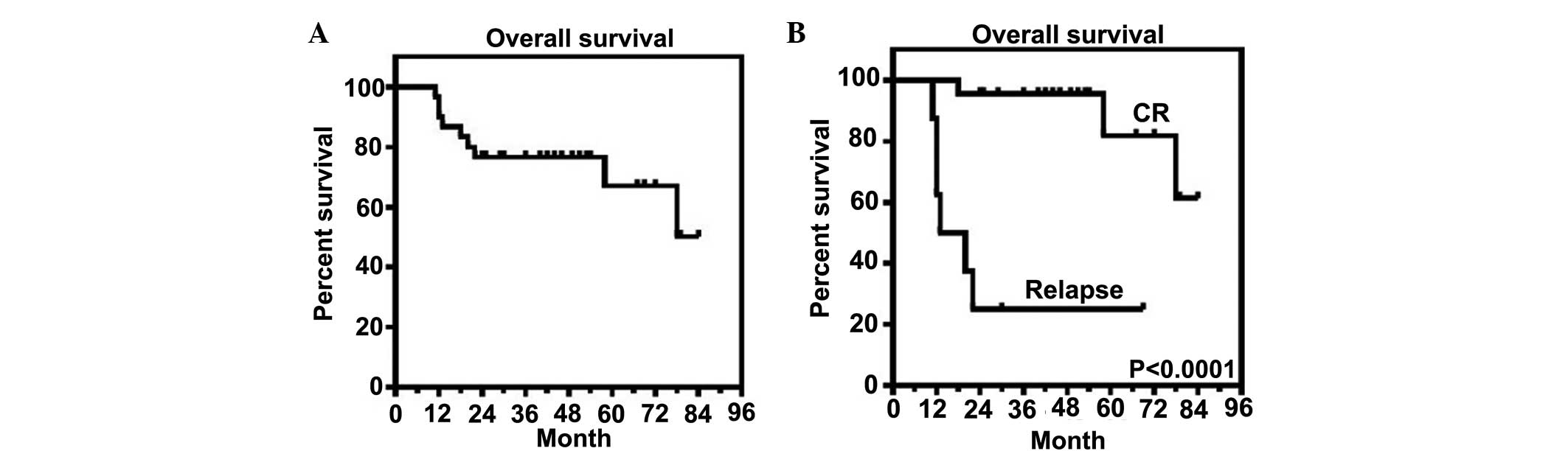
Survival analysis
According to the Kaplan-Meier method, the three-year
overall survival rate for patients with DLBCL was recorded as 77%
(Fig. 4A). When the patients were
divided into groups for either the attainment of CR or the
incidence of early relapse, the CR group had a higher survival rate
(Fig. 4B). This suggests that the
prediction of early relapse would be critical during initial
disease prognosis. The patients were also divided into two groups
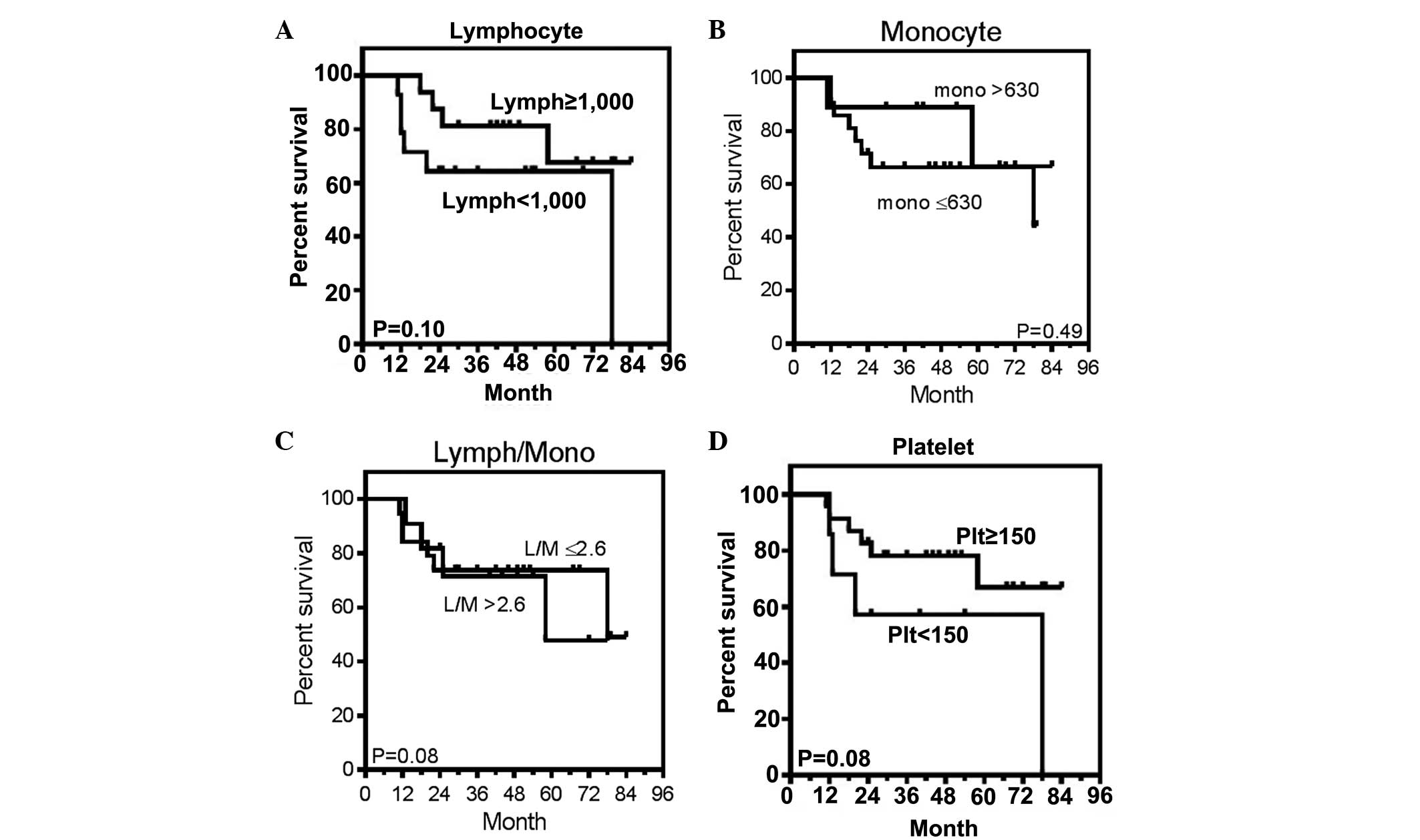
according to the cut-off values of each of the peripheral counts of
lymphocytes, monocytes and platelets, and the lymphocyte-monocyte
ratio. The cut-off values were set at 1,000/μl for lymphocytes
(14,17), 630/μl for monocytes (20), 2.6 for the lymphocyte/monocyte ratio
(22) and 150,000/μl for platelets
(25), as determined by previous
studies. As shown in Fig. 5, they
were not correlated with prognosis. Thus, these results suggested
that the peripheral blood cell counts were not predictive of the
prognosis of patients with advanced DLBCL who attained CR using
standard R-CHOP-like chemotherapy.
Discussion
The CHOP regimen is the most frequently implemented
combination chemotherapy to treat DLBCL, as more intense
chemotherapy methods have not demonstrated any additional patient
benefits (27). The addition of
rituximab to the CHOP regimen has improved the therapeutic outcome,
and the superiority of R-CHOP has been confirmed by several
clinical studies (2,3). Nevertheless, primary refractoriness is
identified in 10–15% of patients, and relapse occurs in 20–25% of
those who initially responded to therapy (8,9). In
particular, the prediction of relapse may stratify patients
according to risk and establish more appropriate treatment
schedules, thereby improving clinical outcomes. Numerous prognostic
markers have been proposed for patients with DLBCL. These include
gene expression profiling, several biological markers, and clinical
markers represented by the IPI and its variants (4,6,7,11–13).
However, these can be expensive to test and are not always
applicable in daily practice. Therefore, inexpensive and readily
available parameters are more practical in a clinical context.
The peripheral blood cell count and white blood cell
differentials have also been evaluated in terms of their
association with the prognosis of DLBCL (14–25). A
low absolute lymphocyte count at diagnosis has been revealed to be
an important prognostic marker in follicular lymphoma and DLBCL
(14–18). Kim et al (14) demonstrated a significant impact of
an absolute lymphocyte count of <1.0×109/l on
event-free survival and OS rates in a group of patients receiving
the R-CHOP or CHOP regimen. The two-year OS rates were 80% for the
patients with higher lymphocyte counts and 46% for the patients
with lower lymphocyte counts (14).
