Introduction
Leiomyomatosis peritonealis disseminata (LPD) is a
specific type leiomyomatosis that is rarely identified by clinical
evaluation. To date, <200 cases have been reported in the
literature. Accurate diagnosis is difficult prior to surgery, as
diagnosis relies on medical history, observations during surgery
and pathological results. At present, no standard treatment for LPD
has been identified. The prognosis of LPD is good as only 9 of ~200
cases that has been reported so far have been malignant (1–8). It
has been reported that the majority of LPD patients have previously
been exposed to laparoscopic power morcellation during hysterectomy
or myomectomy for uterine fibroids (9). The Food and Drug Administration (FDA)
(10) have indicated that the use
of laparoscopic power morcellation may be associated with the
development of LPD. The present study analyzes the clinical
procedure and treatment strategy received by an LPD patient who was
treated in the Second Hospital of Jilin University (Changchun,
China) to improve our understanding of this disease. Written
informed consent was obtained from the patient.
Case report
The present study reports the case of a 34-year-old
female (gravida 1, para 0, aborta 1) who underwent laparoscopic
myomectomy for uterine fibroids at the Second Hospital of Jilin
University in August 2009. During the surgery, a myoma that was 5
cm in diameter was identified in the posterior uterine wall and was
removed using a morcellator. The post-operative pathology report
determined that the uterus was rich in leiomyoma cells.
In May 2012, the patient underwent an exploratory
laparotomy due to relapse of the uterine fibroid. During the
surgery, a myoma that was 10 cm in diameter was identified at the
uterine bladder peritoneal reflection, numerous unequally sized
leiomyoma tubercles were identified on the uterine surface and a
leiomyoma tubercle that was 2 cm in diameter was identified on the
surface of the right fallopian tube. The mass was removed from the
uterine bladder peritoneal reflection and frozen section analysis
determined a diagnosis of uterine leiomyoma, therefore, a
myomectomy was performed. Post-operative pathology determined that
the lesion was a leiomyoma and immunohistochemical staining
resulted in positivity for α-smooth muscle antibody (α-SMA),
h-caldesmon and desmin, with a Ki-67 labeling index of 5%.
In September 2013, a gynecological ultrasound
revealed a 5.0×5.0-cm mass in the left lower abdomen. As the
patient presented with no specific symptoms, the patient decided to
continue with follow-up examinations only. The patient was
hospitalized again on 6 June, 2014, following identification of a
bilateral adnexal cyst in a patient review. A gynecological
ultrasound determined that the endometrium measured 0.5 cm and that
the uterus and ovaries were of normal size. However, a
heterogeneous hypoechoic mass was identified on each ovary,
measuring 5.2×4.3 cm and 3.7×2.3 cm, respectively, and exhibiting a
regular form with clear boundaries. Furthermore, color Doppler flow
imaging revealed no abnormal blood flow signals and the mass
activity was good under gynecological examination, demonstrating no
tenderness. No abnormalities were identified in the liver, gall
bladder or spleen ultrasound examinations, heart and lung
assessments or gastrointestinal endoscopy. In addition, a cancer
antigen 125 level of 9 U/ml (normal range, <35 U/ml), a human
epididymis protein 4 level of 60.36 pM (normal range, <150 pM)
and an α-fetoprotein level of 3.27 IU/ml (normal range, <10
IU/ml) were recorded.
Based on the clinical manifestation and auxiliary
examinations, ovarian neoplasm was considered as a possible
diagnosis, therefore, an exploratory laparotomy was performed.
