Introduction
Breast cancer represents the most common type of
malignancy in females, worldwide. Despite earlier diagnosis and
improvement in adjuvant therapies, a number of patients present
with metastatic recurrence, which has a two to three year median
overall survival time (1,2). Hormonal therapy, chemotherapy and more
recently biological treatment are systemic therapies designed to
reduce the size of tumors, improve patient survival and preserve
quality of life. However, in a metastatic setting, the majority of
patients will relapse regardless of the initial efficacy of the
treatment strategy undertaken. The most important therapeutic goals
in metastatic breast cancer (MBC) are palliative and aim to improve
progression free survival (PFS). However, this management of MBC is
a clinical challenge for healthcare workers, as the optimal type
and duration of chemotherapy, and the benefits of maintenance
chemotherapy versus maintenance hormonal treatment required, have
yet to be determined. Thus, the present retrospective study aimed
to investigate the impact of HRT and capecitabin, two types of
maintenance therapy, on MBC patient PFS.
Following a response to rescue chemotherapy,
maintenance treatment with HRT or targeted agents may be considered
for the treatment of MBC; however, maintenance HRT is limited to
MBC patients with hormone receptor-positive disease (3,4). A
number of targeted agents are widely accepted as a type of
maintenance therapy for MBC, for example trastuzumab is
administered for human epidermal growth factor receptor 2
(Her-2)-positive MBC (5,6). However, targeted agents are relatively
high in cost and, thus, are not routinely selected as maintenance
treatment in developing countries. In addition to efficacy, the
convenience and tolerability of the maintenance treatment must be
considered; for example, intravenous chemotherapy requires frequent
hospital visits for the patient, which are associated decreased
quality of life for patients and increased healthcare worker costs.
Therefore, the majority of patients prefer oral as opossed to
intravenous chemotherapy (7,8), for
example oral capecitabine.
Capecitabine is approved by the US Food and Drug
Administration for the treatment of patients with locally advanced
breast cancer or MBC. It has a favorable safety profile with
adverse events effectively managed by dose modification (9) and it can conveniently be administered
by oral dosing (10). Furthermore,
capecitabine typically lacks cumulative toxicity with prolonged use
and, thus, is suitable for long-term administration. A number of
clinical trials of capecitabine for the treatment of MBC indicate
that capecitabine is effective when combined with a variety of
agents, including taxanes, vinorelbine, gemcitabine, trastuzumab or
bevacizumab (11–16). However, it is unclear how the
therapeutic effects of capecitabine-based first-line combination
chemotherapy may be maintained. Thus, the current study presents
the results of an analysis of MBC patients receiving capecitabine
or hormone replacement therapy (HRT) as maintenance treatment
following initial response to capecitabine-based combination
therapy.
Patients and methods
Patient selection
From January 2008 to June 2013, 226 MBC patients
received TX combination therapy at the Department of Breast
Oncology of Beijing Cancer Hospital (Beijing, China). Of these, 79
patients were eligible to receive maintenance treatment according
to the following inclusion criteria: Female patients aged ≥18 years
with histologically confirmed primary breast cancer; patients must
have a minimum of one measurable lesion, according to Response
Evaluation Criteria in Solid Tumors guidelines (RECIST) 1.0
(17), and an Eastern Cooperative
Oncology Group score of ≤2 (18);
patients must not have undergone prior chemotherapy for advanced
disease; and patients must have completed four to eight cycles and
achieved disease control [complete relief (CR), partial relief (PR)
or stable disease (SD)]. Furthermore, patients were allowed to
receive one-line endocrine treatment for advanced disease prior to
docetaxel plus capexitabine (TX) chemotherapy. This study was
approved by the ethics committee of Beijing Cancer Hospital
(Beijing, China) and written informed consent was obtained from all
patients.
Treatment strategy
Capexitabine was administered as the combination and
maintenance therapy at a dose of 1,000 mg/m2 twice daily
on days 1–14 followed by a 7-day rest period. In the combination
regimen, docetaxel was coadministered, as a 1-h 75 mg/m2
intravenous infusion on day 1 of every 3-week cycle. Following a
response to chemotherapy, 39 patients continued to receive
single-agent capecitabine with the abovementioned dose, whilst 40
patients received hormonal therapy with tamoxifen (n=3), toremifene
(n=7), exemestane (n=15), letrozle (n=6) or anastrozole (n=9). To
relieve the symptoms of hand-foot syndrome during maintenance
therapy, all patients were coadministered with 100 mg vitamin B6
three times daily.
Efficacy and safety assessments
The PFS time of the 79 patients was determined as
the interval from the day of combined TX chemotherapy commencement
to cancer progression, cancer-related mortality, mortality from an
unknown cause during therapy, or the final day of follow-up for
patients who had not progressed at the date of analysis. By
contrast, for maintenance treatment PFS, the start time was defined
as the day of capecitabine or hormonal agent maintenance therapy
commencement. Additionally, the clinical efficacy and major adverse
events were investigated, with response assessed using RECIST and
adverse events graded according to the National Cancer Institute
Common Toxicity Criteria version 3.0 (19).
Statistical analysis
The duration of response was defined as the period
between CR or PR onset and evidence of disease progression, and the
duration of response and PFS were estimated using the Kaplan-Meier
method. Additionally, the baseline characteristics of the patients
and the incidence of adverse events between capecitabine and HRT
maintenance therapy were compared using Pearson’s χ2
test. All statistical analyses were performed using SPSS software
(version 15.0; SPSS Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Patient characteristics
The 79 patients investigated in the present study
were divided into two groups, with 39 patients receiving
capecitabine maintenance therapy and 40 patients receiving hormone
maintenance therapy. The baseline patient characteristics of the 79
patients are summarized in Table I.
