Introduction
The increase in the incidence of cancer has become a
worldwide health concern, with gastric cancer accounting for a
large proportion of cancer-related mortalities. A global annual
case-fatality ratio (CFR) of 0.75 exists for gastric cancer, with
~934,000 new cases diagnosed and 700,349 associated fatalities. The
CFR of gastric cancer exceeds that of colon (CFR, 0.52), breast
(CFR, 0.36) and prostate (CFR, 0.33) cancer (1,2). In
2006, gastric cancer was the fifth most common newly diagnosed
cancer and the fourth most common cause of mortality in Europe
(3). The majority of gastric cancer
patients are diagnosed with locally advanced or metastatic disease.
Furthermore, gastric cancer commonly has a poor prognosis,
particularly if it localized to the cardia and gastroesophageal
junction. It is estimated that 40–60% of patients undergoing
treatment for tumor resection, develop recurrence (4). Despite this, using chemotherapy to
treat other non-hematological cancers, such as breast and
colorectal cancer, has taken priority over gastric cancer in recent
decades. Therefore, a requirement exists for renewable,
environmentally friendly and accessible therapeutic agents to treat
gastric cancer (5).
Prunella vulgaris L. belongs to the
Prunella genus (Lamiaceae) and has been used worldwide for
centuries as an alternative medicine in the treatment of fungal and
bacterial infections. In addition, anti-oxidant (6,7),
anti-allergy (8), immunomodulatory
(9), anti-diabetic (10) and anticancer (including lymphoma
(11), breast cancer (12) and esophageal cancer) (13) activities have been observed in
recent years. As previously reported, the injection of Prunella
vulgaris L. induces a significant effect on the human gastric
cancer SGC 7901 cell line (14),
however, few studies have been performed on the endophytic fungi
XKC-S03, extracted from the Prunella vulgaris L. plant. In
addition, compared with their counterparts derived from
conventional medicinal plants, the use of active metabolites from
endophytic fungi has a number of advantages. These include minimal
destruction of resources, sustainable utilization, straightforward
large-scale industrial production and simple quality control. At
present, an increasing number of compounds with various
bioactivities are being isolated from endophytic fungi (15–18).
Therefore, the objective of the present study was to investigate
the therapeutic potential of the endophytic fungi from Prunella
vulgaris L. in vivo and in vitro, and to examine
the molecular targets for treating gastric cancer.
Materials and methods
Samples
During summer 2009, the endophytic fungus XKC-S03
was isolated from the spicae of Prunella vulgaris L., which
were collected from the Second Hospital of Zhongnan University,
Changsha, China. In total, 10 liters of XKC-S03 fermentation broth
was isolated using 30 liters of petroleum ether (S03-PE), ethyl
acetate (S03-EA), dichloromethane (S03-DM) and n-butyl alcohol
(S03-BA) (all obtained from Santa Cruz Biotechnology, Inc., Santa
Cruz, CA, USA), to obtain 3.9-, 10.3-, 3.0- and 7.2-g extracts,
respectively.
Reagents
The
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay was purchased from Amresco (Solon, OH, USA). Polyclonal
rabbit anti-human vascular endothelial growth factor (VEGF;
dilution 1:2,000; catalogue number, ab9571), polyclonal mouse
anti-human B-cell lymphoma protein-2 (Bcl-2; dilution, 1:3,000;
catalogue number, ab117115) and polyclonal rabbit anti-human
Bcl-2-associated X protein (Bax; dilution, 1:2,000; catalogue
number, ab7977) antibodies were purchased from Abcam (Cambridge,
UK). Cyclophosphamide (CTX) was purchased from the National
Institutes for Food and Drug Control, (Beijing, China).
Cell lines and culture
The SGC-7901 cell line was purchased from the
Beijing Institute for Cancer Research (Beijing, China) and
maintained in RMPI 1640 medium (Beijing Solarbio Science and
Technology Co., Ltd., Beijing, China), which contained 100 U/ml
penicillin and 100 mg/ml streptomycin. The medium was supplemented
with 15% fetal bovine serum (Zhejiang Tianhang Biological
Technology Co., Ltd., Hangzhou, China) and cultures were incubated
at 37°C in a humidified atmosphere of 5% CO2.
Cell proliferation assay
Cell proliferation was analyzed using the MTT assay
as previously described (19).