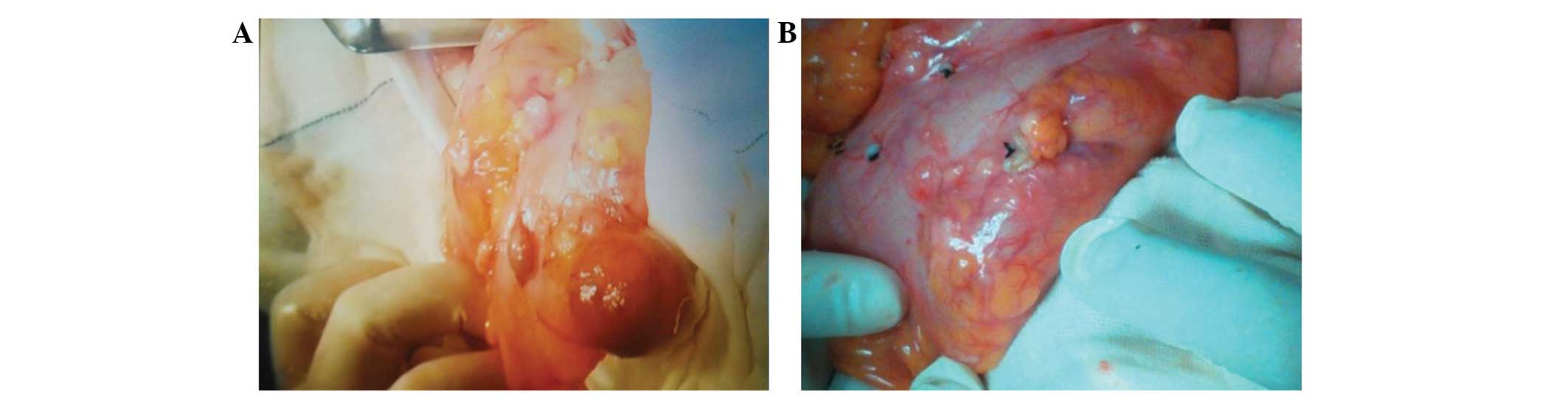
During the surgery, concentrated myoma tubercle-like cysts were
identified on the surface of the intestine and mesentery, with
sizes ranging from 0.4×0.3×0.3 cm to 5.0×4.0×3.0 cm (Fig. 1). Additionally, a 1.5-cm diameter
endometriotic cyst was identified on left ovary and a 1-cm
endometriosis lesion was identified on the left posterior
uterosacral ligaments. The cysts on the surface were partially
removed. The frozen section indicated a diagnosis of mesenchymal
tumors, however, intestinal leiomyoma could not be excluded. During
the surgery, the following diagnoses were made: Intestinal
leiomyoma, endometriotic cysts on the left ovary and pelvic
endometriosis. The majority of the leiomyoma on the intestine and
mesentery was removed, however, the myomas were compact and
therefore, not all of them could be removed. The endometriotic
cysts on the left ovary and the pelvic endometriosis lesion were
removed concurrently. Post-operative pathology determined a
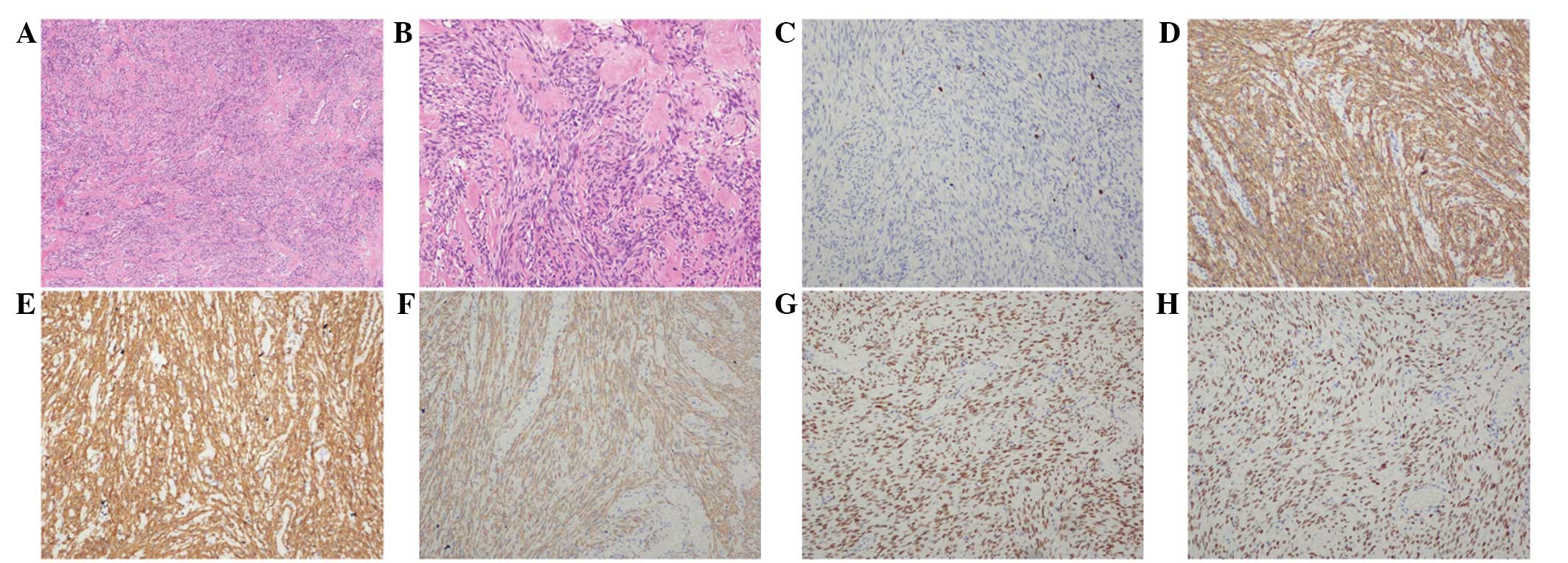
diagnosis of leiomyomatosis peritonealis disseminate, with
occasional mitosis and no necrosis (Fig. 2A and B). Immunohistochemical
staining revealed the resected tissues to be positive for
h-caldesmon, α-SMA, desmin, estrogen receptor and progesterone
receptor (PR) (Fig. 2C–H), and
negative for cluster of differentiation (CD)117, discovered on
gastrointestinal stromal tumor-1, CD34, neuron-specific enolase and
S-100, with a Ki-67 labeling index of 1%. The post-operative
procedure was successful and the patient was monitored by follow-up
every six months. Follow-up is scheduled for five years, after
which gynecological sonography examination will be performed
annually. In October 2014, a gynecological sonography examination
revealed no abnormalities and at the time of writing, the patient
remains alive and well.