The median patient age was 55 years (range, 34–75 years), the
majority of patients exhibited hormone receptor-positive tumors
(79.8%; HR-positive status indicates estrogen receptor-positive
and/or progesterone receptor-positive), and Her-2-negative disease
(83.5%). The most common sites of metastasis were the bone and lung
(51.9%). The majority of patients had received prior
anthracycline-based chemotherapy (78.4%), with more than half
(50.6%) receiving prior taxane-based chemotherapy. Additionally,
palliative hormonal therapy due to metastasis had been administered
prior to DX chemotherapy in 30 patients, including 17 patients
(43.5%) in the capecitabine maintenance group and 13 patients
(32.5%) in the HRT group. Of the 40 patients who received endocrine
agent maintenance, 28 patients received aromatase inhibitors (AIs),
five patients received toremifene, six received goserelin plus AIs
and one patient received tamoxifene.
 | Table IBaseline characteristics of all
patients (n=79). |
Table I
Baseline characteristics of all
patients (n=79).
| Capecitabine
maintenance | HRT maintenance | |
|---|
|
|
| |
|---|
| Characteristics | n | % | n | % | P-value |
|---|
| Menopause status | | | | | 0.406 |
| Pre | 12 | 30.8 | 9 | 22.5 | |
| Post | 27 | 69.2 | 31 | 77.5 | |
| ECOG PS | | | | | 0.372 |
| 0 | 23 | 59.0 | 30 | 75.0 | |
| 1 | 14 | 35.9 | 9 | 22.5 | |
| 2 | 2 | 5.1 | 1 | 2.5 | |
| HR status | | | | | 0.082 |
| Positive | 28 | 71.8 | 35 | 87.5 | |
| Negetive | 11 | 28.2 | 5 | 12.5 | |
| Lymph nodes,
na | | | | | 0.516 |
| 0–3 | 28 | 71.8 | 26 | 65.0 | |
| ≥4 | 11 | 28.2 | 14 | 35.0 | |
| Her-2 status | | | | | 0.876 |
| Positiveb | 6 | 15.4 | 5 | 12.5 | |
| Negativec | 32 | 82.1 | 34 | 85.0 | |
| Unknown | 1 | 2,4 | 1 | 2.5 | |
| Metastatic
site |
| Liver | 14 | 35.9 | 12 | 30.0 | 0.577 |
| Lung | 23 | 59.0 | 18 | 45.0 | 0.214 |
| Bone | 20 | 51.3 | 21 | 52.5 | 0.914 |
| Brain | 3 | 7.7 | 4 | 10 | 1.000 |
| Soft tissue | 22 | 56.4 | 30 | 75.0 | 0.082 |
| Visceral
metastasis | | | | | 0.210 |
| Yes | 32 | 82.1 | 28 | 70.0 | |
| No | 7 | 17.9 | 12 | 30.0 | |
| Metastatic sites,
n | | | | | 0.943 |
| 1 | 7 | 17.9 | 8 | 20.0 | |
| 2 | 18 | 46.2 | 17 | 42.5 | |
| ≥3 | 14 | 35.9 | 15 | 37.5 | |