After 12h, the SGC 7901 cells (1×105) were seeded into a
96-well plate and equal volumes of different concentrations of
XKC-S03 extracts (25, 50, 100, 250 and 500 μg/ml) were added to the
cells for 24, 48 and 72 h. Following this, MTT (final
concentration, 5 mg/ml) was added and incubated at 37°C for 4 h.
The medium was removed, formazan was dissolved in dimethylsulfoxide
and the optical density was measured at 570 nm using an ELISA plate
reader (MQX200; BioTek Co., Ltd, Winooski, CA, USA). All the
experiments were performed in triplicate, and three independent
experiments were conducted. The rate of cell viability to control
(I%) was calculated as: I% = A570(treated)/A570(control) × 100
(20). CTX and phosphate-buffered
saline were used as positive and negative controls,
respectively.
Antitumor activity of S03-EA in vivo
In total, 40 male nude BALB/c mice, aged 6–8 weeks
old and weighing between 20 and 22 g, were obtained from the Animal
Research Center, Capital Medical University (Beijing, China). The
SGC-7901 cells (5×106 in 0.5 ml 0.9% saline) were
subcutaneously injected into the flanks of the mice. When the
tumors reached 0.2–0.3 cm3, the mice were randomly
assigned to one of three groups. For the treatment group, 50 or 100
mg/kg/day S03-EA was administered daily for 15 days by
intraperitoneal (i.p.) injection. The negative and positive control
groups were treated with 0.9% saline and CTX, respectively, at a
dose of 10 mg/kg/day every 3 days, according to the same schedule.
On the last day, the mice in the treatment and control groups (n=10
in each group) were anaesthetized with pentobarbital sodium
intravenously, and sacrificed. The tumor volumes were recorded
using Vernier calipers every two days and calculated by the
following equation: V = ab2/2, where ‘a’ represents the
length and ‘b’ represents the width (21), and then transformed into relative
values (V) using the formula: V = Vt/V0,
where V0 is the initial tumor volume and Vt is the final
tumor volume after sacrifice (22).
The body and tumor weight of the mice were recorded following 15
days of treatment. All surgical procedures and care administered to
the animals were in accordance with institutional guidelines of the
of Second Hospital of Zhongnan University. The study was approved
by the Committee on the Use of Live Animals in Teaching and
Research of Capital Medical University (Beijing, China).
Observation of Bcl-2, Bax and VEGF
expression in tumor cells treated with S03-EA
Two slices of the tumor tissue were selected
randomly from each treatment group and the cells were observed in
five fields. The expression rates of cells positive for Bcl-2, Bax
and VEGF were calculated to obtain a conclusion. Paraffin sections
were performed as previously described (23) and immunostained by the
streptavidin-peroxidase conjugate method.
Statistical analysis
The results are expressed as the mean ± standard
deviation and are representative of at least three independent
experiments. The individual comparisons were obtained by Duncan’s
multiple range test subsequent to the demonstration of homogeneity
of variance with a one-way analysis of variance for more than two
groups using SPSS version 13.0 (SPSS, Inc., Chicago, IL, USA).
P<0.001 was considered to indicate a statistically significant
difference between groups.
Results
S03-EA inhibits the proliferation of SGC
7901 cells in vitro
The extracts of XKC-S03 inhibited the growth of the
human gastric cancer cells. The growth inhibitory effects of four
different extractions of XKC-S03 (S03-PE, EA, DM and BA) on the
human gastric cancer SGC 7901 cell line were evaluated using MTT
assays. The SGC-7901 cells were treated with four crude extractions
of XKC-S03 at concentrations of 500, 250, 100, 50 and 25 μg/ml. The
results demonstrated that treatment with PE, EA and DM extractions
induced growth inhibition in the SGC-7901 cells, and that S03-EA
had the greatest effect on cell viability (Fig. 1), with a half maximal inhibitory
concentration (IC50) of 25.89 μg/ml. The dose-dependent
cell proliferation column diagram indicates that S03-EA is an
effective growth inhibitor of the gastric cancer SGC 7901 cell line
in vitro (Fig. 1).
