Introduction
Gastric carcinoma is one of the most common
malignancies worldwide. Although the early diagnosis and treatment
of gastric cancer has improved in recent years, it remains one of
the leading causes of cancer-related mortality in countries, such
as China and Japan (1). The
aggressive behavior of cancer cells is predominantly attributed to
their capacity to invade and metastasize. Metastasis is a complex
process that involves the spread of a cancer to a site distant from
its origin. Reduced cell-cell adhesion and degradation of the
extracellular matrix (ECM) are essential mechanisms for the
occurrence of cell invasion and metastasis, leading to the
dissemination of a cancer via the blood vessels or the lymph nodes
(2).
Matrix metalloproteinase (MMP)-9 is the largest
member of the MMPs, an extensive family of zinc-dependent
proteolytic enzymes. Previous studies have validated that MMP-9
degrades the principal components of the ECM, collagen types IV and
V, and gelatin and, thus, is closely associated with tumor cell
invasion and metastasis (3–5). Furthermore, it has been reported that
MMP-9 serum levels in the peripheral blood can be used for
preoperative prognosis (6,7). The MMP-9 expression level is elevated
in non-small cell lung cancer and is associated with lymph node
metastasis, although, MMP-9 expression levels are not associated
with postoperative survival rate (8). MMP-9 is associated with the growth,
invasion and metastasis of gastric carcinoma (9), however, there are few reports
regarding preoperative serum levels of MMP-9 in gastric carcinoma.
In the current study, the expression of MMP-9 in gastric carcinoma
samples was analyzed and the correlation between preoperative serum
levels of the MMP-9 protein and the clinicopathological
characteristics of gastric carcinoma was investigated.
Patients and methods
Patients and sample collection
Forty-five primary gastric carcinoma patients, who
had undergone a gastrectomy at The First Affiliated Hospital of
Shantou University Medical College (Shantou, China) between June
2005 and January 2006, were involved in the present study. Thirty
cases were male and 15 cases were female, with a mean age of 60.4
years. Fourteen patients had a family history of cancer. None of
the patients had received chemotherapy or radiotherapy prior to the
surgery and no additional malignancies were evident. All patients
were informed of the nature of the study and provided written
informed consent, and the study was approved by the ethics commitee
of the Medical College of Shantou University.
Peripheral venous blood (3 ml) was collected from
each patient prior to the surgery and stored at −75°C for the
enzyme-linked immunosorbent assay (ELISA; Sino Biological Inc.,
Bejing, China). Tumor tissue samples were obtained immediately from
the resected specimens and distal healthy gastric mucosa tissue was
collected from a distance of >5 cm from the tumor. For
immunohistochemistry, the tissue samples were fixed with 10%
formalin, dehydrated with gradient alcohol, cleared with xylene and
embedded in paraffin. In addition, 10 specimens of peripheral serum
and gastric mucosa tissue from 10 healthy individuals were randomly
selected to serve as controls. Classification of the tumors was
conducted according to the tumor-node-metastasis (TNM) staging
system issued by the International Union Against Cancer in 1997
(10).
Semi-quantitative reverse
transcription-polymerase chain reaction (RT-PCR) analysis of MMP-9
expression
Total RNA was extracted using TRI
Reagent® (Molecular Research Center, Inc., Cincinnati,
OH, USA) followed by complementary DNA synthesis using the Moloney
murine leukemia virus reverse transcriptase (Thermo Fisher
Scientific, Pittsburgh, PA, USA). PCR was performed using Taq
polymerase (Takara Biotechnology Co., Ltd., Dalian, China) under
the following conditions: 94°C for 2 min; 22 cycles (for β-actin)
or 32 cycles (for MMP-9) of 94°C for 30 sec, 57°C for 30 sec and
72°C for 40 sec; followed by 72°C for 5 min. β-actin served as an
internal control. The PCR product lengths for MMP-9 and β-actin
were 462 and 592 bp, respectively and were sequenced by Invitrogen
Biotechnology Co., Ltd. (Shanghai, China). The primers for the PCR
were as follows: Sense, 5′-CACCCTTGTGCTCTTCCCTG-3′ and anti-sense,
5′-GGATACCCGTCTCCGTGCT-3′ for MMP-9; sense,
5′-CCAAGGCCAACCGCGAGAAGATGAC-3′ and anti-sense,
5′-AGGGTACATGGTGGTGCCGCCAGAC-3′ for β-actin. Each PCR product was
examined by agarose gel electrophoresis and images were captured
using a digital camera (PowerShot A2400; Canon Inc., Tokyo, Japan).
