Introduction
Myofibroblasts are a type of interstitial spindle
cell with the ultrastructural characteristics of both fibroblasts
and smooth muscle cells. Myofibroblasts were first identified
electromagnetically by Gabbiani et al (1) in 1971. Montgomery et al
(2) initially reported
myofibroblastic sarcoma (MS) in 2001. MS originates from
undifferentiated mesenchymal cells with the potential to
differentiate into fibrocytes and histiocytes. According to the
classification of the World Health Organization 2002,
myofibrosarcoma was defined as low-grade MS (LGMS) (3). LGMS is a neoplasm of atypical
myofibroblasts with fibromatoses like features and a predilection
for head and neck sites (4). LGMS
is rare in clinical practice and can occur at any site. The head
and neck, extremities and femoral bone are the most common sites of
origin (5–8), whereas occurrence in the abdominal
cavity is extremely rare and thus, only a small number of cases
have been reported in the literature (9–14).
To the best of our knowledge, no cases of MS of the
liver have been reported. The current study presents the
pathological observations, as well as the clinical manifestations,
biochemical blood test results and imaging results of a patient
with MS in the right posterior liver. Written informed consent for
the publication of this study was obtained from the patient.
Case report
A 38 year old female presented to the Clinic of
General Surgery, Department of Infectious Diseases, The First
Affiliated Hospital of Xiamen University (Xiamen, China), after
experiencing backache for three months previously. The patient was
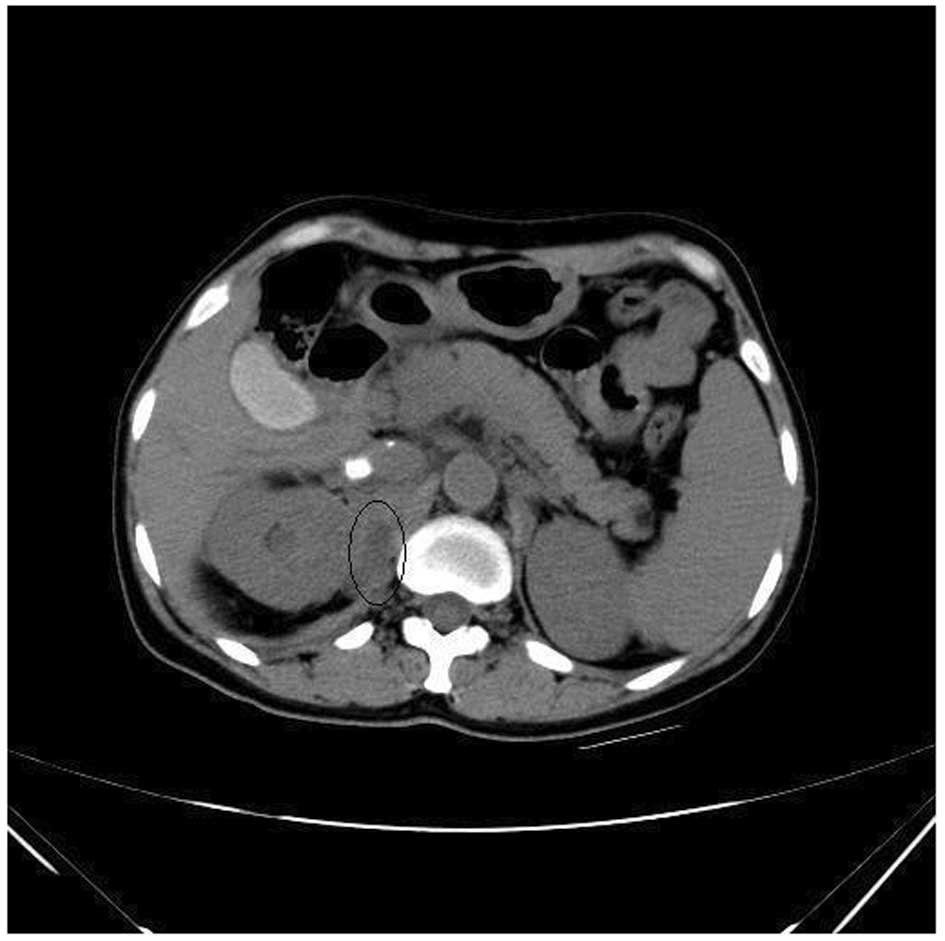
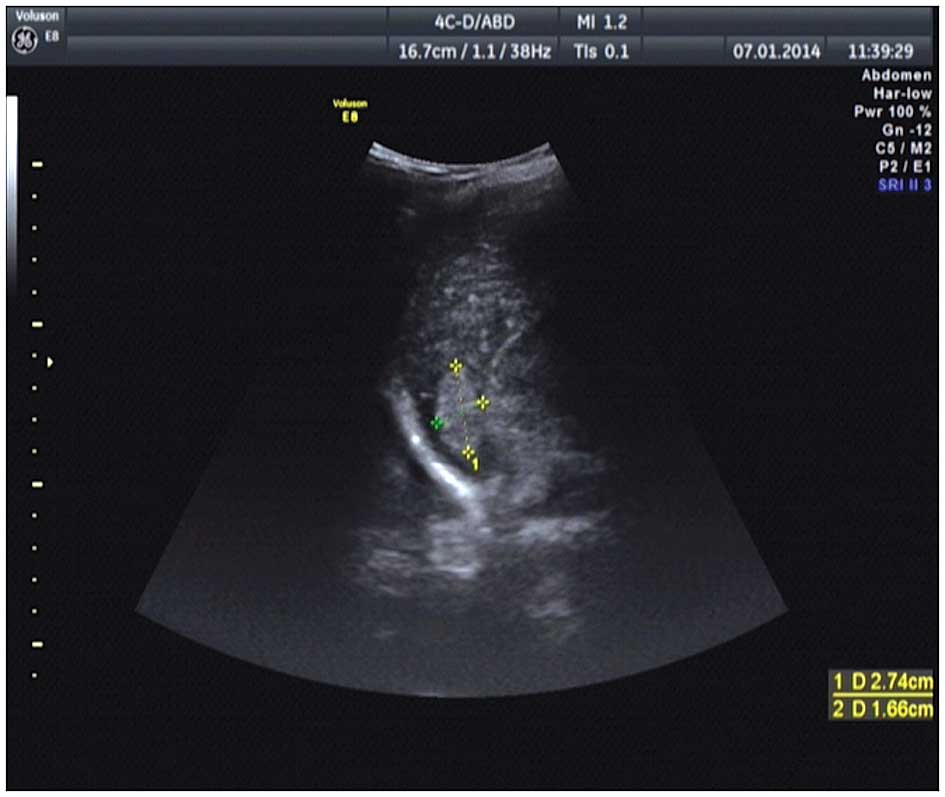
diagnosed with a retroperitoneal mass following computed tomography
(CT) and ultrasonic tests (Figs. 1
and 2), which was removed
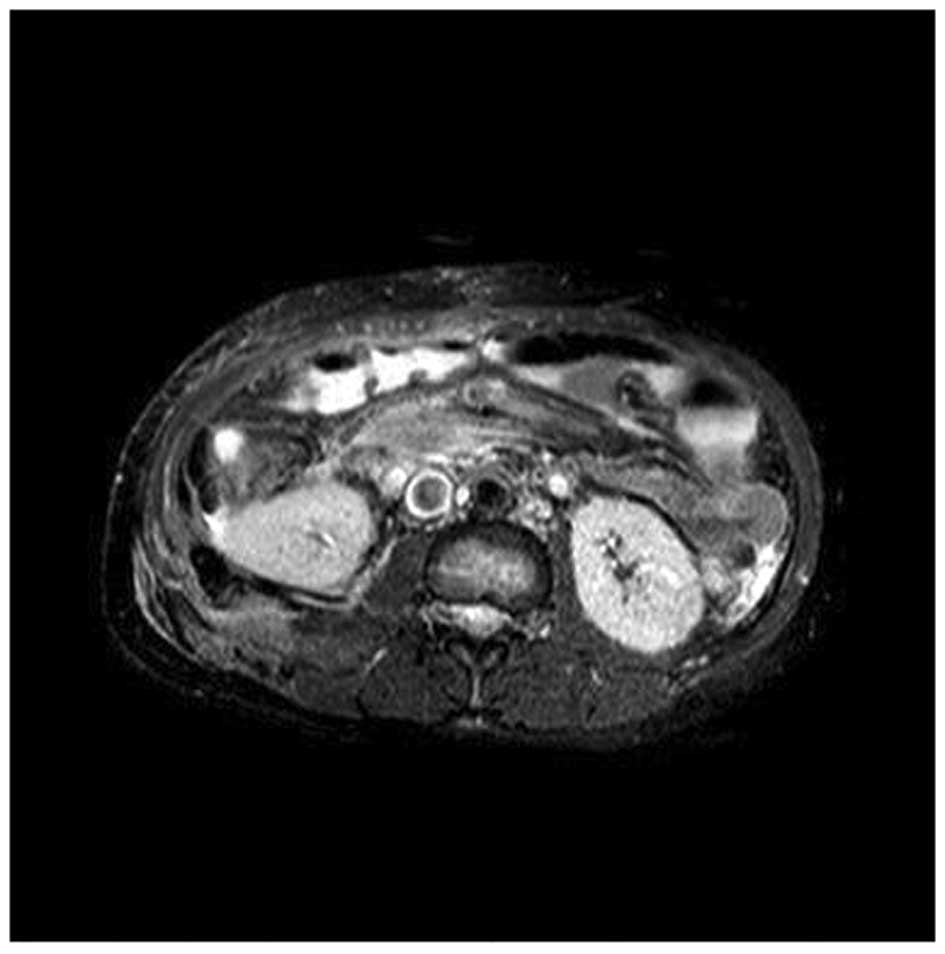
surgically. Postoperative magnetic resonance imaging (Fig. 3) and pathology revealed an
inflammatory myofibroblastic tumor, borderline type, according to
the World Health Organization Classification of Tumors (3).
Following surgery, the patient was treated with
reduced glutathione (300 mg/day) and phosphatidylcholine (200
mg/day) for a month. A liver function test one month after surgery
revealed total bilirubin (TBIL) levels of 13.8 μmol/l (normal
range, 0–20.5 μmol/l), alanine aminotransferase (ALT) levels of 78
μ/l (normal range, 0–45 U/l), aspartate aminotransferase (AST)
levels of 105 μ/l (normal range, 0–35 U/l), alkaline phosphatase
(ALP) levels of 458 μ/l (normal range, 40–150 U/l), and gamma
glutamyltransferase (GGT) levels of 225 μ/l (normal range, 0–50
U/l). The patient exhibited no symptoms of fever, jaundice, nausea,
loss of appetite, fatigue or diarrhea, however, no significant
improvement in liver function was observed when compared with that
prior to surgery. Three months following surgery, the patient’s
liver function was evaluated, which revealed TBIL levels of 16.1
μmol/l, ALT levels of 165 μ/l, AST levels of 127 μ/l, GGT levels of
438 μ/l and ALP levels of 1426 μ/l. The patient was transferred
from surgical clinic to our department (the Department of
Infectious Diseases, The First Affiliated Hospital of Xiamen
University), and was admitted with a diagnosis of liver damage of
unknown origin.
Physical examination on admission to our department
revealed that the patient showed no mental or intellectual
abnormalities, with no jaundice of the skin or sclera and no liver
palm or spider angiomas. Lung sounds were clear, with no dry or
moist rales. The patient’s heart rhythm was regular and no murmurs
were heard. The abdomen was flat and soft and a diagonal surgical
incision 20 cm in length was identified on the abdomen. No
tenderness or rebound tenderness was identified. The liver was
palpable under the inferior margin of the rib. However, xiphoid
bone and the spleen were not palpable under the left rib. The
patient was negative for Murphy syndrome and no shifting dullness
of the abdomen or swelling of the lower extremities was identified.