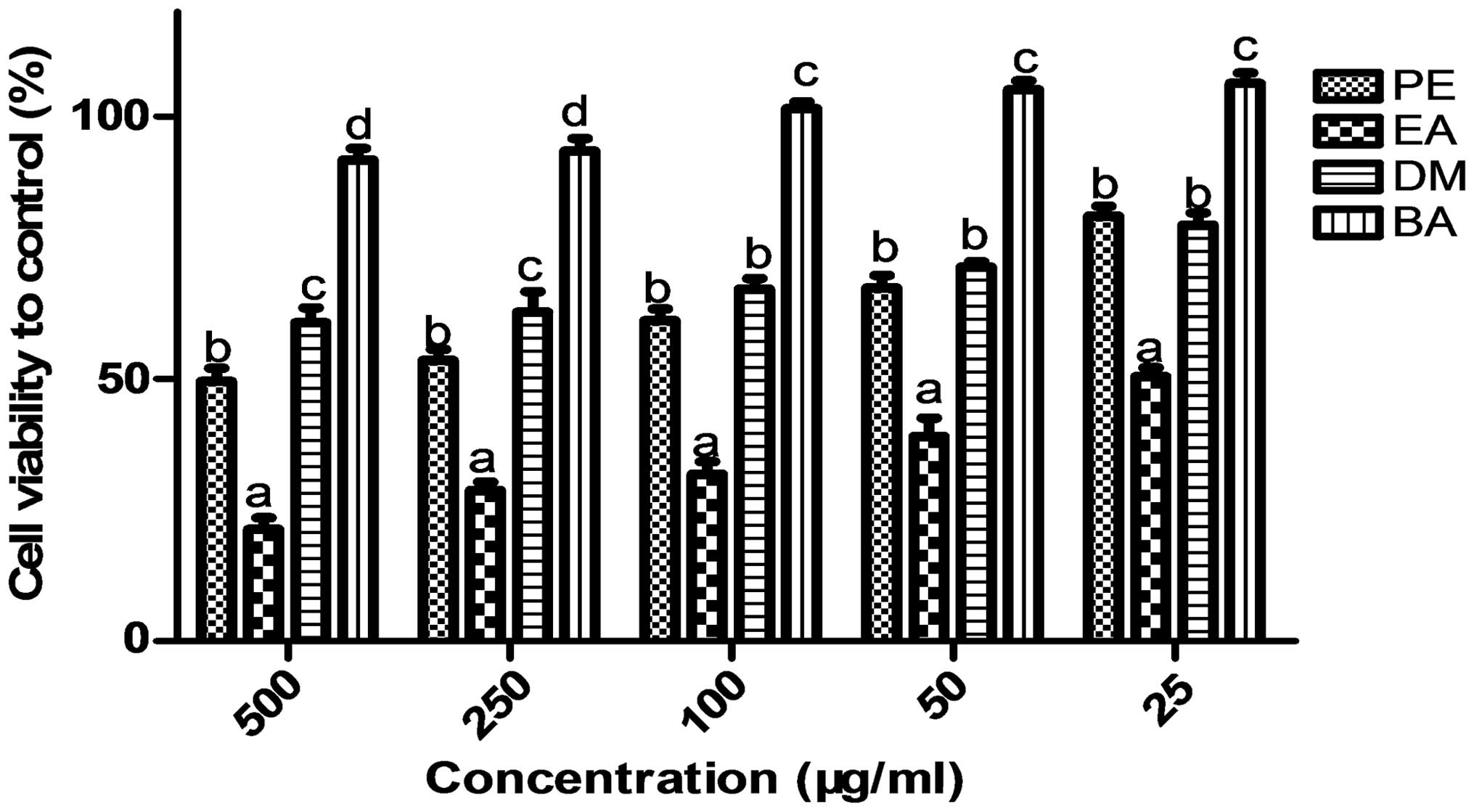 | Figure 1Four different extracts of XKC-S03
inhibit the proliferation of the SGC 7901 cell line. SGC 7901 cells
were exposed to serial dilutions of S03-PE, -EA, -DM and -BA
(25–500 μg/ml) for 24, 48 or 72 h followed by analysis by MTT
assay. Values not sharing a common superscript (a,
b, c and d) differ significantly
(Duncan’s multiple range test). Results are expressed as the mean ±
standard deviation (n=3), P<0.001 denotes statistically