The protein band intensities were analyzed using Image J software
(National Institutes of Health, Bethesda, MD, USA).
Immunohistochemical staining of
MMP-9
Serial sections were cut from the paraffin-embedded
tissues at a thickness of 5 μm, and routine hematoxylin and eosin
(H&E) staining was conducted prior to immunohistochemical
staining. The staining procedure was performed according to the
immunohistochemical EliVision™ two-step method (11) and a tissue section with a known
MMP-9 expression level served as the positive control. Staining
with phosphate-buffered saline, as a substitute for the primary
antibody, served as the negative control. Cells exhibiting a
brown-stained cytoplasm, at a higher intensity than the
non-specific background staining, were regarded as positively
stained and tumors exhibiting positive staining in >10% of the
total number of tumor cells were regarded as positive for MMP-9
expression. The human monoclonal anti-MMP-9 and poly-horseradish
peroxidase secondary antibodies were purchased from Maxim
Biotechnology Development Co., Ltd. (Fuzhou, China).
ELISA of MMP-9
The concentration of the MMP-9 protein in the
peripheral serum was measured using a double-antibody sandwich
ELISA method (Wuhan Boster Biological Technology, Ltd, Wuhan,
China) according to the manufacturer’s instructions. Following
coloration with 3,3′-diaminobenzidine, the optical density value
was measured at a wavelength of 450 nm and a standard curve was
determined to calculate the concentration of MMP-9 protein.
Statistical analysis
Statistical analysis was performed using SPSS