Discussion
In 1952, Willson and Peale (11) reported the first case of LPD. LPD
predominantly occurs in females of reproductive age, however, the
pathogenesis of LPD is poorly understood. The condition may be
caused by changes in estrogen and progesterone levels, peritoneal
metaplasia, growth factors, iatrogenic factors or genetics
(12). LPD is benign, however, it
exhibits recurrent and malignant tendencies. LPD lesions always
appear at the omentum majus, mesentery and surface of the small
intestine, or at the colon, uterus, ovary, oviduct or pouch of
Douglas. LPD generally presents with no clinical symptoms, however,
gastralgia or abdominal distension do occasionally occur. Previous
studies, including one conducted by Larraín et al (9), propose that LPD may be caused by the
remaining uterine fibroid fragments in the pelvic cavity, as the
use of laparoscopic power morcellation during surgery could cause
the fragments to be parasitized by the blood vessels of the
peritoneum and mesentery (13).
In the present study, the patient was a female of
reproductive age, with a surgical history of laparoscopic
myomectomy for uterine fibroid, in which laparoscopic power
morcellation was used. The development of myoma at the uterine
bladder peritoneal reflection, leiomyoma tubercles on the uterine
surface identified two years previously and multiple leiomyoma on
the intestinal surface, could all be explained by the theory
suggested by Larraín et al (9). Currently available data from an
analysis conducted by the FDA (10)
estimates that ~1/350 female individuals who undergo hysterectomy
or myomectomy to treat fibroids also have an unsuspected uterine
sarcoma. Furthermore, if these female patients with unsuspected
uterine sarcomas undergo laparoscopic power morcellation during
surgery, there is an increased risk of cancerous tissue
dissemination within the abdomen and pelvis.
Therefore, in April 2014, the FDA published the
following advice: i) Laparoscopic power morcellation during
hysterectomy or myomectomy for uterine fibroids should be
discouraged; ii) laparoscopic uterine power morcellation should not
be performed in female patients with suspected or diagnosed uterine
cancer; iii) all the possible treatment strategies should be
considered for the treatment of female patients with symptomatic
uterine fibroids, and the benefits and risks of each should be
discussed with the patient; and iv) for those patients in whom
laparoscopic power morcellation is considered to be the most
appropriate therapeutic procedure, a specimen bag should be used
during morcellation, with the aim of containing the uterine tissue
and minimizing the risk of dissemination throughout the abdomen and
pelvis (10).
Currently, there is a lack of pre-operative
diagnostic methodology for LPD. Instead, intraoperative diagnosis,
and intraoperative or post-operative pathology results are
predominantly relied on to diagnose the condition.
In addition, no standard treatment currently exists
for LPD. Certain studies have considered individualized treatments.
An animal experiment demonstrated that long-term high-level
progesterone administration may cause mesenchymal stem cells to
develop into leiomyomatosis peritoneal lesions (14). However, a clinical study determined
that estrogen stimulates subcelomic mesenchymal cells to
proliferate and differentiate into myoblasts, myofibroblasts,
fibroblasts and decidua-like cells (15). Therefore, the majority of studies
propose that females of reproductive age should undergo lesion
excision and omentectomy followed by discontinuation of hormonal
disruptions (12), such as the
termination of pregnancy and oral contraceptives, or the
administration of oral gonadotropin-releasing hormone agonists
(GnRh-a) (16), aromatase
inhibitors (17) or estrogen
receptor antagonists (18). A
number of studies have reported that GnRh-a hormone therapy
following surgery will prevent the appearance of new lesions for
five years (19,20). Other studies have reported that the
tumor will itself be diminished after childbirth or discontinuation
of oral contraceptive (21,22), however, for young patients who
desire to bear children, close follow-up visits are necessary to
provide gestation guidance and to observe the change in
progesterone levels.