significant differences. PE, petroleum ether; EA, ethyl acetate;
DM, dichloride methane; BA, n-butyl alcohol. |
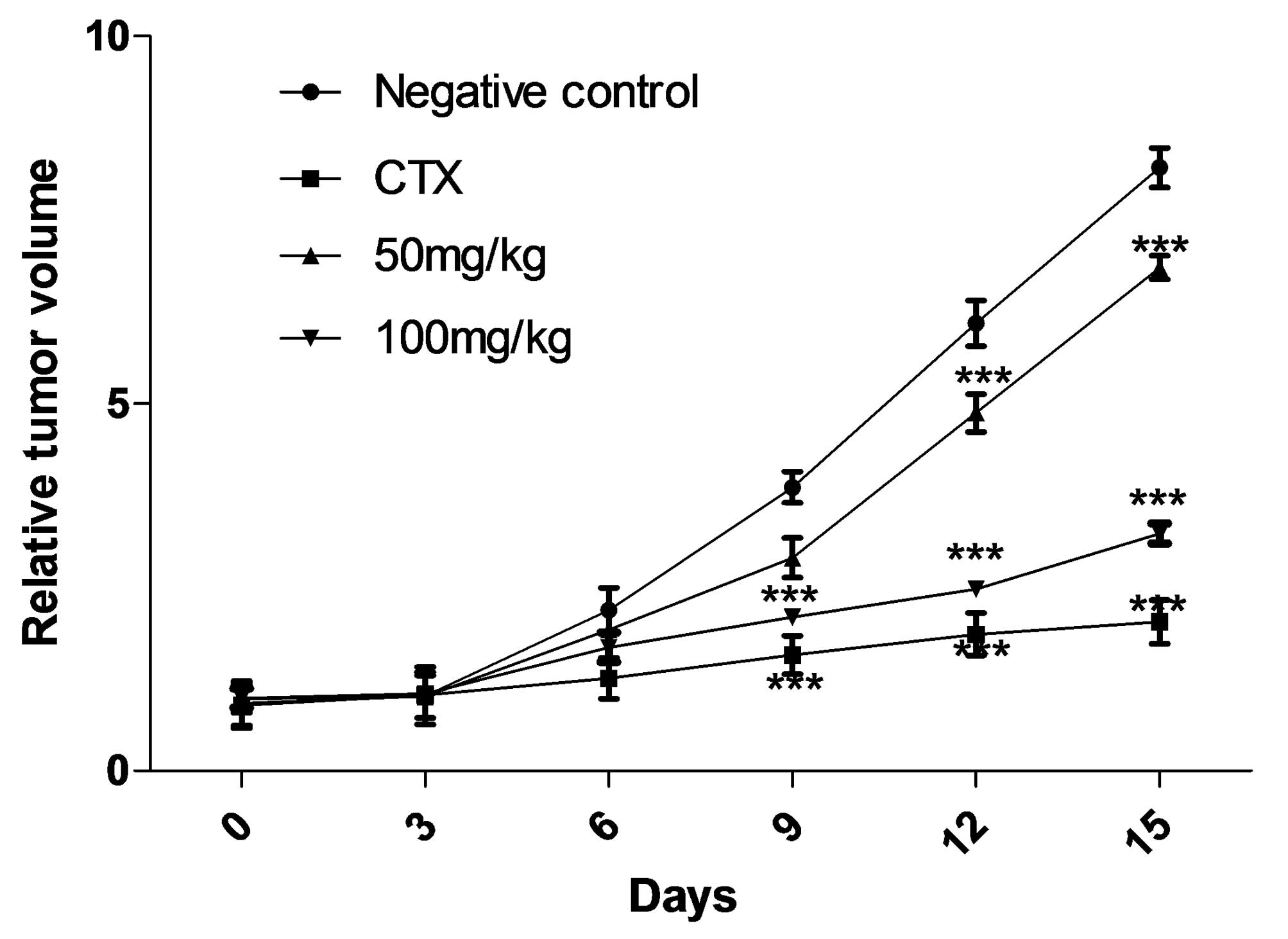
S03-EA inhibits the proliferation of SGC
7901 cells in vivo
S03-EA showed dose-dependent suppression of SGC 7901
tumor growth compared with the negative saline control. At the end
of the study (day 15), the established SGC 7901 xenograft mouse
model (average tumor volume, 200 mm3) injected with i.p.