software (version 10.0; SPSS, Inc., Chicago, IL, USA). Student’s
t-test was applied for data analysis between the two groups,
χ2 and Fisher’s exact tests were applied for numeration
data, and a Pearson correlation coefficient was used to analyze the
correlation between gene expression and the protein level.
P<0.05 was considered to indicate a statistically significant
difference.
Results
MMP-9 expression levels in tissue
samples
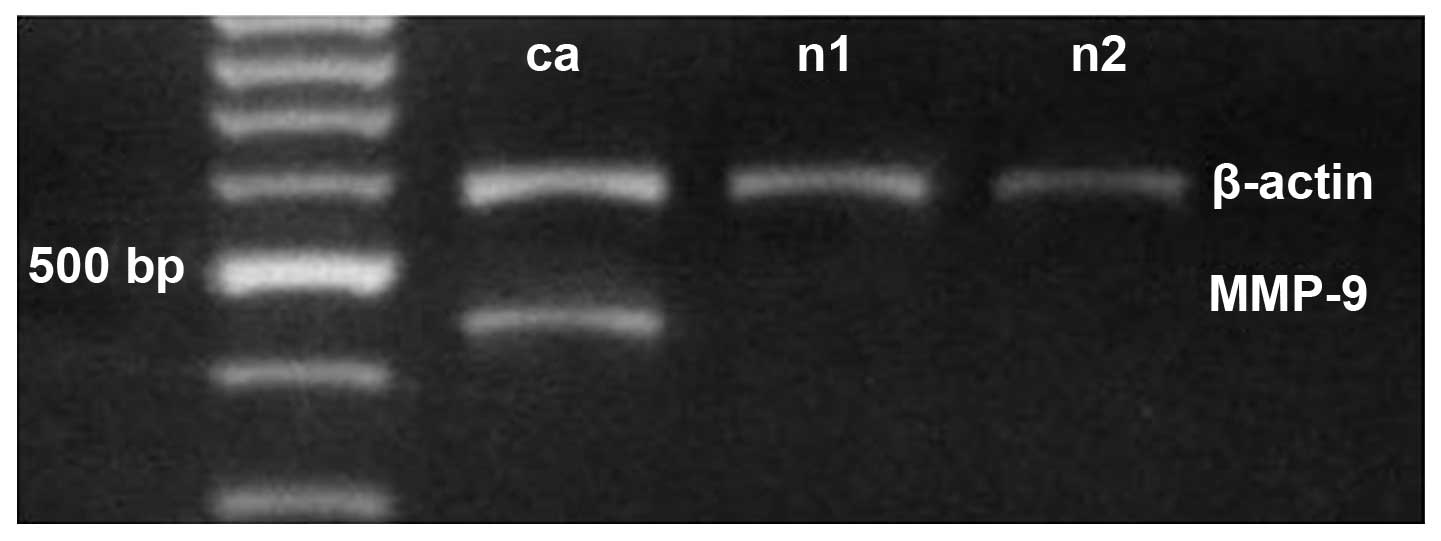
Semi-quantitative RT-PCR (Fig. 1) identified MMP-9 expression in
48.9% (22/45) of the gastric carcinoma tissues, 13.3% (6/45) of the
distal gastric mucosa tissues (>5 cm from the gastric tumor) and
10% (1/10) of the healthy gastric mucosa control tissues. The mean
level of MMP-9 mRNA in the gastric carcinoma tissues (0.42±0.65)
was significantly higher than in the distal (0.22±0.76; P<0.05)
and control tissues (0.03±0.06; P<0.05; Table I).
 | Table IMatrix metalloproteinase-9 parameters
of the gastric carcinoma, distal tissue and control groups (mean ±
standard deviation). |
Table I
Matrix metalloproteinase-9 parameters
of the gastric carcinoma, distal tissue and control groups (mean ±
standard deviation).
| Group | Cases, n | mRNA value | Positive expression,
n (%) | Protein
concentration, μg/ml |
|---|
| Gastric
carcinoma | 45 | 0.42±0.65a | 39 (86.67)a | 0.41±0.26a |
| Distal tissue | 45 | 0.22±0.76 | 12 (26.67)b | |
| Control | 10 | 0.03±0.06 | 1 (10.00)b | 0.15±0.04 |
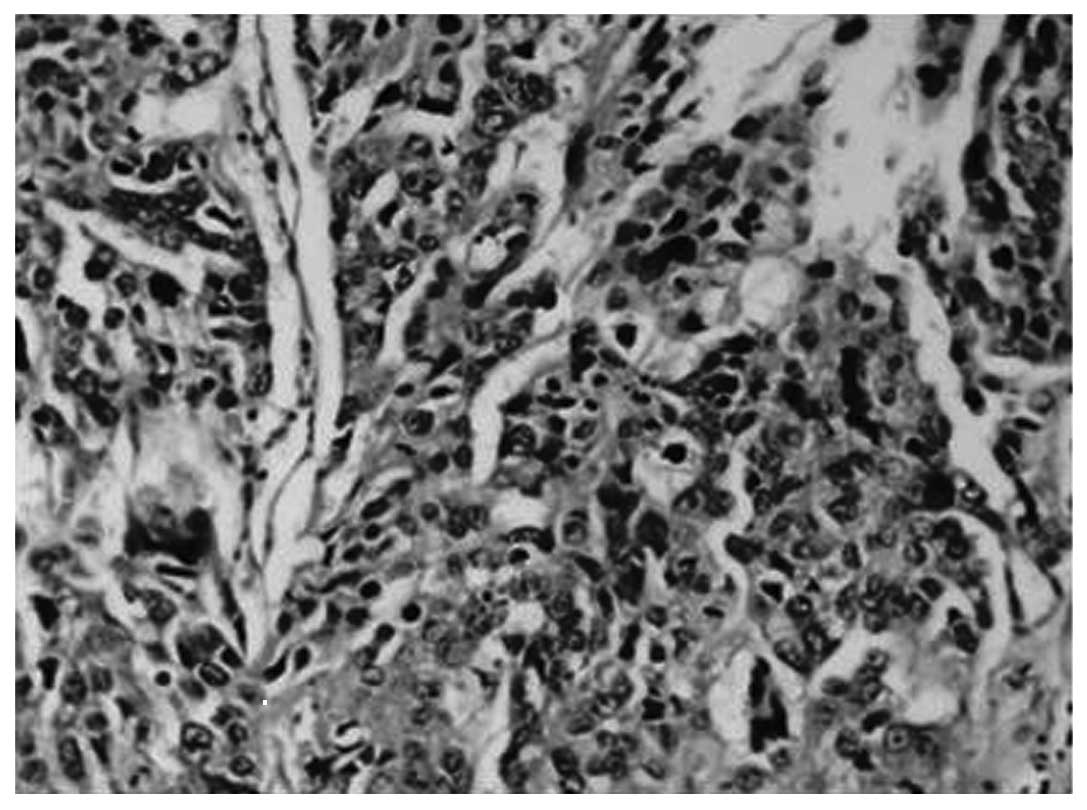
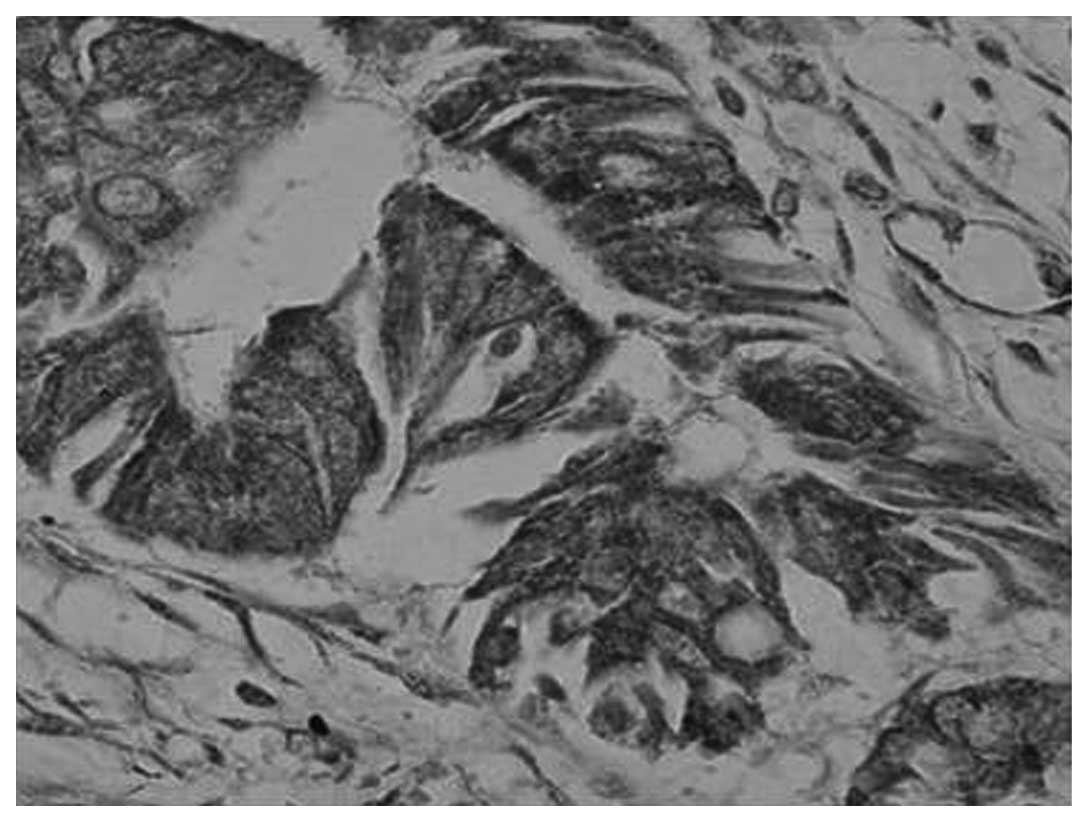
H&E (Fig. 2) and
immunohistochemical (Fig. 3)
staining for MMP-9 was observed in the majority of gastric
