Introduction
The mechanism of carcinoma resistance to
chemoradiotherapy may involve multiple factors. Among these,
hypoxia is an important factor in the chemoresistance of head and
neck carcinomas, such as oral squamous cell carcinoma and
nasopharyngeal carcinoma (1,2). Under
conditions of hypoxic stress, carcinoma cells require more energy
to support cell proliferation. Glucose is an important source of
energy. Therefore, increasing glucose transporter-1 (GLUT-1)
activity is one of the most important ways to increase the cellular
influx of glucose (3). In our
previous study, it was demonstrated that increased GLUT-1
expression was an independent predictor of survival in patients
with laryngeal carcinoma (4). Thus,
GLUT-1 may present a novel therapeutic target in laryngeal
carcinoma (5,6). However, few studies have investigated
GLUT-1 expression and tumor drug resistance (7–10).
Another important factor in tumor resistance to
chemotherapy is intrinsic chemotherapy resistance (11–14).
Various drug transporter proteins inside tumor cells are involved
in intrinsic chemotherapy resistance, including P-glycoprotein
(P-gp), multidrug resistance-associated protein (MRP) and
glutathione-s-transferase-π (GST-π). These drug transporters are
overexpressed in a number of cancer types, such as liver cancer,
lung cancer, glioma and gallbladder cancer (11–14),
and overexpression of these proteins is associated with hypoxia
(15,16). In the present study, the expression
of GLUT-1, P-gp, MRP1 and GST-π in laryngeal carcinomas was
investigated by immunohistochemistry (IHC). The present study
investigated the correlations between the expression of these
proteins, with respect to various clinical and pathological
features of laryngeal carcinoma.
Materials and methods
Patients and tissues
A total of 34 paraffin-embedded archival tissue
blocks from laryngeal squamous cell carcinoma patients were
obtained from The Second Hospital of Shaoxing City (Shaoxing,
China) between May 2005 and January 2012. A total of 34
paraffin-embedded archival tissue blocks from patients with
precancerous lesions were also obtained from The First Affiliated
Hospital, College of Medicine, Zhejiang University (Zhejiang,
China). A representative paraffin block from each tumor was
selected for immunohistochemical analysis. The diagnosis was
confirmed after all hematoxylin and eosin-stained sections were
reviewed blindly. No patients had received preoperative
radiotherapy or chemotherapy. Demographic and clinicopathological
data, including gender, age, tumor-node-metastasis (TNM) stage were
retrospectively collected. The study protocol was approved by the
institutional review board of The Second Hospital of Shaoxing City
and The First Affiliated Hospital, College of Medicine, Zhejiang
University and all patients provided consent.
IHC
Formalin-fixed and paraffin-embedded tissue blocks
from primary lesions were cut into 4-μm sections, and
representative sections were analyzed immunohistochemically using
an EliVision™ Plus IHC kit (Fuzhou Maixin Biotechnology Development
Co., Ltd., Fuzhou, China) for GLUT-1 (cat. no. ab14683; 1:50)
rabbit polyclonal; a mouse monoclonal antibody against P-gp (cat.
no. ab3366; 1:100), a mouse monoclonal antibody against MRP1 (cat.
no. ab63987; 1:100), and a mouse monoclonal antibody against GST-π
(cat. no. ab131059; 1:50; all antibodies purchased from Abcam,
Cambridge, MA, USA). Primary antibodies were applied for 1 h at
room temperature, and then sections were washed three times with
0.05 mol/l Tris-buffered saline (pH 7.2) and incubated with 50 μl
of polymer enhancer (Fuzhou Maixin Biotechnology Development Co.,
Ltd.) for 20 min. This was followed by incubation with 50 μl
polymerized horseradish peroxidase-conjugated anti-mouse
immunoglobulin G (Fuzhou Maixin Biotechnology Development Co.,
Ltd.) for 30 min at room temperature. GLUT-1 expression was
considered positive if distinct membrane staining was identified.