| Disease-free
interval, years | | | | | 0.539 |
| <2 | 13 | 33.3 | 16 | 40.0 | |
| ≥2 | 26 | 66.7 | 24 | 60.0 | |
| Prior adjuvant
chemotherapy | | | | | 0.523 |
| Taxane | 20 | 51.3 | 20 | 50.0 | |
| Anthracycline | 35 | 89.7 | 27 | 67.5 | |
| Prior adjuvant
endocrine therapy | 25 | 64.1 | 27 | 67.5 | 0.764 |
| Prior palliative
endocrine therapy | 17 | 43.5 | 13 | 32.5 | 0.310 |
Efficacy of combined DX chemotherapy plus
maintenance treatment
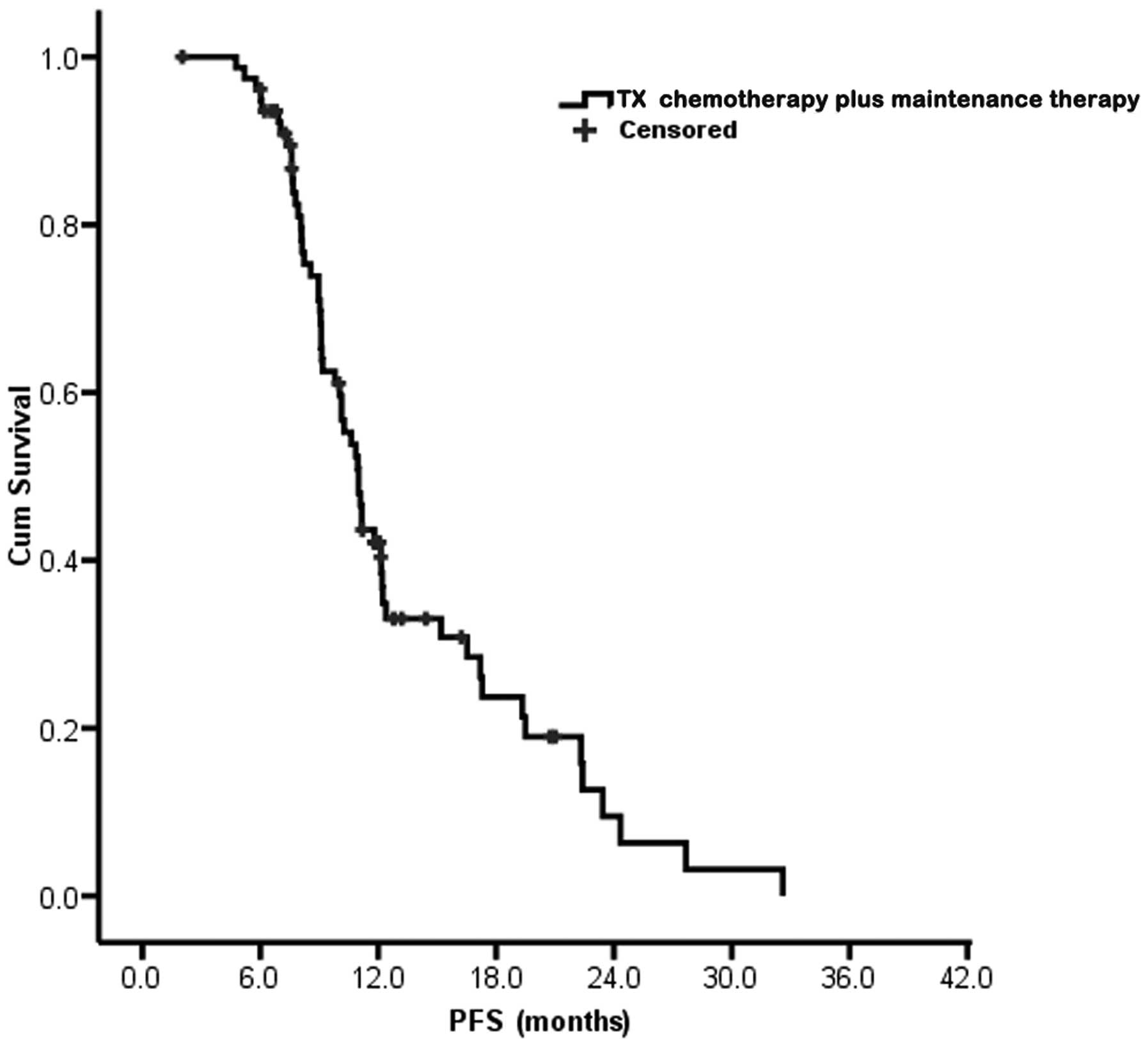
Combined agents chemotherapy plus maintenance
therapy was received by all 79 patients and resulted in a median
PFS of 11.0 months [95% confidence interval (CI), 10.1–11.9 months;
Fig. 1]. Dependent on the
nonprogressive response, eight patients (10.1%) received eight
cycles of combined chemotherapy, 14 patients (17.7%) received four
cycles and 57 patients (72.2%) recevied six cycles. The baseline
response to the combination chemotherapy was a CR in two patients
(2.5%), a PR in 32 patients (40.5%) and SD in 45 patients (57.0%).
For the 39 patients following the single-agent capecitabine
maintenance treatment, the baseline was as follows: Two patients
(5.1%) achieved a CR, 20 patients (51.3%) exhibited SD and PR
occured in 17 patients (43.6%), whilst in the 40 HRT patients, PR
occured in 15 patients (37.5%) and SD in 25 patients (62.5%). The
rate of CR and PR were not significantly different between the two
groups (48.7 vs. 37.5%, respectively; P=0.314).
Efficacy of capecitabine maintenance
therapy and HRT
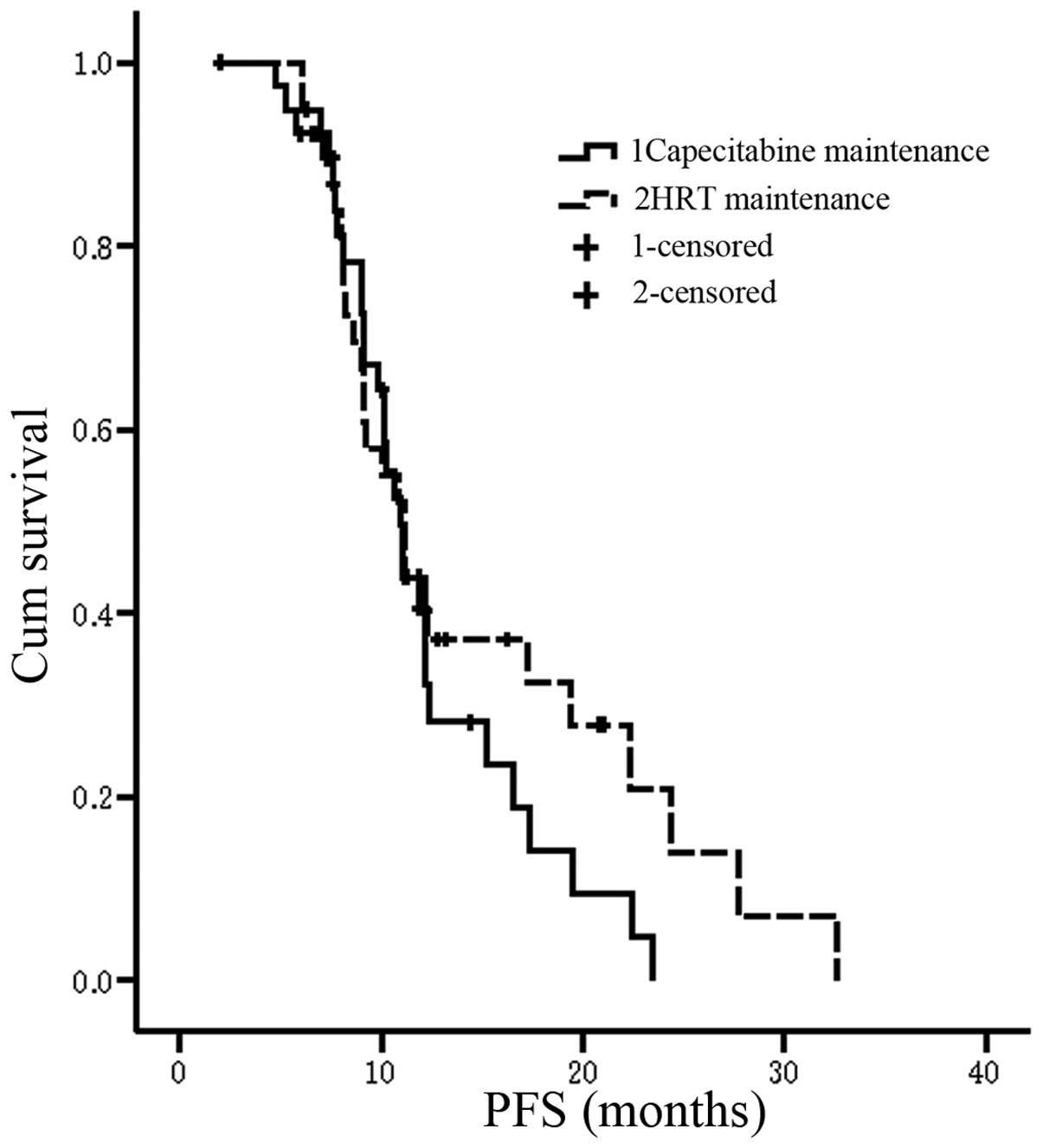
The median PFS time of patients in the TX
chemotherapy plus capecitabine maintenance therapy group was 10.9
months (95% CI, 9.9–12.0 months) and for the TX chemotherapy plus
HRT group was 11.1 months (95% CI, 8.8–13.4 months; P=0.28;
Fig. 2). Compared with the PFS time
of maintenance treatment only, TX chemotherapy plus single-agent
capecitabine treatment prolonged survival by 6.8 months (95% CI,
5.7–7.9 months), which was not significantly different to the PFS
time of TX chemotherapy plus HRT (5.8 months; 95% CI, 4.0–7.6
months; P=0.55; Fig. 3). The
6-month PFS rate of the two types of maintenance treatment were
similar (95% CI, 51.3 for capecitabine vs. 42.5% for HRT;
P=0.434).