carcinoma tissues. The expression rate of MMP-9 was 86.67% (39/45)
in the gastric carcinoma tissues, 26.67% (12/45) in the distal
gastric mucosa tissues and 10% (1/10) in the healthy gastric mucosa
tissues (Table I). Correlation
between MMP-9 expression levels and the clinicopathological
characteristics of the tumor revealed that patients with
seromembranous invasion had a significantly higher proportion of
MMP-9-positive tumor cells (92.31%; 36/39) than patients without
seromembranous invasion (50.00%; 3/6) (P<0.05). However, no
significant difference in the levels of MMP-9 expression was
identified with respect to the tumor size, family history of
tumors, TNM stage, or occurrence of lymph node or distant
metastasis (Table II).
 | Table IICorrelation between the MMP-9 protein
levels in the peripheral serum and clinicopathological features
(mean ± standard deviation). |
Table II
Correlation between the MMP-9 protein
levels in the peripheral serum and clinicopathological features
(mean ± standard deviation).
| Feature | Cases, n | Mean gray value | Positive rate of
MMP-9 protein, % (n) | Serum (μg/ml) |
|---|
| Tumor size, cm |
| <5 | 22 | 0.47±0.61 | 81.82 (18/22) | 378.14±278.61 |
| ≥5 | 23 | 0.75±0.59 | 91.30 (21/23) | 432.71±238.05 |
| Family history of
tumor |
| Negative | 31 | 0.55±0.56 | 83.87 (26/31) | 391.81±283.28 |
| Positive | 14 | 0.75±0.72 | 92.86 (13/14) | 437.52±192.69 |
| Affected region |
| Upper part | 21 | 0.48±0.44 | 85.71 (18/21) | 450.39±289.75 |
| Middle-lower
part | 24 | 0.73±0.72 | 87.5 (21/24) | 367.22±223.97 |
| Differentiation |
| Well and