P-gp, MRP1, and GST-π were identified in the membrane and/or
cytoplasm. Protein analysis was performed in 10 random high-power
fields; a total of 100 tumor cells were counted from each
high-power field for each case and for all antibodies analyzed. The
percentage of positive cells was calculated by dividing the number
of positive tumor cells by the total number of tumor cells counted.
Staining intensity was scored as follows: Negative staining (−),
<10% cells were stained positive; weak staining (+), ≥10 but
<25% cells were stained positive; moderate staining (++), ≥25
but <75% cells were stained positive; and intense staining
(+++), 75–100% cells were stained positive.
Statistical analysis
Associations between GLUT-1, P-gp, MRP1 and GST-π
immunostaining and other parameters were analyzed using the
χ2 and Fisher’s exact tests. P<0.05 was considered to
indicate a statistically significant difference. The associations
between GLUT-1 and P-gp, MRP1 and GST-π were analyzed by Spearman’s
correlation. Overall survival, which was defined as the time from
surgery until mortality from any cause, was plotted as a
Kaplan-Meier curve. Univariate survival analysis was performed
using the log-rank test and multivariate analysis was performed
using Cox proportional-hazards regression analysis. All analyses
were conducted using SPSS version 19.0 software (SPSS, Inc.,
Chicago, IL, USA).
Results
Patient characteristics
All patients had squamous cell carcinoma. The
subjects included 33 males and one female with a mean age of 62.1
years (range, 45–76 years). A total of 28 (82.4%), five (14.7%),
and one (2.9%) patients had tumors located in the glottis,
supraglottis and subglottis, respectively. A total of 25 patients
received partial laryngetomy (21 vertical partial laryngetomies and
four supraglottic partial laryngetomies) and nine patients received
total laryngetomy in addition to postoperative radiotherapy. TNM,
clinical stage and other clinopathological parameters of the
patients are shown in Table I. Six
patients were lost to follow-up. Seven patients (20.6%) developed
local recurrence and two (5.9%) developed distant metastases.
Twenty-two patients were alive at the last follow-up (December
2012). The three- and five-year cumulative survival rates were 76.0