For those patients who do not desire to bear
children, total abdominal hysterectomy, salpingo-oophorectomy,
omentectomy and debulking may be the most appropriate alternatives
(23,24). For those patients with developing
disease who cannot undergo surgery, such as PR(-) patients with
liver or lung lesions, systemic chemotherapy may be undertaken
(25). In the present study, the
patient was a female of reproductive age who desired children. LPD
was not diagnosed during surgery by frozen section, the myoma did
not exhibit any uterus or adnexa involvement and concentrated myoma
tubercle-like cysts were identified on the surface of the
intestine; therefore, myoma resection was performed, however, the
omentum, uterus and bilateral adnexa were not removed.
A British study reported that LPD is always
associated with endometriosis (12); a possible explanation for this
association is that the two diseases may be derived from the same
cellular origin (23,25–28).
During the present patient’s third surgery, an endometriotic cyst
was identified on left ovary and an endometrial lesion was
identified on the left posterior uterosacral ligaments. As the
patient was a female of reproductive age who desired children, only
the endometriotic cyst on the left ovary and the pelvic endometrial
lesion were removed. The pathology results of the three surgeries
undertaken by the patient were all benign, however, the disease
relapsed and exhibited malignant tendencies. The pathology results
of the first surgery reported abundant uterine leiomyoma cells. In
the third surgery, the lesion exhibited intestinal and mesentery
involvement, however, not all of the leiomyoma was removed,
therefore, close follow-up examinations are required.
In conclusion, LPD is rare and difficult to
diagnose, however, the prognosis is good, as the minority become
malignant. The majority of LPD patients have a medical history of
laparoscopic myomectomy for uterine fibroid. The use of
laparoscopic power morcellation may contribute to the development
of LPD, therefore, the specific surgical approach used in
laparoscopic myomectomy should be carefully considered, and
protective measures should be taken to prevent myoma fragments
spreading if laparoscopic power morcellation is used.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (grant nos. 81272875
and 81302242), the Jilin Science and Technology Fund (grant nos.
20110755, 20130102094JC and 20140204022YY) and the Basic Science
Research Fund of Jilin University (grant no. 20142116).
References
|
1
|
Akkersdijk GJ, Flu PK, Giard RW, van Lent
M and Wallenburg HC: Malignant leiomyomatosis peritonealis
disseminata. Am J Obstet Gynecol. 163:591–593. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Alaniz Sanchez A and Castañeda Delgado Y:
Disseminated peritoneal leiomyomatosis with malignant degeneration.
A case report. Ginecol Obstet Mex. 62:336–340. 1994.(In Spanish).
PubMed/NCBI
|
|
3
|
Raspagliesi F, Quattrone P, Grosso G,
Cobellis L and Di Re E: Malignant degeneration in leiomyomatosis
peritonealis disseminata. Gynecol Oncol. 61:272–274. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Borsellino G, Zante P and Ciraldo MC:
Diffuse peritoneal leiomyomatosis. A clinical case report. Minerva
Ginecol. 49:53–57. 1997.(In Italian). PubMed/NCBI
|
|
5
|
Yamaguchi T, Imamura Y, Yamamoto T and
Fukuda M: Leiomyomatosis peritonealis disseminata with malignant
change in a man. Pathol Int. 53:179–185. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sharma P, Chaturvedi KU, Gupta R and Nigam
S: Leiomyomatosis peritonealis disseminata with malignant change in
a post-menopausal woman. Gynecol Oncol. 95:742–745. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yu RS, Wang ZK, Sun JZ and Chen LR:
Computed tomography of pancreatic implantation with malignant
transformation of leiomyomatosis peritonealis disseminata in a man.
Dig Dis Sci. 52:1954–1957. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lamarca M, Rubio P, Andrés P and Rodrigo
C: Leiomyomatosis peritonealis disseminata with malignant
degeneration. A case report. Eur J Gynaecol Oncol. 32:702–704.
2011.
|
|
9
|
Larraín D, Rabischong B, Khoo CK,
Botchorishvili R, Canis M and Mage G: “Iatrogenic” parasitic
myomas: unusual late complication of laparoscopic morcellation
procedures”. J Minim Invasive Gynecol. 17:719–724. 2010. View Article : Google Scholar
|
|
10
|
US Food and Drug Administration.
Laparoscopic uterine power morcellation in hysterectomy and
myomectomy: FDA safety communication. http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm393576.htm.