moderate | 27 | 0.60±0.62 | 81.48 (22/27) | 380.22±257.50 |
| Poor or none | 18 | 0.79±0.61 | 94.44 (17/18) | 444.75±259.12 |
| Depth of
invasion |
| No seromembranous
invasion | 6 | 0.25±0.28 | 50.00 (3/6)b | 303.47±202.06 |
| Seromembranous
invasion | 39 | 0.67±0.63 | 92.31 (36/39)b | 421.81±250.33 |
| TNM stage |
| I + II | 23 | 0.54±0.60 | 78.26 (18/23) | 305.22±223.99a |
| III + IV | 22 | 0.68±0.63 | 95.45 (21/22) | 511.42±251.52a |
| Lymphatic
metastasis |
| Negative | 21 | 0.58±0.61 | 85.71 (18/21) | 300.30±205.62a |
| Positive | 24 | 0.64±0.62 | 87.50 (21/24) | 498.55±265.77a |
| Distant
metastasis |
| Absent | 43 | 0.59±0.62 | 86.05 (37/43) | 396.28±254.94 |
| Present | 2 | 1.00±0.11 | 100.00 (2/2) | 615.72±297.53 |
Correlation between preoperative serum
MMP-9 protein levels and clinicopathological features of gastric
carcinoma
The mean preoperative serum MMP-9 protein levels in
gastric carcinoma patients (0.41±0.26 μg/ml) was significantly
higher than those of the control group (0.15±0.04 μg/ml; P<0.05)
(Table I). In addition, the serum
concentration of MMP-9 protein in patients with an advanced TNM
stage (511.42±251.52), and lymph node metastasis (498.55±265.77)
was significantly higher than that in the patients at an early TNM
stage (305.22±223.99) and exhibiting no lymph node metastasis
(300.30±205.62), respectively (P<0.01). However, there was no
significant correlation between the serum MMP-9 protein level and
other clinicopathological characteristics, such as the tumor size,
a family history of tumors, localization of the tumor, tumor
differentiation, degree of invasion or distal metastasis (Table II).