and 61.0%, respectively.
 | Table IClinicopathological parameters and
GLUT-1, P-gp, MRP, and GST-π expression in 34 laryngeal
carcinomas. |
Table I
Clinicopathological parameters and
GLUT-1, P-gp, MRP, and GST-π expression in 34 laryngeal
carcinomas.
| Pt | Gender | Age (years) | Location | DIF | TNM stage | Treatment | Clinical
stagea | DM | Follow-up | GLUT-1 | MRP1 | P-gp | GST-π |
|---|
| 1 | M | 70 | Glottis | W-M | T1N0M0 | VPL | I | No | 12 months, lost | − | − | − | − |
| 2 | M | 61 | Glottis | W | T4N1M0 | TL+PR | IV | Yes | 24 months, DOD | + | + | + | + |
| 3 | M | 60 | Glottis | W-M | T2N0M0 | VPL | II | No | 81 months, alive | + | + | − | − |
| 4 | M | 68 | Glottis | W-M | T3N0M0 | VPL | III | No | 23 months, DOD | + | − | − | + |
| 5 | M | 55 | Subglottis | W | T3N1M0 | TL+PR | III | No | 78 months, alive | + | + | + | + |
| 6 | M | 72 | Supraglottis | M | T3N0M0 | TL+PR | III | No | 76 months, alive | + | + | − | + |
| 7 | M | 76 | Glottis | M | T3N1M0 | TL+PR | III | No | 15 months, DOD | + | + | + | + |
| 8 | M | 63 | Glottis | W | T3N0M0 | VPL | III | No | 71 months, alive | + | + | + | + |
| 9 | M | 54 | Supraglottis | M | T2N1M0 | SL+PR | III | No | 69 months, alive | − | − | − | + |
| 10 | M | 50 | Glottis | W-M | T2N0M0 | VPL | II | No | 43 months, lost | − | − | − | − |
| 11 | M | 68 | Glottis | W | T2N0M0 | VPL | II | No | 56 months, lost | − | + | − | − |
| 12 | M | 55 | Supraglottis | M | T2N0M0 | SL+PR | II | No | 65 months, alive | − | + | − | + |
| 13 | M | 76 | Glottis | W | T1N0M0 | VPL | I | No | 57 months,
alive | + | + | − | − |
| 14 | M | 75 | Glottis | W-M | T3N0M0 | TL+RP | III | No | 57 months,
alive | + | + | − | − |
| 15 | M | 65 | Glottis | W | T1N0M0 | VPL | I | No | 56 months,
alive | + | + | − | + |
| 16 | M | 58 | Glottis | W | T2N0M0 | VPL | II | No | 56 months,
alive | − | + | − | + |
| 17 | M | 72 | Glottis | M | T1N0M0 | VPL | I | No | 55 months,
alive | − | + | − | + |
| 18 | M | 57 | Glottis | M | T3N0M0 | TL | III | No | 52 months,
alive | − | − | − | − |
| 19 | M | 67 | Glottis | M | T3N2M0 | TL+PR | IV | No | 40 months, DOD | + | + | − | + |
| 20 | M | 69 | Glottis | W | T1N0M0 | VPL | I | No | 55 months,
alive | − | − | − | − |