Levels of α-fetoprotein (AFP) were <1.0 ng/ml (normal range, 0–9
ng/ml). Levels of AFP glycosylation heterogeneity were normal
(<10%). Tests for viral markers of hepatitis A, B, C, D and E
were negative. Furthermore, antibody profiles of autoimmune liver
disease and auto-antibodies were negative: IgG, 22.200 g/l; IgA,
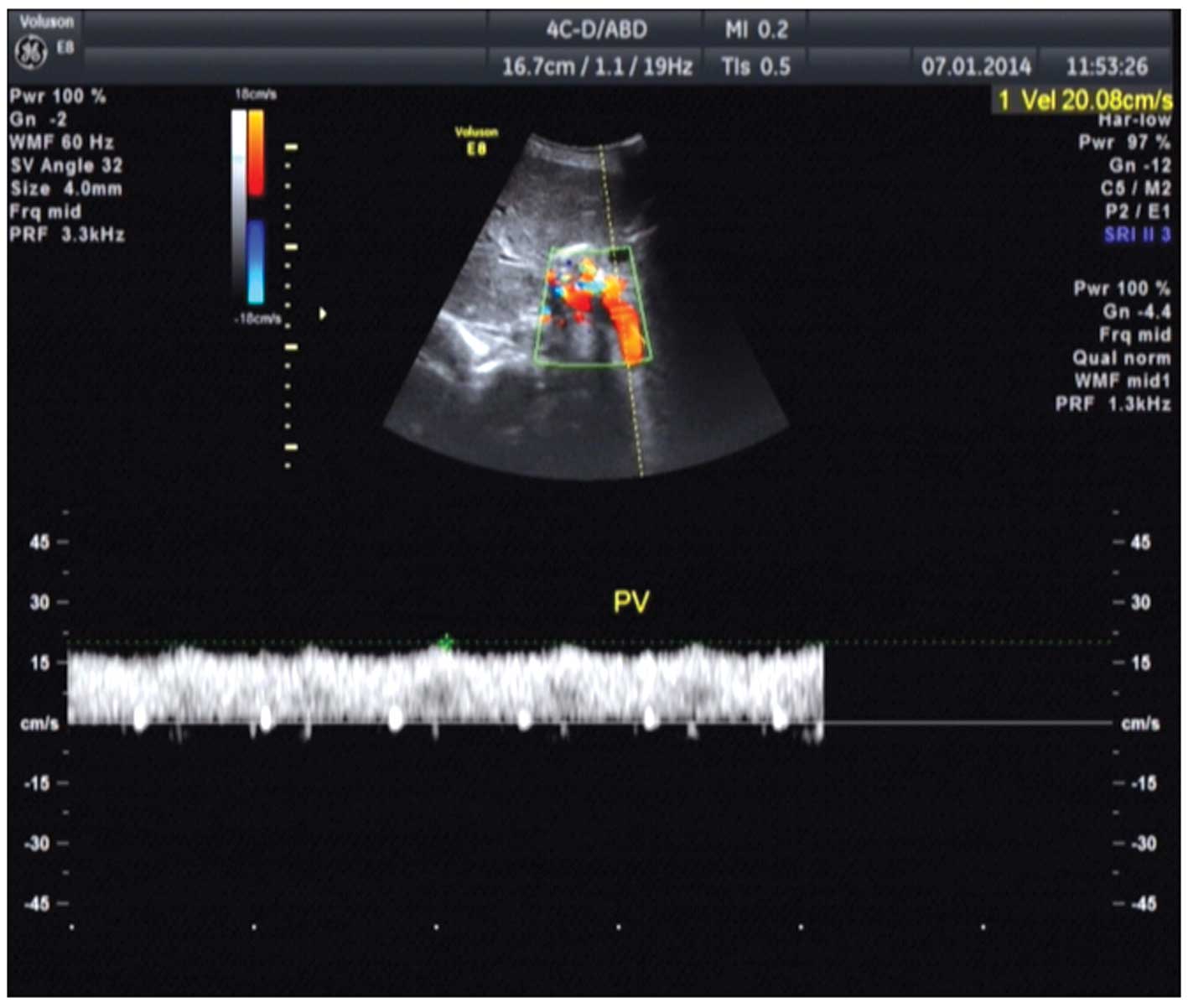
3.8200 g/l; IgM, 2.4200 g/l; and IgG2, 11.0 g/l. Color ultrasound
of the upper abdomen showed diffuse disease of the hepatic
parenchyma (Fig. 4). Plain and
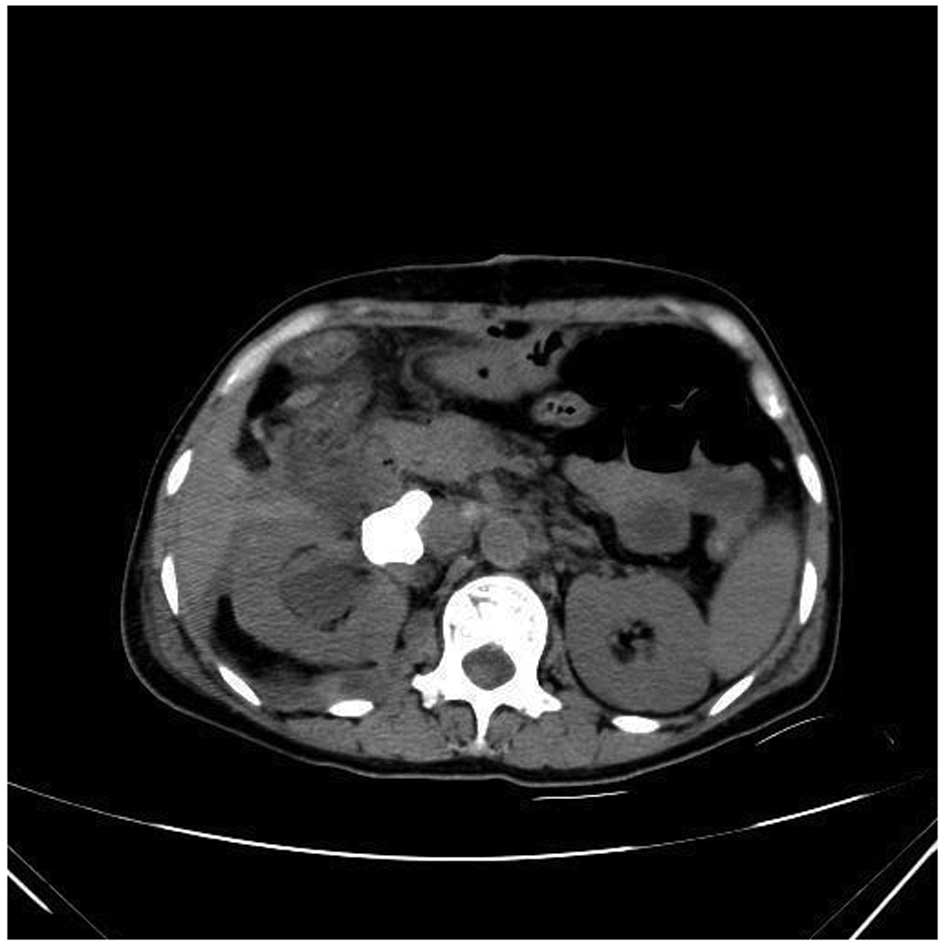
enhanced CT scans of the liver revealed a marginal reduction in
volume and cavernous hemangioma in the right posterior liver
(Fig. 5). Liver biopsy was
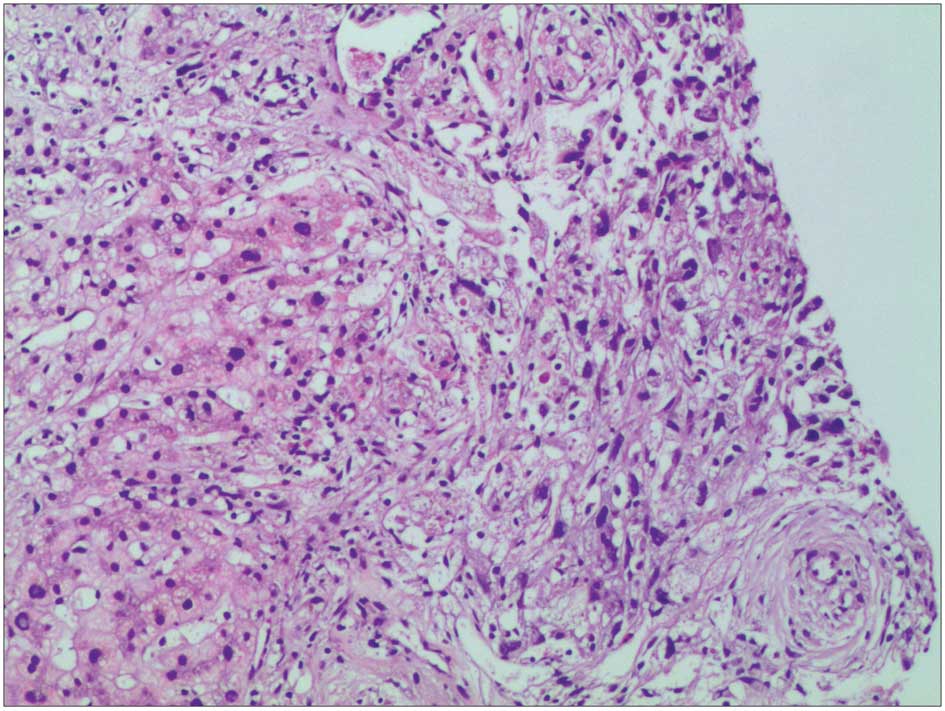
performed to clarify the nature of the lesion (Fig. 6). Pathological observation revealed
widespread infiltration of spindle-shaped cells, with abundant and
eosinophilic cytoplasm, unclear cell borders and low and moderate
range atypia of the nuclei. A small number of lymphocytes and
eosinophilic granulocytes were also observed in the liver tissue.
In the surrounding liver tissue lesions, the dividing lines of