Correlation between MMP-9 expression
levels in gastric carcinoma tissues and preoperative serum MMP-9
protein levels
The expression level of MMP-9 in gastric carcinoma
tissues and the preoperative serum was higher when compared with
the healthy tissues. However, the correlation between MMP-9 mRNA
levels in gastric carcinoma tissues and the preoperative serum
MMP-9 levels was not identified to be statistically significant
(r=0.13; P=0.394).
Discussion
Degradation of the ECM and basement membranes is a
critical step in tumor invasion and metastasis. The process of
metastasis depends on the activity of various proteolytic enzymes,
particularly serine and cysteine proteases, and MMP. MMPs, a family
of closely associated enzymes that degrade the ECM, are considered
to be important in facilitating tumor invasion and metastasis, and
are associated with tumor invasion, lymph node metastasis and
survival of gastric carcinoma (12). Previously, studies have identified a
significant correlation between the expression of MMP-2, -7 and -9,
and the depth of tumor invasion, vessel invasion, lymph node and
distant metastasis, TNM stage and microvessel density, indicating
that these markers may be significant in cell migration in gastric
carcinoma (13–15). Zymographic analysis of the
expression levels of MMP-9 and active MMP-2 demonstrated a
proportional increase with tumor grade and invasiveness in bladder
cancer (16). MMP-9 mRNA expression
levels in lymphocytes tended to be higher in malignant pleural
effusions of lung cancer when compared with healthy pleural
effusions (17). Consistent with
the findings of the above-mentioned reports, the present study
demonstrated that MMP-9 was highly expressed in gastric carcinoma
tissues and preoperative serum when compared with distal and
healthy tissue.
Correlation between the MMP-9 expression levels and
the clinicopathological parameters of tumors did not identify any
significant difference, with the exception of the depth of tumor
invasion. The incidence of MMP-9 positive expression was observed
to be greater in tumors with seromembranous invasion compared with
non-invasive tumors, indicating that MMP-9 may contribute to
invasion and tissue infiltration in gastric carcinoma. Furthermore,
the present study identified that patients with gastric carcinoma
exhibited higher preoperative peripheral serum MMP-9 levels
compared with the control groups, and demonstrated that the serum
MMP-9 levels correlated with the TNM stage and occurrence of lymph
node metastasis. These data indicate that serum protein expression
levels may contribute to the malignant tumor phenotype, as reported
in previous studies (15,18). MMP-9 mRNA and serum expression were
upregulated in gastric carcinoma, however, the present study did
not observe a correlation between MMP-9 expression levels in the
gastric carcinoma tissues and those in the preoperative serum. This
may be due to mechanisms, in addition to the influence of cancerous
tissue on serum MMP-9 protein secretion (19), such as the action of macrophages,
leucocytes and other inflammatory cells (20,21).
In the present study, it was identified that MMP-9 expression
tended to be higher in patients with a positive family history of
cancer compared with patients without, however, no significant
correlation was observed. Additionally, the present study
demonstrated that MMP-9 expression levels in distal tissues were
significantly higher than those of the healthy control group,
indicating that abnormal gene activation may be involved, possibly
in a similar manner to MMP-9 overexpression in benign gastric
lesions (22).