| 21 | M | 45 | Glottis | W | T2N0M0 | VPL | II | No | 48 months,
alive | + | − | − | − |
| 22 | M | 53 | Glottis | W | T4N0M0 | TL+PR | IV | No | 100 months,
lost | + | + | − | − |
| 23 | F | 54 | Glottis | M | T2N0M0 | VPL | II | No | 43 months,
alive | + | + | + | + |
| 24 | M | 69 | Glottis | W-M | T2N0M0 | VPL | II | No | 42 months,
alive | − | − | − | + |
| 25 | M | 64 | Glottis | W-M | T4N0M0 | TL+PR | IV | No | 42 months,
alive | + | − | − | + |
| 26 | M | 61 | Supraglottis | W | T2N0M0 | SL+PR | II | No | 37 months,
alive | − | + | − | + |
| 27 | M | 48 | Supraglottis | W-M | T2N0M0 | SL+PR | II | No | 26 months, DOD | + | − | − | + |
| 28 | M | 74 | Glottis | W | T2N0M0 | VPL | II | No | 31 months,
alive | − | + | − | − |
| 29 | M | 65 | Glottis | M-P | T1N0M0 | VPL | I | No | 13 months, DOD | − | + | − | + |
| 30 | M | 47 | Glottis | W | T2N0M0 | VPL | II | No | 24 months,
alive | + | + | + | + |
| 31 | M | 47 | Glottis | W-M | T2N1M0 | VPL+PR | III | No | 24 months,
alive | − | − | − | − |
| 32 | M | 55 | Glottis | W | T2N0M0 | VPL | II | No | 14 months,
alive | − | − | − | − |
| 33 | M | 71 | Glottis | W | T2N0M0 | VPL | II | No | 14 months,
alive | − | − | − | − |
| 34 | M | 66 | Glottis | W-M | T2N2M0 | VPL+PR | IV | Yes | 12 months,
lost | − | − | + | + |
Expression of GLUT-1, MRP1, P-gp and
GST-π
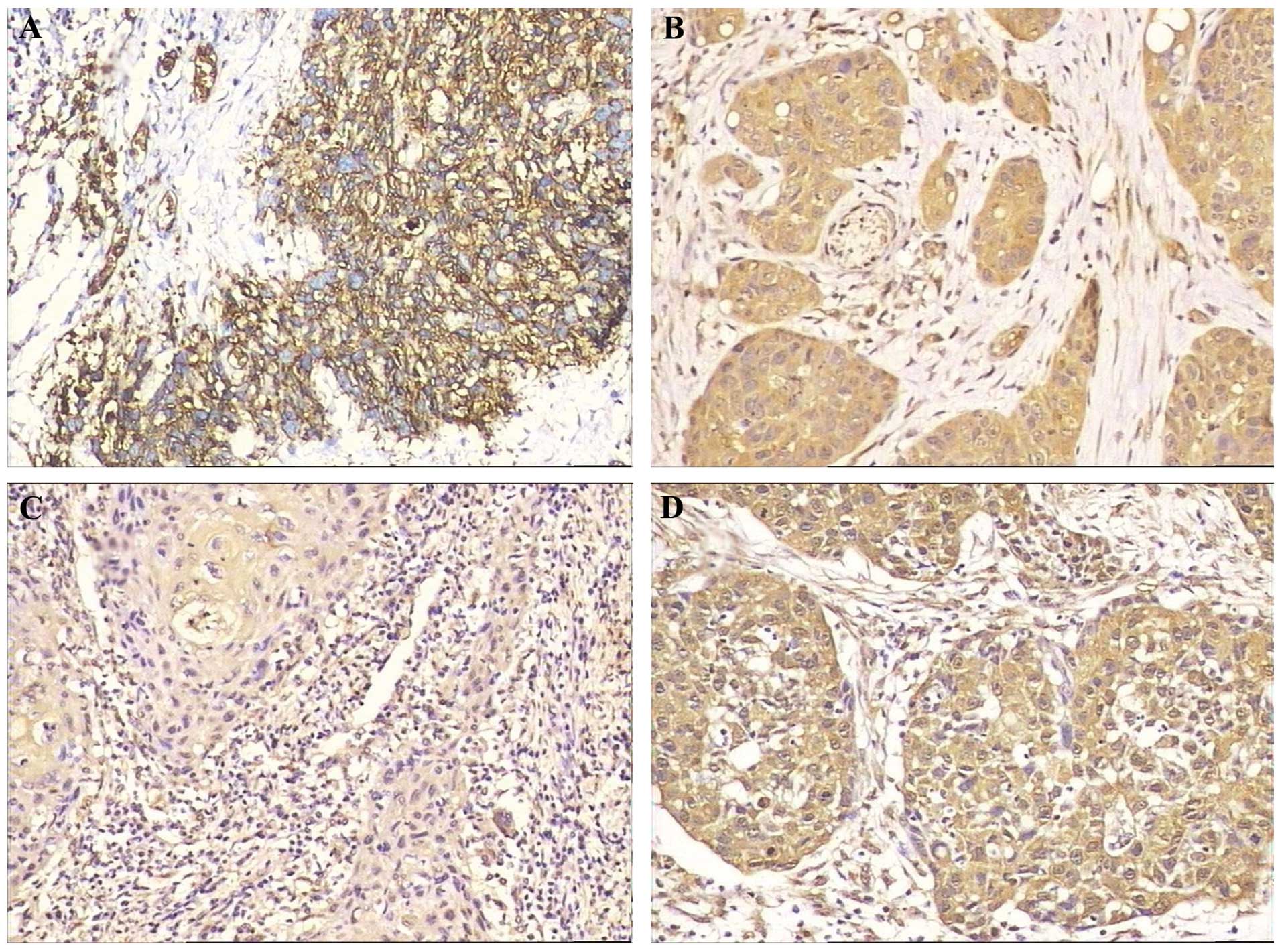
In this study, 52.9 (18/34), 58.8 (20/34), 20.6
(7/34) and 58.8% (20/34) of the laryngeal carcinomas were positive
for GLUT-1, P-gp, MRP1 and GST-π, respectively (Fig. 1). Pearson’s correlation analysis
showed correlations between GLUT-1 and P-gp (r=0.364; P=0.034),
GLUT-1 and MRP1 (r=0.359; P=0.037), and P-gp and GST-π (r=0.426;
P=0.012).
Association between GLUT-1, MRP1, P-gp
and GST-π expression in laryngeal carcinoma and clinicopathological
parameters and prognosis
GLUT-1 expression was found to significantly
correlate with TNM stage (P=0.02) and clinical stage (P=0.037).
P-gp was found to significantly correlate with clinical stage
(P=0.026). No significant difference was identified between GLUT-1
and P-gp expression and the remaining clinicopathological factors
investigated. No significant difference was identified between MRP1
and GST-π expression and any of the clinicopathological factors
investigated.