Efficacy of maintenance therapy with or
without palliative endocrine therapy prior to chemotherapy
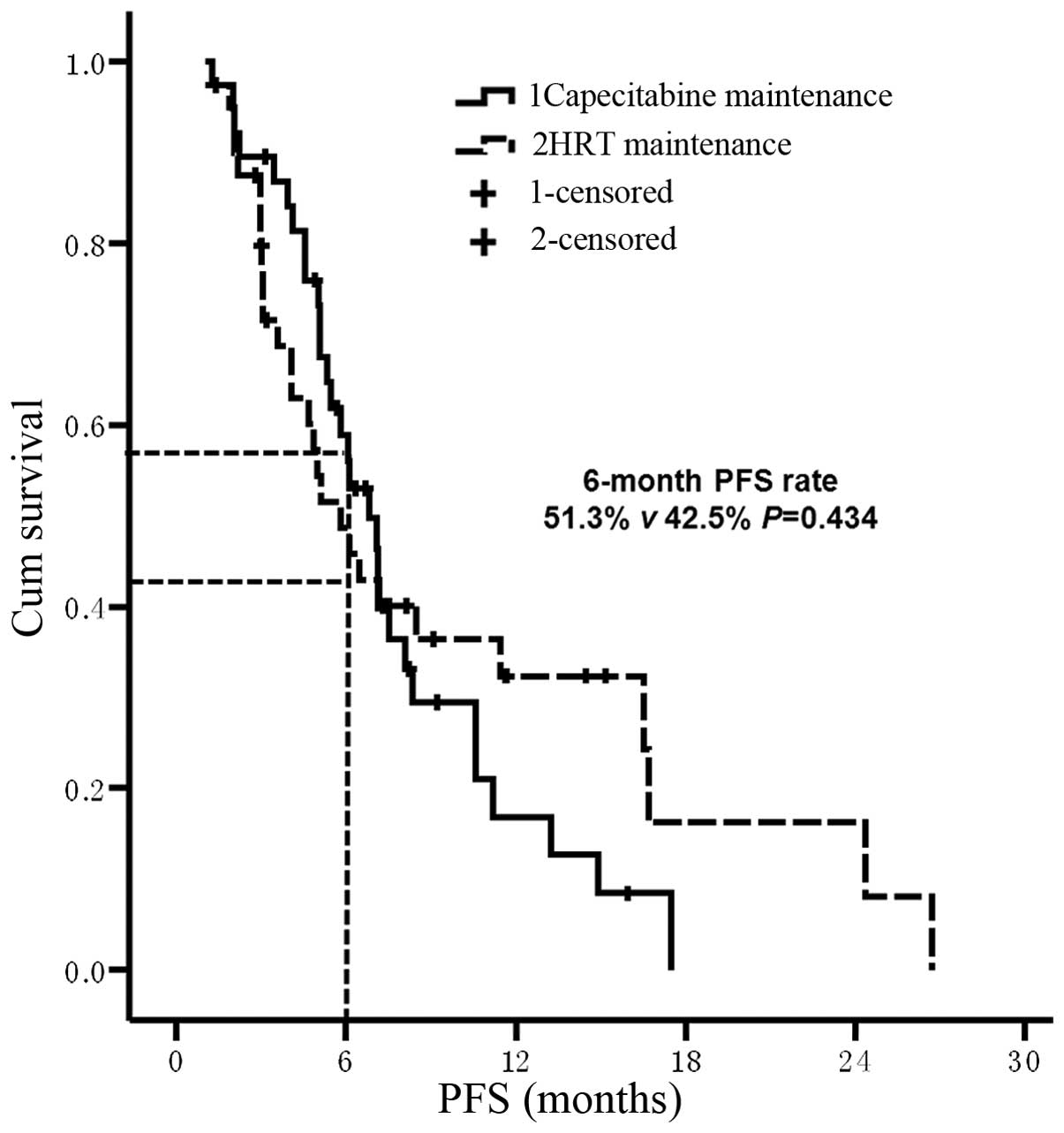
In 49 patients, the first-line treatment strategy
was not palliative hormonal therapy; this included 22 patients in
the capecitabine maintenance group and 27 patients in the HRT
maintenance group. For these 49 patients, the median PFS time from
maintenance treatment was 6.1 months in the capecitabine group and
11.5 months in the HRT group (P=0.045; Fig. 4). Prior to the administration of TX
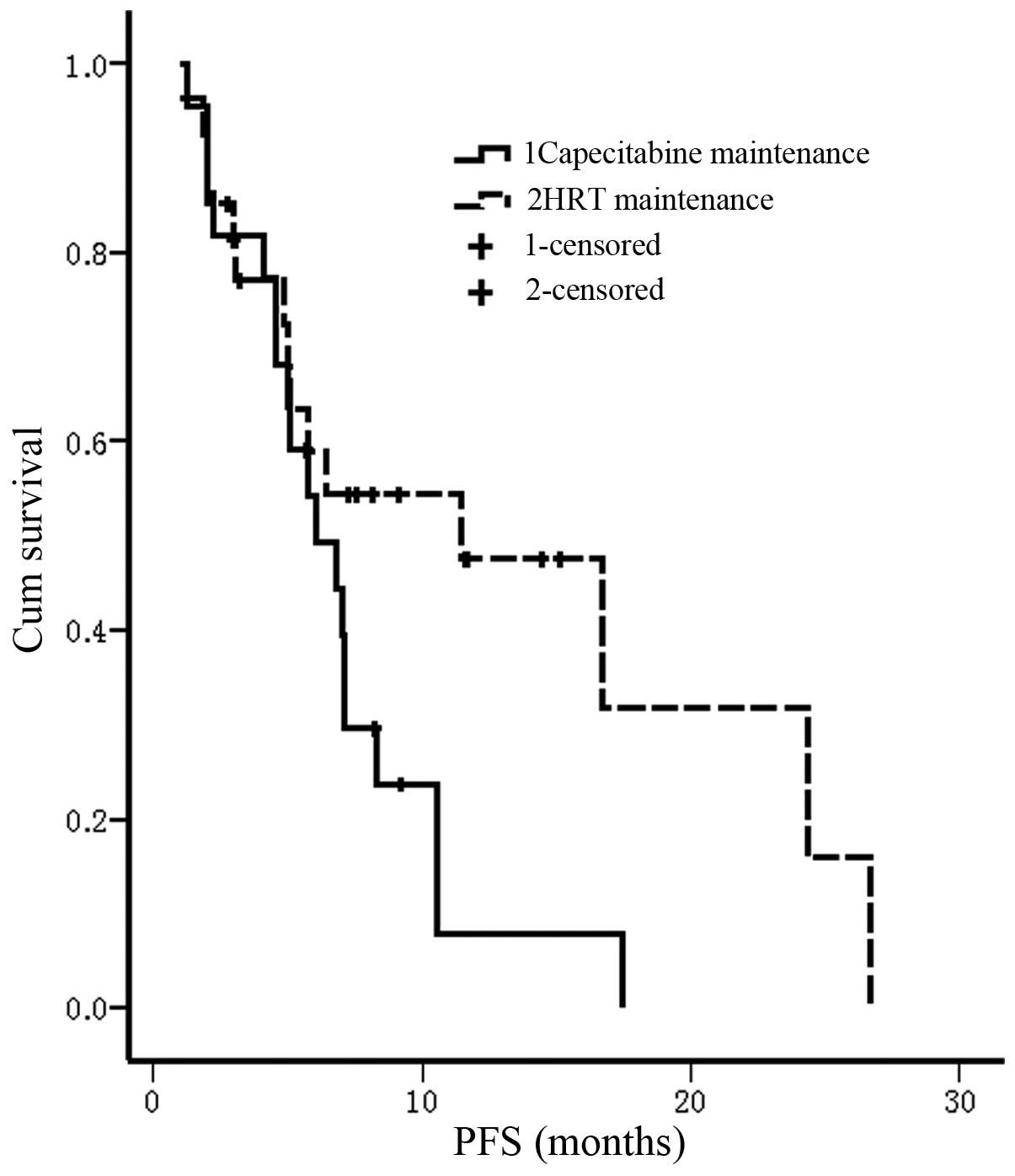
chemotherapy, 30 patients had received palliative hormonal therapy
as first-line therapy for metastatic breast cancer, including 17
patients in the capecitabine maintenance group and 13 in the HRT
group. The median PFS time of these 30 patients from maintenance
treatment was 7.5 months in the capecitabine group and 4.1 months
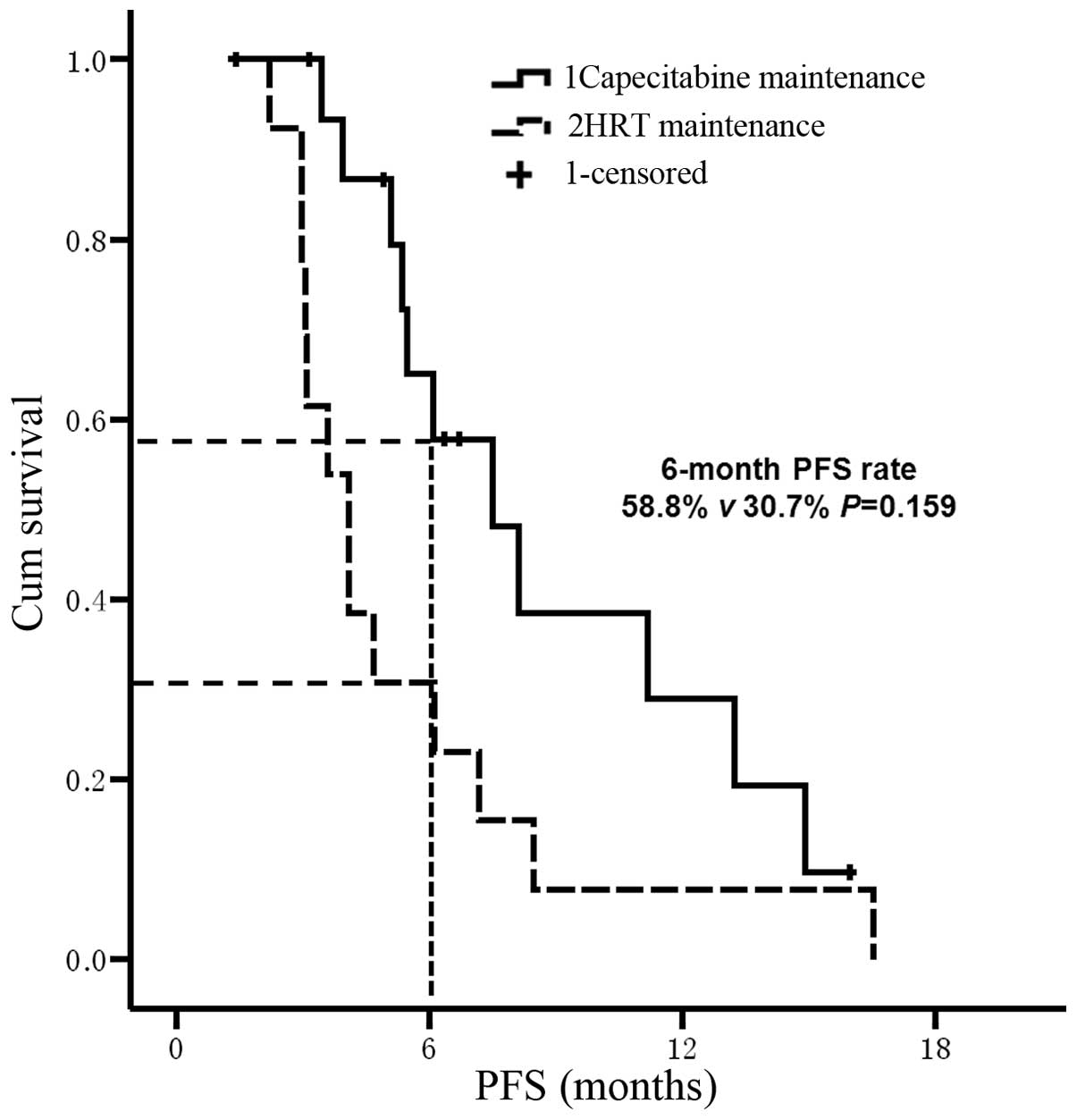
in the HRT group (P=0.043; Fig. 5).
Furthermore, the 6-month PFS rate was 58.8% in the capecitabine
maintenance group and 30.7% in the HRT group (P=0.159; Fig. 5). No significant difference was
identified between the two maintenance groups; however, this may
have been due to an insufficient number of cases being
investigated, as it was observed that the 6-month PFS rate of the
capecitabine maintenance group was almost twice that of the HRT
group.
Toxicity analysis
Table II indicates
the treatment-associated toxicities [according to National Cancer
Institute Common Terminology Criteria for Adverse Events (19)] of 79 patients observed in the
present study. Hematologic and gastrointestinal toxicities, as well