S03-EA (50 or 100 mg/kg/day) demonstrated inhibition of tumor
volume growth by 17.91 and 65.67%, and of tumor weight growth by
25.70 and 64.79%, respectively (P<0.001 vs. 0.9% saline
controls). By day 15, the positive control, CTX, had reduced tumor
volume and weight by 73.63 and 72.18%, respectively. No significant
difference was identified between this result and that obtained
with 100 mg/kg S03-EA (Table. I). Suppression of tumor growth was
evident from the sixth day following treatment with S03-EA
(Fig. 2), similar to the results
observed in the CTX-treated group. In addition, no significant loss
in body weight was identified in the S03-EA- and CTX-treated groups
(Table I). The present in
vivo study indicates that S03-EA is a potential therapeutic
treatment of gastric cancer and is relatively non-toxic to nude
mice.
 | Table ITumor volume and weight of BALB/c nude
mice treated with S03-EA and CTX. |
Table I
Tumor volume and weight of BALB/c nude
mice treated with S03-EA and CTX.
| Group | Body weight, g | Final tumor volume,
cm3 | Final tumor weight,
g | Inhibition rate, %
(volume/weight) |
|---|
|
|---|
| Pre-treatment | Post-treatment |
|---|
| Negative control | 21.85±0.99 | 20.67±1.29 | 2.01±0.52 | 2.84±0.39 | |
| CTX | 21.46±0.63 | 31.49±2.26 | 0.53±0.12 | 0.9±0.28 | 73.63/72.18a |
| 50 mg/kg | 21.37±1.11 | 30.75±2.39 | 1.65±0.43 | 2.11±0.61 | 17.91/25.70b |
| 100 mg/kg | 20.11±0.68 | 28.68±3.12 | 0.69±0.21 | 1.00±0.38 | 65.67/64.79a |
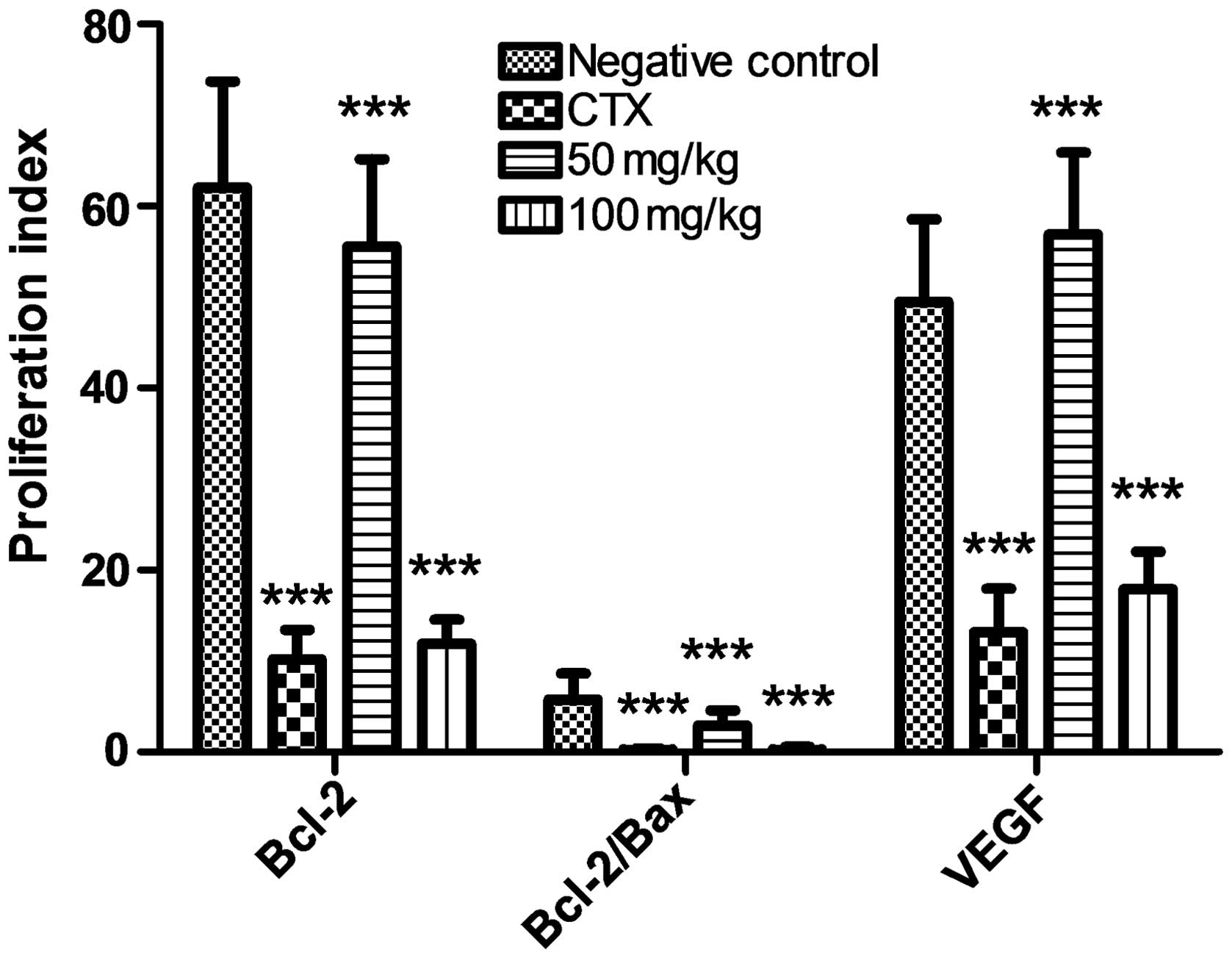
S03-EA downregulates Bcl-2 and VEGF
expression, and actives Bax expression in gastric cancer cells
The effect of S03-EA on the expression levels of
Bcl-2, VEGF and Bax was investigated to examine the mechanism
behind its inhibition of gastric cancer cell proliferation. As
shown in Fig. 3, Bcl-2 and VEGF
expression in the 100-mg S03-EA- and CTX-treated groups were
suppressed in the gastric cancer cells. However, the expression of
Bax was higher in these treatment groups, which correlates with the
decrease in the Bcl-2/Bax ratio observed. This result of the
present study is consistent with previous studies investigating
other tumors (24).
Discussion
Prunella vulgaris L. has been demonstrated to
possess a variety of anticancer activities. In addition, there have
been studies investigating its endophytic fungi as a potential
source of novel drug development. Prunella vulgaris L. was
reported to be used in the treatment of gastric cancer as early as
1979 and revealed good curative effects (25). Subsequently, a Prunella