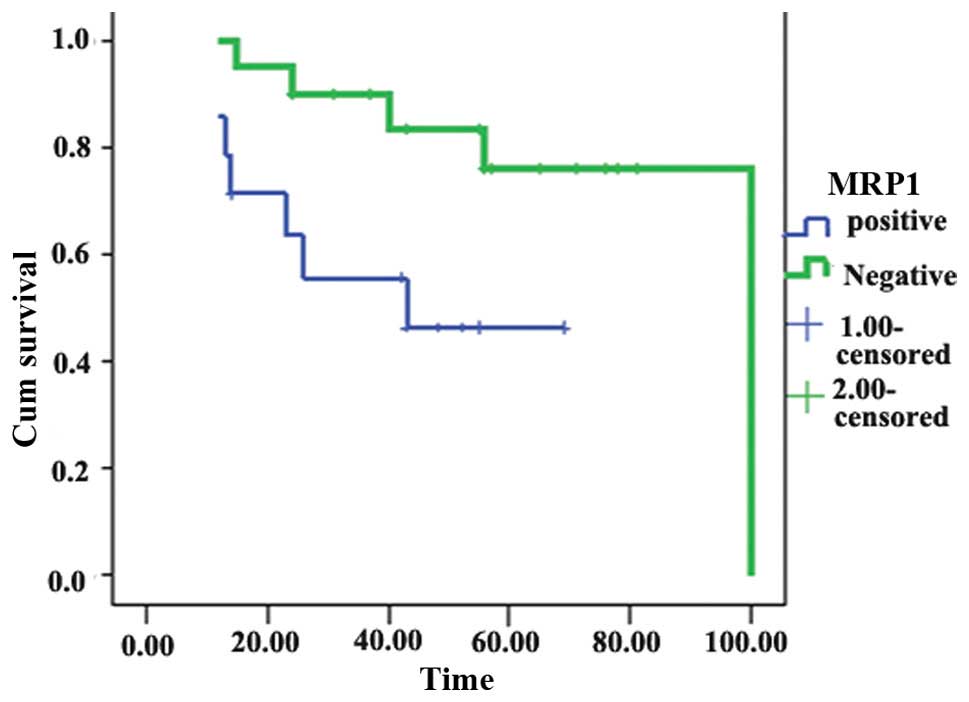
Univariate analysis showed that MRP1 expression was
significantly associated with reduced survival (χ2=5.16;
P=0.023; Fig. 2). By contrast,
GLUT-1, P-gp and GST-π expression were not associated with
survival. Multivariate analysis revealed that lymph node metastasis
(P=0.009) and MRP1 overexpression (P=0.023) were significant
predictors of poor survival.
Discussion
P-gp, MRP1 and GST-π are associated with intrinsic
chemotherapy resistance (11–14).
P-gp and MRP1, two important ATP-binding cassette transporters,
affect the intracellular drug concentration by altering drug influx
or efflux (12). GST-π is a member
of the GST family, which catalyzes the conjugation of glutathione
and leads to the inactivation of cytotoxic drugs (12,13).
The majority of studies have investigated P-gp, MRPs and GST-π in
human solid malignant tumors (11–14).
In the present study, P-gp, MRP1 and GST-π expression were
investigated in laryngeal carcinomas. The expression of P-gp, MRP1
and GST-π was higher than that in the laryngeal precancerous
lesions (P<0.05). Among these proteins, P-gp was found to
significantly correlate with clinical stage (P=0.026) and MRP1
overexpression was significantly associated with poor survival
(P=0.023). These results are similar to those for other human solid
cancers. Yu et al (11)
found that multidrug resistance protein 3 and MRP1 were poor
prognostic factors in liver cancer. In four lung cancer cell lines,
SK-MES-1, SPCA-1, NCI-H-460 and NCI-H-446, the expression of P-gp,
MRP1 and GST-π was different; the level of GST-π in the SK-MES-1
cells was the highest, whereas the level of P-gp in the SPCA-1
cells was the lowest. The chemoresistance to cisplatin, doxorubicin
and VP-16 in the four cell lines was also different; the SPCA-1
cell line was most resistant to cisplatin, and the SK-MES-1 cell
line was most resistant to VP-16, but most sensitive to
doxorubicin. There was a positive correlation between GST-π
expression and resistance to cisplatin, between TopoIIα expression
and resistance to VP-16, and a negative correlation was noted
between TopoIIα expression and resistance to doxorubicin. Among
these proteins, GST-π may be useful for the prediction of intrinsic
resistance to cisplatin (12). P-gp
and MDR have been found to be highly expressed in gallbladder
carcinoma (14). Similarly, P-gp,
MRP1 and GST-π were highly expressed in gliomas (13). However, the regulatory mechanism
underlying the high level of expression of these proteins in cancer
remains unclear.