as hand-foot syndrome did not occur at significantly different
rates in the two groups. For example, the rate of grade III
neutropenia was marginally higher in the capecitabine maintenance
group compared with the HRT group (20.5 vs. 10.0%, respectively;
P=0.225), and the mean incidence of hand-foot syndrome was markedly
greater in the capecitabine group compared with the HRT group (48.7
vs. 27.5%, respectively; P=0.052).
 | Table IITreatment-associated toxicities. |
Table II
Treatment-associated toxicities.
| Adverse event | Capecitabine
maintenance, n (%) | HRT maintenance, n
(%) | P-value |
|---|
| Neutropenia,
grade | | | 0.492 |
| 0 | 17 (43.6) | 23 (57.5) | |
| 1 | 4 (10.3) | 3 (7.5) | |
| 2 | 10 (25.6) | 10 (25.0) | |
| 3 | 8 (20.5) | 4 (10.0) | |
| 4 | 0 (0.0) | 0 (0.0) | |
| Vomiting/diarrhea,
grade | | | 0.433 |
| 0 | 27 (69.2) | 29 (72.5) | |
| 1 | 6 (15.4) | 7 (17.5) | |
| 2 | 3 (7.7) | 4 (10.0) | |
| 3 | 3 (7.7) | 0 (0.0) | |
| 4 | 0 (0.0) | 0 (0.0) | |
| Hand-foot syndrome,
grade | | | 0.052a |
| 0 | 20 (51.3) | 29 (72.5) | |
| 1 | 7 (17.9) | 3 (7.5) | |
| 2 | 4 (10.3) | 2 (5.0) | |
| 3 | 8 (20.5) | 6 (15.0) | 0.521b |
Discussion
The long-term survival of female MBC patients
remains poor, despite decades of research into systemic therapy
(20). Systemic therapy uses
chemotherapy or hormonal therapy, depending on factors, such as
hormone receptor status, performance status, disease bulk, number
of disease sites and patient age. For HR-positive patients, initial
chemotherapy may be selected as the treatment modality due to the
aggressive nature of the disease; in particular, combination
chemotherapy has demonstrated a number of potential benefits,