vulgaris L. injection was developed by Shanghai Shuguang
Hospital (Shanghai, China) and proved to be effective in the
treatment of advanced gastric cancer and in SGC 7901 cells
(14,26). In view of these studies, the
endophytic fungi from Prunella vulgaris L was investigated
in the present study. Firstly, the activity of the endophytes
isolated from the spicae of Prunella vulgaris L. against the
SGC 7901 cell line was screened. The crude extract of the XKC-S03
fermentation broth demonstrated the most promising result. In the
present study, it was identified that the S03-EA extract of XKC-S03
inhibited SGC 7901 cell proliferation in vitro and tumor
growth in vivo.
The results of the present study indicated that
S03-EA suppressed SGC 7901 cell growth in a dose- and
time-dependent manner, with an IC50 value of 25.89
μg/ml. A significant difference in inhibition activity was
identified compared with the other three groups (S03-PE, -DM and
-BA). According to the subsequent in vivo study, it was
identified that the tumor volume and weight could be significantly
reduced in mice following the sixth day of treatment with S03-EA.
In addition, the average change in body weight subsequent to S03-EA
treatment was not significant compared with that following CTX
treatment (Table I). No
gastrointestinal bleeding or other severe organ damage was
identified in the S03-EA-treated mice, suggesting that it is safe
for longer-term use. In order to explore the therapeutic mechanism
of S03-EA-mediated inhibition, the expression of the three
proteins, Bcl-2, Bax and VEGF, was analyzed in SGC 7901 cells.
The occurrence of tumors has a close association
with apoptosis, a cellular event controlled by a balance between
pro- and anti-apoptotic molecules, such as those belonging to the
proto-oncogene Bcl-2 family. Among the Bcl-2 family, the Bcl-2
protein and its associated protein X (Bax) are delegates. In
contrast to Bcl-2, Bax protein plays a pro-apoptotic role,
activating apoptosis and initiating cell death (27,28).
The results of the present study demonstrated that S03-EA inhibited
the proliferation of the SGC 7901 cells by upregulating Bax
expression and downregulating Bcl-2 expression. In addition,
pro-angiogenic factors, such as VEGF, are also important to tumor
cell proliferation, as they stimulate tumor progression, invasion
and metastasis (29,30). In the present study, the suppression
of VEGF expression may therefore have reduced the proliferation of
the gastric cancer cells.