Previous studies have shown that the overexpression
of these proteins may be associated with hypoxia (15,16).
Hypoxia is an important factor in chemoresistance (1,2). A
small number of studies have demonstrated co-expression of GLUT-1
and P-gp in the capillaries of the blood-brain barrier (18,19).
However, the association between GLUT-1, P-gp, MRP1 and GST-π
expression in human cancers has not been reported. In the present
study, correlations were identified between GLUT-1 and P-gp, GLUT-1
and MRP1 and P-gp and GST-π in laryngeal carcinoma.
In addition, GLUT-1 is associated with a poor
response to chemoradiotherapy, and the silencing of GLUT-1
expression may increase sensitivity to chemotherapeutic agents
(7–10). Solid cancers grow rapidly and cause
hypoxia due to an insufficient supply of blood and oxygen. Under
hypoxic conditions, GLUT-1 may supply glucose to meet the energy
requirements of cancer cells (4,7–10). In
the present study, high levels of GLUT-1 expression were identified
in laryngeal carcinomas, which was similar to the results of our
previous study regarding laryngeal carcinoma (4). However, GLUT-1 expression was not
associated with any clinicopathological parameters. These results
differ from our previous and other studies, and these differences
may be due to variation in histopathological type,
immunohistochemical techniques, tumor stage and sample size
(4,10). GLUT-1 expression has been found to
be significantly associated with a reduced response to
chemoradiotherapy, in oesophageal cancer, rectal cancer and ovarian
carcinoma (9). These differences
may be due to variation in histopathological type,
immunohistochemical techniques, tumor stage and sample size.
In the present study, the expression and
correlations between GLUT-1, P-gp, MRP1 and GST-π in laryngeal
carcinoma samples was investigated. Whether these proteins are
involved in resistance to chemotherapy in patients with laryngeal
carcinoma requires further study.
In conclusion, this is the first study to
investigate the expression of GLUT-1, P-gp, MRP1 and GST-π in
laryngeal carcinomas and the correlations between these proteins.
P-gp was found to significantly correlate with clinical stage,
while MRP1 overexpression was significantly associated with poor
survival. In our future studies, we will further investigate
whether these proteins may be resistant to chemotherapy in
laryngeal carcinoma in vivo. Inhibition of these proteins by
targeted treatment may enhance the sensitivity of chemotherapy in
laryngeal carcinoma.
Acknowledgements
This study was supported by the Department of
Science and Technology of Zhejiang Provincial (grant no.
2010C33011) and the National Natural Science Foundation of China
(grant nos. 81172562 and 81372903).
References
|
1
|
Pérez-Sayáns M, Supuran CT, Pastorekova S,
et al: The role of carbonic anhydrase IX in hypoxia control in
OSCC. J Oral Pathol Med. 42:1–8. 2013. View Article : Google Scholar
|
|
2
|
Hong B, Lui VW, Hashiguchi M, Hui EP and
Chan AT: Targeting tumor hypoxia in nasopharyngeal carcinoma. Head
Neck. 35:133–145. 2013. View Article : Google Scholar
|
|
3
|
Gu J, Yamamoto H, Fukunaga H, et al:
Correlation of GLUT-1 overexpression, tumor size, and depth of
invasion with 18F-2-fluoro-2-deoxy-D-glucose uptake by positron
emission tomography in colorectal cancer. Dig Dis Sci.
51:2198–2205. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wu XH, Chen SP, Mao JY, Ji XX, Yao HT and
Zhou SH: Expression and significance of hypoxia-inducible factor-1α
and glucose transporter-1 in laryngeal carcinoma. Oncol Lett.