including an increased therapeutic response, a shorter time to
progression and the possibility of improved overall survival. Thus,
chemotherapy is often selected as the the priority treatment
strategy in patients exhibiting visceral metastasis (21). However, upon the termination of
chemotherapy for metastatic disease, disease progression occurs
quickly. For example, studies conducted by Park et al
(22) and Alba et al
(4) demonstrated that the median
PFS time following chemotherapy termination was 3.8 and 5.1 months,
respectively. Therefore, it is important that maintenance therapy
for MBC patients is conducted. If a patient exhibits a hormone
receptor-positive tumor, the majority of healthcare workers would
initiate treatment with maintenance hormonal therapy following the
completion of chemotherapy, despite the lack of prospective
randomized trials regarding its efficacy (23). However, for patients with
HR-negative tumors, endocrine-resistant disease of the luminal
subtype or rapidly proliferative and/or symptomatic disease, there
is no preferred method for maintaining stable disease. Recently,
the Korean Cancer Study Group conducted a phase III clinical trial
of HER2-negative MBC patients who had achieved disease control
following six cycles of first-line paclitaxel/gemcitabine
chemotherapy (22). The study
determined that subsequent gemcitabine/paclitaxel maintenance
chemotherapy was associated with a statistically significant
increase in the median and 6-month PFS rates, as well as an
increase in the overall survival period (22). Furthermore, single-agent
chemotherapy was considered to be an effective maintenance
treatment and was the preferred choice compared with combination
agents.