Tadmor et al (20) assessed
the prognostic significance of an absolute monocyte count in 1,017
patients with DLBCL. The best absolute monocyte count cut-off level
was <630/mm3 and the five-year OS was 71% for these
patients, but 59% for those with a count of >630/mm3
(P=0.0002) (20). Li et al
(22) investigated the peripheral
blood lymphocyte to monocyte ratio at diagnosis in 438 patients
with DLBCL treated with R-CHOP. Using a cut-off value of 2.6, those
patients above this threshold demonstrated a better clinical
outcome (five-year OS of >80%) than those below it (five-year OS
of ~70%) (22). Chen et al
(25) evaluated the correlation of
the peripheral platelet count at disease onset with the therapeutic
outcomes of patients with DLBCL treated with the R-CHOP-like
regimen. When thrombocytopenia was defined as a platelet count of
<150×109/l, the one- and three-year OS rates were
80.2 and 16.7%, respectively, in patients with a platelet count of
<150×109/l; these rates were significantly lower than
the one- and three-year OS rates of 90.2 and 75.6%, respectively,
in those with a platelet count of ≥150×109/l (P=0.0003)
(25).
The present retrospective study evaluated peripheral
blood cell counts and white blood cell differentials in 30 patients
with advanced DLBCL who achieved CR following R-CHOP-like regimens
(Table I). The absolute counts of
lymphocytes, monocytes and platelets, and the lymphocyte-monocyte
ratio were evaluated. However, these parameters were not associated
with the IPI or R-IPI scores (Figs.
1 and 2). Furthermore, they
were not predictive of early disease relapse and patient OS
(Figs. 3–5). The failure to determine any
significance of these parameters that have been identified to be of
prognostic value in previous studies may be due to the patient
selection in the present study. The patients evaluated in this
study had advanced DLBCL and had all attained CR by the first-line
treatment, while patients at an early disease stage or those with
primary refractory disease were excluded. This selection was made
due to the importance of predicting the incidence of post-remission
relapse in order to stratify treatment schedules to each patient.
The small sample size of the present study may also fail to provide
significance to a likely parameter, such as the lymphocyte count
(Fig. 3).
In conclusion, peripheral blood cell counts were not
predictive of disease prognosis or the incidence of early disease
relapse of advanced DLBCL that had reached CR using a standard
R-CHOP-like regimen. The prediction of relapse following CR would
allow for an improved treatment schedule, including the use of
up-front high-dose chemotherapy with autologous stem cell
transplantation and/or novel investigational agents.
Abbreviations:
|
DLBCL
|
diffuse large B-cell lymphoma
|
|
IPI
|
International Prognostic Index
|
|
CR
|
complete remission
|
|
OS
|
overall survival
|
References
|
1
|
Morton LM, Wang SS, Devesa SS, et al:
Lymphoma incidence patterns by WHO subtype in the United States,
1992–2001. Blood. 107:265–276. 2006. View Article : Google Scholar
|
|
2
|
Coiffier B, Lepage E, Briere J, et al:
CHOP chemotherapy plus rituximab compared with CHOP alone in
elderly patients with diffuse large-B-cell lymphoma. N Engl J Med.
346:235–242. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pfreundschuh M, Trümper L, Osterborg A, et
al: CHOP-like chemotherapy plus rituximab versus CHOP-like
chemotherapy alone in young patients with good-prognosis diffuse
large-B-cell lymphoma: a randomised controlled trial by the
MabThera International Trial (MInT) Group. Lancet Oncol. 7:379–391.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ziepert M, Hasenclever D, Kuhnt E, et al:
Standard International prognostic index remains a valid predictor
of outcome for patients with aggressive CD20+ B-cell
lymphoma in the rituximab era. J Clin Oncol. 28:2373–2380. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gisselbrecht C, Glass B, Mounier N, et al:
Salvage regimens with autologous transplantation for relapsed large
B-cell lymphoma in the rituximab era. J Clin Oncol. 28:4184–4190.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
No authors listed. A predictive model for
aggressive non-Hodgkin’s lymphoma. The International Non-Hodgkin’s
Lymphoma Prognostic Factors Project N Engl J Med. 329:987–994.
1993.
|
|
7
|
Sehn LH, Berry B, Chhanabhai M, et al: The
revised International Prognostic index (R-IPI) is a better
predictor of outcome than standard IPI for patients with diffuse
large B-cell lymphoma treated with R-CHOP. Blood. 109:1857–1861.
2007. View Article : Google Scholar
|
|
8
|
Bari A, Marcheselli L, Sacchi S, et al:
Prognostic models for diffuse large B-cell lymphoma in the
rituximab era: a never-ending story. Ann Oncol. 21:1486–1491. 2010.
View Article : Google Scholar
|
|
9
|
Sehn LH: Paramount prognostic factors that
guide therapeutic strategies in diffuse large B-cell lymphoma.
Hematology Am Soc Hematol Educ Program. 2012:402–409.