5:261–266. 2013.
|
|
5
|
Xu YY, Bao YY, Zhou SH and Fan J: Effect
on the expression of MMP-2, MT-MMP in laryngeal carcinoma Hep-2
cell line by antisense glucose transporter-1. Arch Med Res.
43:395–401. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhou SH, Fan J, Chen XM, Cheng KJ and Wang
SQ: Inhibition of cell proliferation and glucose uptake in human
laryngeal carcinoma cells by antisense oligonucleotides against
glucose transporter-1. Head Neck. 31:1624–1633. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shimanishi M, Ogi K, Sogabe Y, et al:
Silencing of GLUT-1 inhibits sensitization of oral cancer cells to
cisplatin during hypoxia. J Oral Pathol Med. 42:382–388. 2013.
View Article : Google Scholar
|
|
8
|
Chiba I, Ogawa K, Morioka T, et al:
Clinical significance of GLUT-1 expression in patients with
esophageal cancer treated with concurrent chemoradiotherapy. Oncol
Lett. 2:21–28. 2011.PubMed/NCBI
|
|
9
|
Brophy S, Sheehan KM, McNamara DA, Deasy
J, Bouchier-Hayes DJ and Kay EW: GLUT-1 expression and response to
chemoradiotherapy in rectal cancer. Int J Cancer. 125:2778–2782.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cantuaria G, Fagotti A, Ferrandina G, et
al: GLUT-1 expression in ovarian carcinoma: association with
survival and response to chemotherapy. Cancer. 92:1144–1150. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yu Z, Peng S, Hong-Ming P and Kai-Feng W:
Expression of multi-drug resistance-related genes MDR3 and MRP as
prognostic factors in clinical liver cancer patients.
Hepatogastroenterology. 59:1556–1559. 2012.PubMed/NCBI
|
|
12
|
Wang J, Zhang J, Zhang L, et al:
Expression of P-gp, MRP, LRP, GST-π and TopoIIα and intrinsic
resistance in human lung cancer cell lines. Oncol Rep.
26:1081–1089. 2011.PubMed/NCBI
|
|
13
|
Calatozzolo C, Gelati M, Ciusani E, et al:
Expression of drug resistance proteins Pgp, MRP1, MRP3, MRP5 and
GST-pi in human glioma. J Neurooncol. 74:113–121. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang BL, Zhai HY, Chen BY, et al: Clinical
relationship between MDR1 gene and gallbladder cancer.
Hepatobiliary Pancreat Dis Int. 3:296–299. 2004.PubMed/NCBI
|
|
15
|
Min L, Chen Q, He S, Liu S and Ma Y:
Hypoxia-induced increases in A549/CDDP cell drug resistance are
reversed by RNA interference of HIF-1α expression. Mol Med Rep.
5:228–232. 2012.
|
|
16
|
Lelong-Rebel I, Brisson C, Fabre M,
Bergerat JP and Rebel G: Effect of pO2 on antitumor drug
cytotoxicity on MDR and non-MDR variants selected from the LoVo
metastatic colon carcinoma cell line. Anticancer Res. 28:55–68.
2008.PubMed/NCBI
|
|
17
|
Sobin LH, Gospodarowicz MK and Wittekind
C: TNM Classification of Malignant Tumors. 7th ed. Oxford:
Wiley-Blackwell; pp. 3362010
|
|
18
|
Camenzind RS, Chip S, Gutmann H,
Kapfhammer JP, Nitsch C and Bendfeldt K: Preservation of
transendothelial glucose transporter 1 and P-glycoprotein
transporters in a cortical slice culture model of the blood-brain
barrier. Neuroscience. 170:361–371. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bourasset F, Cisternino S, Temsamani J and
Scherrmann JM: Evidence for an active transport of
morphine-6-beta-d-glucuronide but not P-glycoprotein-mediated at
the blood-brain barrier. J Neurochem. 86:1564–1567. 2003.
View Article : Google Scholar : PubMed/NCBI
|