The present study considered DX chemotherapy to be
the preferred treatment stratetgy for MBC patients due to its
positive response and tolerable side effects. In 2002,
O’Shaughnessy et al (14)
conducted a phase III study comparing the effects of docetaxel
administraton alone with docetaxel in combination with capecitabine
(TX chemotherapy). The addition of capecitabine to docetaxe
treatment resulted in an extended time to disease progression,
improved overall survival and more manageable side effects.
Similarly, a PFS time of 11 months for TX chemotherapy was
determined in the present study. Additionally, the total and
maintenance PFS times were similar between the capecitabine and HRT
maintenence groups (10.9 and 6.8 months vs. 11.1 and 5.8 months).
Approximately half of the patients maintained their response to
combination chemotherapy for >6 months and achieved a clinical
benefit in regardless of whether they were in the capecitabine or
HRT maintenence group; however, six patients received HRT for
maintenance treatment >12 months and two patients for >20
months, while four patients received capecitabine >1 year. The
improved response in the HRT group may be because HRT is better
tolerated compared with capecitabine. For the 49 patients who did
not undergo palliative endocrine therapy, the use of HRT for
maintenance therapy demonstrated a longer PFS time (11.5 months vs.
6.1 months), consistent with previous reports (6,24).
Additionally, of the 30 patients who received HRT as first-line
metastasis treatment prior to TX chemotherapy administration, the
capecitabine maintenance group exhibited a higher PFS compared with
the HRT maintenence group. This significant reduction in PFS
(P=0.043) may be associated with endocrine resistance caused by
repeated HRT (25–27). In the present study, ~70% patients
were postmenopausal; and according to the results of several
clinical trials, postmenopausal advanced breast cancer patients are
initially recommended to undergo endocrine therapy predominantly
consisting of a nonsteroidal (letrozole or anastrozole) or
steroidal (exemestane) aromatase inhibitor (28–30).
However, even if this type of hormonal therapy is initially
effective, it considered to be ineffective following relapse caused
by acquired resistance (26).
According to the results of the present study, capecitabine may be
an optional maintenance treatment for patients who are resistant to
endocrine therapy.
Numerous trials have been conducted that indicate
that the use of continuous chemotherapy for the treatment of breast
cancer prolongs the duration of remission; however, its effect on
quality of life and survival are less consistent (3,31,32).
Recently, a meta-analysis was conducted, which analyzed the data
from 11 randomized trials. A longer duration period of first-line
chemotherapy was associated with a markedly improved PFS period
(5); however, it is essential that
the appropriate agent is selected for maintenance treatment by
considering its impact on quality of life and the extent of
toxicity, against the improvement in disease-associated symptoms
and the benefits of tumor regression. Using these considerations,
capecitabine was selected as an appropriate candidate agent for
patients who responded to initial TX chemotherapy. The current
study indicated that single-agent capecitabine maintenance
treatment was well tolerated and its ability to be orally
administered avoids the need for a central venous device, thus,
reducing discomfort and the risk of developing a central venous
catheter infection. Furthermore, the use of oral capecitabine
reduces the hospitalization and administration costs and appears to
improve the patient quality of life.
In conclusion, the results of the present study
indicate that single-agent capecitabine maintenance therapy may be
an a potential therapeutic strategy for MBC patients who have
responded to capecitabine-based chemotherapy prior to disease
progression. In particular, capecitabine may offer a more effective
maintenance treatment duration compared with HRT for patients who
have previously undergone first-line palliative HRT for MBC.
Acknowledgements
The authors thank Dr Youyong Li of the Beijing
Institute for Cancer Research, Professor Xing Zhou of Peking
University and Professor Xingjie Liang of the National Center for
Nanoscience and Technology for reviewing the manuscript.
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Thun MJ, Jemal A and Ward E: Global cancer
incidence and mortality. Cancer: Principles and Practice of
Oncology. DeVita VT Jr: 9th edition. Lippincott Williams &
Wilkins; Philadelphia, PA: pp. 241–266. 2011
|
|
3
|
Gennari A, Amadori D, De Lena M, et al:
Lack of benefit of maintenance paclitaxel in first-line
chemotherapy in metastatic breast cancer. J Clin Oncol.
24:3912–3918. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Alba E, Ruiz-Borrego M, Margelí M, et al:
Maintenance treatment with pegylated liposomal doxorubicin versus
observation following induction chemotherapy for metastatic breast
cancer: GEICAM 2001–01 study. Breast Cancer Res Treat. 122:169–176.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gennari A, Stockler M, Puntoni M, et al:
Duration of chemotherapy for metastatic breast cancer: a systematic
review and meta-analysis of randomized clinical trials. J Clin
Oncol. 29:2144–2149. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dufresne A, Pivot X, Tournigand C, et al:
Maintenance hormonal treatment improves progression free survival
after a first line chemotherapy in patients with metastatic breast
cancer. Int J Med Sci. 5:100–105. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bertelli G, Garrone O, Bertolotti L, et
al: Maintenance hormone therapy with letrozole after first-line
chemotherapy for advanced breast cancer. Oncology. 68:364–370.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mayer IA: Treatment of HER2-positive
metastatic breast cancer following initial progression. Clin Breast
Cancer. 9(Suppl 2): S50–S57. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Leonard R, Hennessy BT, Blum JL and
O’Shaughnessy J: Dose-adjusting capecitabine minimizes side effects
while maintaining efficacy: a retrospective review of capecitabine
for metastatic breast cancer. Clin Breast Cancer. 11:349–356. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Górnaś M and Szczylik C: Oral treatment of
metastatic breast cancer with capecitabine: what influences the
decision-making process? Eur J Cancer Care (Engl). 19:131–136.