In summary, the present study demonstrated that
treatment with the S03-EA extract of XKC-S03 isolated from
Prunella vulgaris L. can suppress gastric cancer in mice.
This finding lends support to existing studies on the therapeutic
potential of active metabolites from Prunella vulgaris L.,
and the investigation of novel medicines for the treatment of
gastric cancer.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li R, Wu X, Wei H and Tian S:
Characterization of side population cells isolated from the gastric
cancer cell line SGC-7901. Oncol Lett. 5:877–883. 2013.PubMed/NCBI
|
|
3
|
Jackson C, Cunningham D and Oliveira J;
ESMO Guidelines Working Group. Gastric cancer: ESMO clinical
recommendations for diagnosis, treatment and follow-up. Ann Oncol.
20(Suppl 4): 34–36. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Verdecchia A, Mariotto A, Gatta G,
Bustamante-Teixira MT and Ajiki W: Comparison of stomach cancer
incidence and survival in four continents. Eur J Cancer.
39:1603–1609. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu CH, Zou XW, Lu H and Tan RX:
Antifungal activity of Artemisia annua endophyte cultures against
phytopathogenic fungi. J Biotechnol. 88:277–282. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Harikrishnan R, Kim JS, Kim MC,
Balasundaram C and Heo MS: Prunella vulgaris enhances the
non-specific immune response and disease resistance of Paralichthys
olivaceus against Uronema marinum. Aquaculture. 318:61–66. 2011.
View Article : Google Scholar
|
|
7
|
Skottová N, Kazdová L, Oliyarnyk O, Vecera
R, Sobolová L and Ulrichová J: Phenolics-rich extracts from Silybum
marianum and Prunella vulgaris reduce a high-sucrose diet induced
oxidative stress in hereditary hypertriglyceridemic rats. Pharmacol
Res. 50:123–130. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cook NC and Samman S: Flavonoids -
chemistry, metabolism, cardioprotective effects, and dietary
sources. J Nutr Biochem. 7:66–76. 1996. View Article : Google Scholar
|
|
9
|
Lu J, Qin R and Ye S: Prunnela vulgaris L.
extract improves cellular immunity in MDR-TB challenged rats. J Med
Coll PLA. 26:230–237. 2011. View Article : Google Scholar
|
|
10
|
Zheng J, He J, Ji B, Li Y and Zhang X:
Antihyperglycemic activity of Prunella vulgaris L. in
streptozotocin-induced diabetic mice. Asia Pac J Clin Nutr.
16(Suppl 1): 427–431. 2007.PubMed/NCBI
|
|
11
|
Zhang KJ, Zhang MZ, Wang QD and Liu WL:
The experimental research about the effect of Prunella vulgaris L.
on Raji cells growth and expression of apoptosis related protein.
Zhong Yao Cai. 29:1207–1210. 2006.(In Chinese).
|
|
12
|
Collins NH, Lessey EC, DuSell CD,
McDonnell DP, Fowler L, Palomino WA, Illera MJ, Yu X, Mo B, Houwing
AM and Lessey BA: Characterization of antiestrogenic activity of
the Chinese herb, prunella vulgaris, using in vitro and in vivo
(Mouse Xenograft) models. Biol Reprod. 80:375–383. 2009. View Article : Google Scholar :
|
|
13
|
Takahashi O, Okushiba S, Kondo S, Morikawa
T, Hirano S, Miyamoto M, Shichinohe T, Hara T, Kawarada Y, Saito K
and Takeuchi M: Esophageal pemphigus vulgaris with carcinoma:
postoperative steroid therapy based on pemphigus-related
antibodies. Dis Esophagus. 18:413–417. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhao AG, Li T, You SF, et al: Effects of
Wei Chang An on expression of multiple genes in human gastric
cancer onto nude mice. World J Gastroenterol. 14:693–700. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liang HQ, Xing YM, Chen J, Zhang D, Guo S
and Wang C: Antimicrobial activities of endophytic fungi isolated
from Ophiopogon japonicus (Liliaceae). BMC Complement Altern Med.