liver lobules were clear, and edema and spotted and focal necrosis
was present in a small number of liver cells. The portal area had
marginally expanded and the infiltration of lymphocytes and
eosinophilic granulocytes was identified. Immunohistochemical
analysis revealed positivity for smooth muscle actin, CD99, Bcl-2,
P53, calponin and vimentin and negativity for cytokeratin (CK),
CK8, glypican-3, Hep, myoglobin, myosin, desmin, S100, HMB45,
CD117, CD34 and anaplastic lymphoma kinase. MASSON + reticular
fiber staining revealed a mesh stent in the hepatic lobule, with a
clear structure and slight hyperplasia of fibrous tissue in the
portal area. Pathological diagnosis indicated infiltration of low
grade MS in the liver. After diagnosis was confirmed, the patient
was transferred to the Department of Surgical Oncology, The First
Affiliated Hospital of Xiamen University for further treatment. The
patient refused further treatment and was lost to follow-up three
months after discharge from the hospital.
Discussion
Myofibroblasts are a type of interstitial spindle
cell with the ultrastructural characteristics of both fibroblasts
and smooth muscle cells. They occur in a number of normal tissues
and certain benign lesions, including infection and granulation
tissue (15). In the current case,
the pathology of the retroperitoneal mass indicated inflammatory
myofibroblastic tumor (IMT), which is a tumor of intermediate type.
No recurrence of the retroperitoneal mass was identified in the
follow-up. Furthermore, no tumors were detected in the
intra-abdominal organs, and the retroperitoneal IMT was a different
type of tumor to LGMS. The patient initially presented with
abnormal liver function, which was characterized by a marginal
increase in ALT and AST levels and a significant increase in ALP,
GGT and ALP levels.
In this case report, the female patient exhibited no
evidence of viral hepatitis or liver disease induced by alcohol or
drugs. A significant increase in ALP and GGT levels may lead to
misdiagnosis of autoimmune liver disease. Liver iconography of the
patient in the present report revealed no definite signs of space
occupying lesions. Infiltration of tumor cells is easily detectable
in routine liver biopsy, which may reveal widespread infiltration
of tumor cells in the whole liver. However, at present, there are
few reports of this type of liver lesion in the literature.