2010. View Article : Google Scholar
|
|
11
|
Reichardt P, Von Minckwitz G,
Thuss-Patience PC, et al: Multicenter phase II study of oral
capecitabine (Xeloda®) in patients with metastatic
breast cancer relapsing after treatment with a taxane-containing
therapy. Ann Oncol. 14:1227–1233. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kusama M, Nomizu T, Aogi K, et al: Phase
II study of 4-weekly capecitabine monotherapy in
advanced/metastatic breast cancer. Breast Cancer. 17:233–240. 2010.
View Article : Google Scholar
|
|
13
|
Hortobagyi GN, Gomez HL, Li RK, et al:
Analysis of overall survival from a phase III study of ixabepilone
plus capecitabine versus capecitabine in patients with MBC
resistant to anthracyclines and taxanes. Breast Cancer Res Treat.
122:409–418. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
O’Shaughnessy J, Miles D, Vukelja S, et
al: Superior survival with capecitabine plus docetaxel combination
therapy in anthracycline-pretreated patients with advanced breast
cancer: phase III trial results. J Clin Oncol. 20:2812–2823. 2002.
View Article : Google Scholar
|
|
15
|
Blum JL, Dees EC, Vukelja SJ, et al: Phase
II trial of capecitabine and weekly paclitaxel in patients with
metastatic breast cancer previously treated with every-3-week
taxane therapy. Clin Breast Cancer. 7:465–470. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chan A and Verrill M: Capecitabine and
vinorelbine in metastatic breast cancer. Eur J Cancer.
45:2253–2265. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Eisenhauer EA, Therasse P, Bogaerts J, et
al: New response evaluation criteria in solid tumours: revised
RECIST guideline (version 1.1). Eur J Cancer. 45:228–247. 2009.
View Article : Google Scholar
|
|
18
|
Oken MM, Creech RH, Tormey DC, et al:
Toxicity and response criteria of the Eastern Cooperative Oncology
Group. Am J Clin Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Trotti A, Colevas AD, Setser A, et al:
CTCAE v3.0: development of a comprehensive grading system for the
adverse effects of cancer treatment. Semin Radiat Oncol.
13:176–181. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nicolini A, Giardino R, Carpi A, et al:
Metastatic breast cancer: an updating. Biomed Pharmacother.
60:548–556. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cardoso F, Bedard PL, Winer EP, et al;
ESO-MBC Task Force. International guidelines for management of
metastatic breast cancer: combination vs. sequential single-agent
chemotherapy. J Natl Cancer Inst. 101:1174–1181. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Park YH, Jung KH, Im SA, et al: Phase III,
multicenter, randomized trial of maintenance chemotherapy versus
observation in patients with metastatic breast cancer after
achieving disease control with six cycles of gemcitabine plus
paclitaxel as first-line chemotherapy: KCSG-BR07-02. J Clin Oncol.
31:1732–1739. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Martín M and López-Tarruella S:
Chemotherapy: Maintenance therapy in breast cancer - many questions
remain. Nat Rev Clin Oncol. 10:370–372. 2013. View Article : Google Scholar
|
|
24
|
Sledge GW Jr, Hu P, Falkson G, Tormey D
and Abeloff M: Comparison of chemotherapy with chemohormonal
therapy as first-line therapy for metastatic, hormone-sensitive
breast cancer: an Eastern Cooperative Oncology Group Study. J Clin
Oncol. 18:262–266. 2000.PubMed/NCBI
|
|
25
|
Beaver JA and Park BH: The BOLERO-2 trial:
the addition of everolimus to exemestane in the treatment of
postmenopausal hormone receptor-positive advanced breast cancer.
Future Oncol. 8:651–657. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ali S and Coombes RC: Endocrine-responsive
breast cancer and strategies for combating resistance. Nat Rev
Cancer. 2:101–112. 2002. View
Article : Google Scholar
|
|
27
|
Burstein HJ: Novel agents and future
directions for refractory breast cancer. Semin Oncol. 38(Suppl 2):
S17–S24. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mouridsen H, Sun Y, Gershanovich M, et al:
Superiority of letrozole to tamoxifen in the first-line treatment
of advanced breast cancer: evidence from metastatic subgroups and a
test of functional ability. Oncologist. 9:489–496. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nabholtz JM, Buzdar A, Pollak M, et al:
Anastrozole is superior to tamoxifen as first-line therapy for
advanced breast cancer in postmenopausal women: results of a north
american multicenter randomized trial. Arimidex Study Group. J Clin
Oncol. 18:3758–3767. 2000.PubMed/NCBI
|
|
30
|
Mauri D, Pavlidis N, Polyzos NP and
Ioannidis JP: Survival with aromatase inhibitors and inactivators
versus standard hormonal therapy in advanced breast cancer:
meta-analysis. J Natl Cancer Inst. 98:1285–1291. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Falkson G, Gelman RS, Pandya KJ, et al:
Eastern Cooperative Oncology Group randomized trials of observation
versus maintenance therapy for patients with metastatic breast
cancer in complete remission following induction treatment. J Clin
Oncol. 16:1669–1676. 1998.PubMed/NCBI
|
|
32
|
Nooij MA, de Haes JC, Beex LV, et al;
EORTC Breast Cancer Group. Continuing chemotherapy or not after the
induction treatment in advanced breast cancer patients: Clinical
outcomes and oncologists’ preferences. Eur J Cancer. 39:614–621.
2003. View Article : Google Scholar : PubMed/NCBI
|