12:2382012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Santiago C, Sun L, Munro MH and Santhanam
J: Polyketide and benzopyran compounds of an endophytic fungus
isolated from Cinnamomum mollissimum: biological activity and
structure. Asian Pac J Trop Biomed. 4:627–632. 2014.PubMed/NCBI
|
|
17
|
Kim S, Shin DS, Lee T and Oh KB:
Periconicins, two new fusicoccane diterpenes produced by an
endophytic fungus Periconia sp with antibacterial activity. J Nat
Prod. 67:448–450. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Vaz AB, Mota RC, Bomfim MR, Vierira ML,
Zani CL, Rosa CA and Rosa LH: Antimicrobial activity of endophytic
fungi associated with Orchidaceae in Brazil. Can J Microbiol.
55:1381–1391. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rasul A, Khan M, Yu B, Ma T and Yang H:
Xanthoxyletin, a coumarin induces S phase arrest and apoptosis in
human gastric adenocarcinoma SGC-7901 cells. Asian Pac J Cancer
Prev. 12:1219–1223. 2011.PubMed/NCBI
|
|
20
|
Yu J, Guo QL, You QD, Zhao L, Gu HY, Yang
Y, Zhang HW, Tan Z and Wang X: Gambogic acid-induced G2/M phase
cell-cycle arrest via disturbing CDK7-mediated phosphorylation of
CDC2/p34 in human gastric carcinoma BGC-823 cells. Carcinogenesis.
28:632–638. 2007. View Article : Google Scholar
|
|
21
|
Xian XS, Park H, Cho YK, Lee IS, Kim SW,
Choi MG, Chung IS, Han KH and Park JM: Effect of a synthetic
cannabinoid agonist on the proliferation and invasion of gastric
cancer cells. J Cell Biochem. 110:321–332. 2010.PubMed/NCBI
|
|
22
|
Chua CW, Lee DT, Ling MT, Zhou C, Man K,
Ho J, Chan FL, Wang X and Wong YC: FTY720, a fungus metabolite,
inhibits in vivo growth of androgen independent prostate cancer.
Int J Cancer. 117:1039–1048. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shin IS, Jeon WY, Shin HK, Cha SW and Lee
MY: Banhabaekchulchunma-tang, a traditional herbal formula
attenuates absolute ethanol-induced gastric injury by enhancing the
antioxidant status. BMC Complement Altern Med. 13:1702013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Singh NP and Lai H: Selective toxicity of
dihydroartemisinin and holotransferrin toward human breast cancer
cells. Life Sci. 70:49–56. 2001. View Article : Google Scholar
|
|
25
|
Lin W, Zheng L, Zhuang Q, et al: Spica
prunellae promotes cancer cell apoptosis inhibits cell
proliferation and tumor angiogenesis in a mouse model of colorectal
cancer via suppression of stat 3 pathway. BMC Complement Altern
Med. 13:1442013. View Article : Google Scholar
|
|
26
|
Zhang JF, Zhang JG, Kuai XL, Zhang H,
Jiang W, Ding WF, Li ZL, Zhu HJ and Mao ZB: Reactivation of the
homeotic tumor suppressor gene CDX2 by
5-aza-2′-deoxycytidine-induced demethylation inhibits cell
proliferation and induces caspase-independent apoptosis in gastric
cancer cells. Exp Ther Med. 5:735–741. 2013.PubMed/NCBI
|
|
27
|
Yi B, Liu D, He M, Li Q, Liu T and Shao J:
Roles of the ROS/AMPK signaling pathway in
tetramethylpyrazine-induced apoptosis in gastric cancer cells.
Oncol Lett. 6:583–589. 2013.PubMed/NCBI
|
|
28
|
Yang B, Johnson TS, Thomas GL, Watson PF,
Wagner B, Furness PN and EI Nahas AM: A shift in the Bax/Bcl-2
balance may activate caspase-3 and modulate apoptosis in
experimental glomerulonephritis. Kidney Int. 62:1301–1313. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang X, Li L, Wang B and Xiang J: Effects
of ursolic acid on the proliferation and apoptosis of human ovarian
cancer cells. J Huazhong Univ Sci Technolog Med Sci. 29:761–764.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hung KC, Hsieh PM, Yang KL, Lin KJ, Chen
YS and Hung CH: Effect of thalidomide on the expression of vascular
endothelial growth factor in a rat model of liver regeneration.
Oncol Lett. 5:852–856. 2013.PubMed/NCBI
|