Further studies are required to investigate the
bionomics of LGMS due to its rarity in clinical practice. It
remains unclear whether the abnormality of liver function was
caused by the tumor cells directly or by hepatic tissue destroyed
by tumor cells. Therefore, the association between IMT and LGMS and
tumorigenicity requires further study (16).
References
|
1
|
Gabbiani G, Ryan GB and Majne G: Presence
of modified fibroblasts ingranulation tissue and their possible
role in wounded contraction. Experientia. 27:549–550. 1971.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Montgomery E, Goldblum JR and Fisher C:
Myofibrosarcoma: a clinicopathologic study. Am J Surg. 25:219–228.
2001.
|
|
3
|
Fetcher CD, Unni KK, Mertens F, et al:
World Health Organization Classification of Tumors: Pathology and
Genetics of Soft Tissue and Bone. IARC Press; Lyon, France: pp.
101–103. 2002
|
|
4
|
Jay A, Piper K, Farthing PM, Carter J and
Diwakar A: Low-grade myofibroblastic sarcoma of the tongue. Oral
Surg Oral Med Oral Pathol Oral Radiol Endod. 104:e52–e58. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Meng GZ, Zhang HY, Bu H, et al:
Myofibroblastic sarcoma: a clinicopathological study of 20 cases.
Chin Med J (Engl). 120:363–369. 2007.
|
|
6
|
Andersen ND, DiBernardo LR, Linardic CM,
Camitta MG and Lodge AJ: Recurrent inflammatory myofibroblastic
tumor of the heart. Circulation. 125:2379–2381. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Devaney KO, Lafeir DJ, Triantafyllou A, et
al: Inflammatory myofibroblastic tumors of the head and neck:
evaluation of clinicopathologic and prognostic features. Eur Arch
Otorhinolaryngol. 269:2461–2465. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Takahama A Jr, Nascimento AG, Brum MC, et
al: Low-grade myofibroblastic sarcoma of the parapharyngeal space.
Int J Oral Maxillofac Surg. 35:965–968. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mclaughlin SA, Sehmitt T, Huguet KL, et
al: Myofibrosarcoma of the adrenal gland. Am Surg. 71:191–193.
2005.PubMed/NCBI
|
|
10
|
Miyazawa M, Naritaka Y, Miyaki A, et al: A
low-grade myofibroblastic sarcoma in the abdominal cavity.
Anticancer Res. 31:2989–2994. 2011.PubMed/NCBI
|
|
11
|
Sari A, Tunakan M, Ünsal B, et al:
Inflammatory pseudotumor of the liver diagnosed by needle biopsy:
report of three cases (one with neuroendocrine tumor of the rectum
and lung). Turk J Gastroenterol. 21:308–312. 2010.PubMed/NCBI
|
|
12
|
Tang L, Lai EC, Cong WM, et al:
Inflammatory myofibroblastic tumor of the liver: a cohort study.
World J Surg. 34:309–313. 2010. View Article : Google Scholar
|
|
13
|
Agaimy A, Wünsch PH and Schroeder J:
Low-grade abdominopelvic sarcoma with myofibroblastic features
(low-grade myofibroblastic sarcoma): clinicopathological,
immunohistochemical, molecular genetic and ultrastructural study of
two cases with literature review. J Clin Pathol. 61:301–306. 2008.
View Article : Google Scholar
|
|
14
|
Papachristou GI, Wu T, Marsh W and Plevy
SE: Inflammatory pseudotumor of the liver associated with Crohn’s
disease. J Clin Gastroenterol. 38:818–822. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Qiu X, Montgomery E and Sun B:
Inflammatory myofibroblastic tumor and low-grade myofibroblastic
sarcoma: a comparative study of clinicopathologic features and
further observations on the immunohistochemical profile of
myofibroblasts. Hum Pathol. 39:846–856. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xiao Y, Zhou S, Ma C, et al: Radiological
and histopathological features of hepatic inflammatory
myofibroblastic tumour: analysis of 10 cases. Clin Radiol.
68:1114–1120. 2013. View Article : Google Scholar : PubMed/NCBI
|