Accessed April 17, 2014
|
|
11
|
Willson JR and Peale AR: Multiple
peritoneal leiomyomas associated with a granulosa-cell tumor of the
ovary. Am J Obstet Gynecolo. 64:204–208. 1952.
|
|
12
|
Al-Talib A and Tulandi T: Pathophysiology
and possible iatrogenic cause of leiomyomatosis peritonealis
disseminata. Gynecolo Obstet Invest. 69:239–244. 2010. View Article : Google Scholar
|
|
13
|
Kumar S, Sharma JB, Verma D, Gupta P, Roy
KK and Malhotra N: Disseminated peritoneal leiomyomatosis: an
unusual complication of laparoscopic myomectomy. Arch Gynecol
Obstet. 278:93–95. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fujii S: Experimental approach to
leiomyomatosis peritonealis disseminata - progesterone-induced
smooth muscle-like cells in the subperitoneal nodules produced by
estrogen. Acta Obstet Gynaecol Jpn. 33:671–680. 1981.(In Japanese).
PubMed/NCBI
|
|
15
|
Tavassoli FA and Norris HJ: Peritoneal
leiomyomatosis (leiomyomatosis peritonealis disseminata): a
clinicopathologic study of 20 cases with ultrastructural
observations. Int J Gynecol Pathol. 1:59–74. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hales HA, Peterson CM, Jones KP and Quinn
JD: Leiomyomatosis peritonealis disseminata treated with a
gonadotropin-releasing hormone agonist. A case report. Am J Obstet
Gynecol. 167:515–516. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Takeda T, Masuhara K and Kamiura S:
Successful management of a leiomyomatosis peritonealis disseminata
with an aromatase inhibitor. Obstet Gynecol. 112:491–493. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Parente JT, Levy J, Chinea F, Espinosa B
and Brescia MJ: Adjuvant surgical and hormonal treatment of
leiomyomatosis peritonealis disseminata. A case report. J Reprod
Med. 40:468–470. 1995.PubMed/NCBI
|
|
19
|
Bisceglia M, Galliani CA, Pizzolitto S, et
al: Selected case from the Arkadi M. Rywlin International Pathology
Slide series: Leiomyomatosis peritonealis disseminata: report of 3
cases with extensive review of the literature. Adv Anat Pathol.
21:201–215. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sobiczewski P, Bidziński M, Radziszewski
J, Panek G, Olszewski W and Tacikowska M: Disseminated peritoneal
leiomyomatosis - case report and literature review. Ginekol Pol.
75:215–220. 2004.(In Polish). PubMed/NCBI
|
|
21
|
Hoynck van Papendrecht HP and Gratama S:
Leiomyomatosis peritonealis disseminata. Eur J Obstet Gynecol
Reprod Biol. 14:251–259. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Buttner A, Bässler R and Theele C:
Pregnancy-associated ectopic decidua (deciduosis) of the greater
omentum. An analysis of 60 biopsies with cases of fibrosing
deciduosis and leiomyomatosis peritonealis disseminata. Pathol Res
Pract. 189:352–359. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Heinig J, Neff A, Cirkel U and
Klockenbusch W: Recurrent leiomyomatosis peritonealis disseminata
after hysterectomy and bilateral salpingo-oophorectomy during
combined hormone replacement therapy. Eur J Obstet Gynecol Reprod
Biol. 111:216–218. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ghosh K, Dorigo O, Bristow R and Berek J:
A radical debulking of leiomyomatosis peritonealis disseminata from
a colonic obstruction: a case report and review of the literature.
J Am Coll Surg. 191:212–215. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lin YC, Wei LH, Shun CT, Cheng AL and Hsu
CH: Disseminated peritoneal leiomyomatosis responds to systemic
chemotherapy. Oncology. 76:55–58. 2009. View Article : Google Scholar
|
|
26
|
Valente PT: Leiomyomatosis peritonealis
disseminata. A report of two cases and review of the literature.
Arch Pathol Lab Med. 108:669–672. 1984.PubMed/NCBI
|
|
27
|
Guarch R, Puras A, Ceres R, Isaac MA and
Nogales FF: Ovarian endometriosis and clear cell carcinoma,
leiomyomatosis peritonealis disseminata, and endometrial
adenocarcinoma: an unusual, pathogenetically related association.
Int J Gynecol Pathol. 20:267–270. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Haberal A, Kayikcioglu F, Caglar GS and
Cavusoglu D: Leiomyomatosis peritonealis disseminata presenting
with intravascular extension and coexisting with endometriosis: a
case report. J Reprod Med. 52:422–424. 2007.PubMed/NCBI
|