Previous investigations, regarding how differences
between plasma and serum samples influence the diagnostic and
prognostic performance of MMP-9, identified that plasma MMP-9
levels were elevated in gastric cancer patients when compared with
control subjects, whilst serum MMP-9 levels did not differ between
the groups (23–25). By contrast, in non-small cell lung
cancer, serum MMP-9 levels were demonstrated to be higher in
patients with metastatic cancer (26,27),
indicating that detection of the preoperative plasma MMP-9
expression level may serve as an improved marker for tumor
progression when compared with serum MMP-9 levels.
In conclusion, the present study demonstrated that
MMP-9 protein levels in the preoperative serum and mRNA expression
in carcinoma tissue were higher than the healthy controls, and were
correlated with specific clinicopathological features of gastric
carcinoma. The data indicates that MMP-9 has potential as a
diagnostic marker and therapeutic molecular target for management
of gastric carcinoma.
Acknowledgements
The present study was supported by the Science and
Technology Planning Project of Guangdong Province, China (grant no.
2004B31201012).
References
|
1
|
Wei F, Huang P, Li S, et al: Enhancement
patterns of gastric carcinoma on contrast-enhanced ultrasonography:
relationship with clinicopathological features. PLoS One.
8:e730502013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Paz H, Pathak N and Yang J: Invading one
step at a time: the role of invadopodia in tumor metastasis.
Oncogene. 33:4193–4202. 2014. View Article : Google Scholar
|
|
3
|
Iizasa T, Fujisawa T, Suzuki M, et al:
Elevated levels of circulating plasma matrix metalloproteinase 9 in
non-small cell lung cancer patients. Clin Cancer Res. 5:149–153.
1999.PubMed/NCBI
|
|
4
|
Hofmann UB, Westphal JR, Van Muijen GN and
Ruiter DJ: Matrix metalloproteinases in human melanoma. J Invest
Dermatol. 115:337–344. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Westermarck J and Kähäri VM: Regulation of
matrix metalloproteinase expression in tumor invasion. FASEB J.
13:781–792. 1999.PubMed/NCBI
|
|
6
|
Tan SY, Wang JY, Shen L, Luo HS and Shen
ZX: Relationship between preoperative staging by endoscopic
ultrasonography and MMP-9 expression in gastric carcinoma. World J
Gastroenterol. 13:2108–2112. 2007.PubMed/NCBI
|
|
7
|
Ylisirniö S, Höyhtyä M and
Turpeenniemi-Hujanen T: Serum matrix metalloproteinases -2, -9 and
tissue inhibitors of metalloproteinases -1, -2 in lung cancer -
TIMP-1 as a prognostic marker. Anticancer Res. 20:1311–1316.
2000.
|
|
8
|
Czyzewska J, Guzińska-Ustymowicz K, Kemona
A and Bandurski R: The expression of matrix metalloproteinase 9 and
cathepsin B in gastric carcinoma is associated with lymph node
metastasis, but not with postoperative survival. Folia Histochem
Cytobiol. 46:57–64. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zheng H, Takahashi H, Murai Y, et al:
Expressions of MMP-2, MMP-9 and VEGF are closely linked to growth,
invasion, metastasis and angiogenesis of gastric carcinoma.
Anticancer Res. 26:3579–3583. 2006.PubMed/NCBI
|
|
10
|
Sobin LH and Fleming ID: TNM
Classification of Malignant Tumors, fifth edition (1997). Union
Internationale Contre le Cancer and the American Joint Committee on
Cancer. Cancer. 80:1803–1804. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Peng SH, Deng H, Yang JF, et al:
Significance and relationship between infiltrating inflammatory
cell and tumor angiogenesis in hepatocellular carcinoma tissues.