2012.PubMed/NCBI
|
|
10
|
Perry AM, Mitrovic Z and Chan WC:
Biological prognostic markers in diffuse large B-cell lymphoma.
Cancer Control. 19:214–226. 2012.PubMed/NCBI
|
|
11
|
Alizadeh AA, Eisen MB, Davis RE, et al:
Distinct types of diffuse large B-cell lymphoma identified by gene
expression profiling. Nature. 403:503–511. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Barrans S, Crouch S, Smith A, et al:
Rearrangement of MYC is associated with poor prognosis in patients
with diffuse large B-cell lymphoma treated in the era of rituximab.
J Clin Oncol. 28:3360–3365. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Iqbal J, Meyer PN, Smith LM, et al: BCL2
predicts survival in germinal center B-cell-like diffuse large
B-cell lymphoma treated with CHOP-like therapy and rituximab. Clin
Cancer Res. 17:7785–7795. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim DH, Baek JH, Chae YS, et al: Absolute
lymphocyte counts predicts response to chemotherapy and survival in
diffuse large B-cell lymphoma. Leukemia. 21:2227–2230. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Porrata LF, Ristow K, Habermann TM, et al:
Absolute lymphocyte count at the time of first relapse predicts
survival in patients with diffuse large B-cell lymphoma. Am J
Hematol. 84:93–97. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Porrata LF, Ristow K, Inwards DJ, et al:
Lymphopenia assessed during routine follow-up after
immunochemotherapy (R-CHOP) is a risk factor for predicting relapse
in patients with diffuse large B-cell lymphoma. Leukemia.
24:1343–1349. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Song MK, Chung JS, Seol YM, et al:
Influence of low absolute lymphocyte count of patients with
nongerminal center type diffuse large B-cell lymphoma with R-CHOP
therapy. Ann Oncol. 21:140–144. 2010. View Article : Google Scholar
|
|
18
|
Feng J, Wang Z, Guo X, et al: Prognostic
significance of absolute lymphocyte count at diagnosis of diffuse
large B-cell lymphoma: a meta-analysis. Int J Hematol. 95:143–148.
2012. View Article : Google Scholar
|
|
19
|
Wilcox RA, Ristow K, Habermann TM, et al:
The absolute monocyte count is associated with overall survival in
patients newly diagnosed with follicular lymphoma. Leuk Lymphoma.
53:575–580. 2012. View Article : Google Scholar
|
|
20
|
Tadmor T, Bari A, Sacchi S, et al:
Monocyte count at diagnosis is a prognostic parameter in diffuse
large B-cell lymphoma: results from a large multicenter study
involving 1191 patients in the pre- and post-rituximab era.
Haematologica. 99:125–130. 2014. View Article : Google Scholar :
|
|
21
|
Wilcox RA, Ristow K, Habermann TM, et al:
The absolute monocyte and lymphocyte prognostic score predicts
survival and identifies high-risk patients in diffuse large-B-cell
lymphoma. Leukemia. 25:1502–1509. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li ZM, Huang JJ, Xia Y, et al: Blood
lymphocyte-to-monocyte ratio identifies high-risk patients in
diffuse large B-cell lymphoma treated with R-CHOP. PLoS One.
7:e416582012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rambaldi A, Boschini C, Gritti G, et al:
The lymphocyte to monocyte ratio improves the IPI-risk definition
of diffuse large B-cell lymphoma when rituximab is added to
chemotherapy. Am J Hematol. 88:1062–1067. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Watanabe R, Tomita N, Itabashi M, et al:
Peripheral blood absolute lymphocyte/monocyte ratio as a useful
prognostic factor in diffuse large B-cell lymphoma in the rituximab
era. Eur J Haematol. 92:204–210. 2014. View Article : Google Scholar
|
|
25
|
Chen LP, Lin SJ and Yu MS: Prognostic
value of platelet count in diffuse large B-cell lymphoma. Clin
Lymphoma Myeloma Leuk. 12:32–37. 2012. View Article : Google Scholar
|
|
26
|
Cheson BD, Horning SJ, Coiffier B, et al:
Report of an international workshop to standardize response
criteria for non-Hodgkin’s lymphomas. NCI Sponsored International
Working Group. J Clin Oncol. 17:12441999.
|
|
27
|
Fisher RI, Gaynor ER, Dahlberg S, et al:
Comparison of a standard regimen (CHOP) with three intensive
chemotherapy regimens for advanced non-Hodgkin’s lymphoma. N Engl J
Med. 328:1002–1006. 1993. View Article : Google Scholar : PubMed/NCBI
|