World J Gastroenterol. 11:6521–6524. 2005.
|
|
12
|
Endo K, Maehara Y, Baba H, et al: Elevated
levels of serum and plasma metalloproteinases in patients with
gastric cancer. Anticancer Res. 17:2253–2258. 1997.PubMed/NCBI
|
|
13
|
Alakus H, Grass G, Hennecken JK, et al:
Clinicopathological significance of MMP-2 and its specific
inhibitor TIMP-2 in gastric cancer. Histol Histopathol. 23:917–923.
2008.PubMed/NCBI
|
|
14
|
Lee KH, Shin SJ, Kim KO, et al:
Relationship between E-cadherin, matrix metalloproteinase-7 gene
expression and clinicopathological features in gastric carcinoma.
Oncol Rep. 16:823–830. 2006.PubMed/NCBI
|
|
15
|
Lee LY, Wu CM, Wang CC, et al: Expression
of matrix metalloproteinases MMP-2 and MMP-9 in gastric cancer and
their relation to claudin-4 expression. Histol Histopathol.
23:515–521. 2008.PubMed/NCBI
|
|
16
|
Papathoma AS, Petraki C, Grigorakis A, et
al: Prognostic significance of matrix metalloproteinases 2 and 9 in
bladder cancer. Anticancer Res. 20:2009–2013. 2000.PubMed/NCBI
|
|
17
|
Park KJ, Hwang SC, Sheen SS, Oh YJ, Han JH
and Lee KB: Expression of matrix metalloproteinase-9 in pleural
effusions of tuberculosis and lung cancer. Respiration. 72:166–175.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Torii A, Kodera Y, Uesaka K, et al: Plasma
concentration of matrix metalloproteinase 9 in gastric cancer. Br J
Surg. 84:133–136. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Unemori EN, Hibbs MS and Amento EP:
Constitutive expression of a 92-kD gelatinase (type V collagenase)
by rheumatoid synovial fibroblasts and its induction in normal
human fibroblasts by inflammatory cytokines. J Clin Invest.
88:1656–1662. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Creemers EE, Cleutjens JP, Smits JF and
Daemen MJ: Matrix metalloproteinase inhibition after myocardial
infarction: a new approach to prevent heart failure? Circ Res.
89:201–210. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Herman MP, Sukhova GK, Libby P, et al:
Expression of neutrophil collagenase (matrix metalloproteinase-8)
in human atheroma: a novel collagenolytic pathway suggested by
transcriptional profiling. Circulation. 104:1899–1904. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bergin PJ, Raghavan S, Svensson H, et al:
Gastric gelatinase B/matrix metalloproteinase-9 is rapidly
increased in Helicobacter felis-induced gastritis. FEMS Immunol Med
Microbiol. 52:88–98. 2008. View Article : Google Scholar
|
|
23
|
Wu CY, Wu MS, Chiang EP, et al: Plasma
matrix metalloproteinase-9 level is better than serum matrix
metalloproteinase-9 level to predict gastric cancer evolution. Clin
Cancer Res. 13:2054–2060. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dragutinović VV, Radovanović NS,
Izrael-Zivković LT and Vrvić MM: Detection of gelatinase B activity
in serum of gastric cancer patients. World J Gastroenterol.
12:105–109. 2006.
|
|
25
|
Gerlach RF, Uzuelli JA, Souza-Tarla CD and
Tanus-Santos JE: Effect of anticoagulants on the determination of
plasma matrix metalloproteinase (MMP)-2 and MMP-9 activities. Anal
Biochem. 344:147–149. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Laack E, Scheffler A, Burkholder I, et al:
Pretreatment vascular endothelial growth factor (VEGF) and matrix
metalloproteinase-9 (MMP-9) serum levels in patients with
metastatic non-small cell lung cancer (NSCLC). Lung Cancer.
50:51–58. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shimanuki Y, Takahashi K, Cui R, et al:
Role of serum vascular endothelial growth factor in the prediction
of angiogenesis and prognosis for non-small cell lung cancer. Lung.
183:29–42. 2005. View Article : Google Scholar : PubMed/NCBI
|