Introduction
Lung cancer remains the leading cause of cancer
morbidity and mortality worldwide (1,2).
Lobectomy by thoracotomy is recognized as a primary procedure in
the treatment of early-stage non-small cell lung cancer (NSCLC)
(3–6). Although lobectomy via thoracotomy
provides optimal locoregional control and long-term survival, this
procedure is associated with high mortality and morbidity rates:
2–10 and 30–50%, respectively (2–6).
Therefore, the identification of alternative techniques that
diminish surgical trauma without compromising oncological outcome
is required. Jacobaeus first performed thoracoscopy for inspecting
the pleural space in 1910 (7).
Until the 1980s, thoracoscopy was only employed for diagnosis of
pleural diseases. However, after McKenna reported his initial
experiences of 44 patients who had undergone video-assisted
thoracoscopic surgery (VATS) in 1994 (8), major advances in VATS, such as VATS
lobectomy and VATS segmentectomy, were achieved in the late 1990s
(9). With the worldwide employment
of VATS, reduced postoperative trauma and postoperative morbidity
has been reported (10–12). Certain surgeons employ VATS
lobectomy to reduce surgical trauma. However, the oncological
outcomes following VATS lobectomy, as measured using mediastinal
lymph node dissection and long-term survival times, have not been
fully elucidated (9,13–19).
In addition, few multi-center randomized controlled trials that
compare the two approaches and the long-term oncological outcomes
have been conducted (16–19).
VATS lobectomy for clinical stage I NSCLC was
introduced to Jingling Hospital (Nanjing, China) in January 2008.
The thoracic surgeons in the Department of Thoracic Surgery of the
hospital have the basic ability to perform VATS. The present study
aimed to assess the oncological outcomes following VATS lobectomy
by reviewing five years of experience performing VATS lobectomy at
the hospital.
Patients and methods
Patient evaluation
The present retrospective study complied with the
Declaration of Helsinki rules and was approved by the Ethics
Committee of Jinling Hospital (Nanjing, China). The requirement for
informed consent from all patients was waived due to the
retrospective nature of the study.
Data from 212 consecutive patients with clinical
stage I NSCLC who underwent lobectomy at the Department of Thoracic
Surgery, Nanjing General Hospital of Nanjing Military Command
(Nanjing, China) between February 2003 and July 2013 were
retrospectively reviewed. All patients underwent bronchoscopy,
endobronchial ultrasound, and computed tomographic scans of the
brain, chest and upper abdomen prior to surgery. Mediastinoscopy
was not required except when positive mediastinal or hilar lymph
nodes were detected using the chest computed tomographic scan.
Positron emission tomography-computerized tomography (CT) and bone
scanning were performed on all patients. Classification of the
tumor clinical stage was determined by the 7th edition of the TNM
classification of lung cancer (20), which was proposed by the Union for
International Cancer Control (UICC) and the International
Association for the Study of Lung Cancer (IASLC). The mediastinal
lymph node staging was determined by the most recent lymph node map
proposed by the IASLC (21). For
those patients who underwent surgery prior to 2009, staging was
recalculated to match the 7th edition of TNM classification of lung
cancer proposed by UICC and IASLC (21).
Surgical technique
All surgical procedures, including VATS and
thoracotomy, were performed by three senior surgeons with proven
expertise in lung cancer, who had conducted >60 VATS lobectomies
and >300 open lobectomies prior to the present study. The
selection of VATS or open lobectomy was decided by the patients and
their families. The resection was considered to be administered
with curative intent (R0) in all cases. Only trocars and endsocopic
instruments were used in the VATS lobectomies and no rib spreading
was performed. All patients underwent one-lung ventilation and were
placed in the lateral decubitus position. Mediastinal lymph node
dissection was routinely performed. We did not perform an
intra-operative lymph node frozen section analysis, due to the
time-consuming nature of the procedure.
On the left side, the 5, 6, 7, 8, 9, 10, 11 and 12
lymph node stations, and on the right side, the 2R, 4R, 7, 8, 9,
10, 11 and 12 lymph node stations were systematically dissected
en bloc. The lymph nodes were dissected with the integrity
of the surrounding structure, with clear recognition of the
anatomical landmarks and with no nodal structures. Lymph node
stations 10, 11 and 12, and the affected lobes were systematically
dissected. On the right side, when the mediastinal pleura were
opened, stations 2R and 4R was dissected up to the lowest visible
section of the subclavian artery. After the lung was retracted
anteriorly, station 7 dissection was performed. Regardless of
middle lobectomy and lower lobectomy, stations 8 and 9 were
systematically harvested. On the left side, stations 5 and 6 were
systematically harvested. Subsequently, dissection of stations 7, 8
and 9 was performed (16).
Surgical outcome and post-operative
complications
The operative time, degree of blood loss,
pathological stage, overall number of lymph nodes dissected,
residual tumor status, post-operative morbidity occurring within 30
postoperative days and length of hospital stay were assessed. The
morbidity occurring within 30 postoperative days, which included
major and minor complications, was graded according to the
Clavien-Dindo classification (22).
Major complications were defined as grades 3b, 4a, 4b and 5. Minor
complications were classified as grades 1, 2 and 3a. The 30-day
mortality was defined as all-cause fatality within 30 postoperative
days.
Follow-up
During the first year following treatment, the
patients were assessed every three months at the outpatient
department. In the second year, follow-up was conducted every six
months and, subsequently, follow-up was performed at the end of
each year. During follow-up, diagnostic investigations were
performed. All patients received CT chest scans prior to discharge
and prior to each follow-up visit. Any post-operative complications
and medical conditions that required hospitalization were reviewed.
The final follow-up assessment occurred in November 2013.
Statistical analysis
For statistical analysis, SPSS 13.0 for Windows
(SPSS, Inc., Chicago, IL, USA) was used. Data are presented as the
mean ± standard deviations for variables following a normal
distribution, and were analyzed by Student’s t-test. For variables
following a non-normal distribution, the results are expressed as
the median and range, and were compared by nonparametric test.
Differences of semi-quantitative results were analyzed by the
Mann-Whitney U test. Differences of qualitative results were
analyzed by the χ2 or Fisher exact test, where
appropriate. The survival rates were analyzed using the
Kaplan-Meier method; the differences between the two groups were
analyzed with the log-rank test. The overall survival time was
classified as the time period between the date of surgery and the
date of the final follow-up or fatality from any cause. The
disease-free survival time was calculated as the time period
between the date of surgery and the date of cancer recurrence or
fatality from any cause. Univariate analysis was performed to
identify prognostic variables associated with overall survival
time. Univariate variables with P<0.05 were selected for
inclusion in the multivariate Cox proportional-hazards regression
model. The adjusted odds ratios along with the corresponding 95%
confidence intervals were calculated. P<0.05 was considered to
indicate a statistically significant difference.
Results
Demographic data
The demographic data are summarized in Table I. During the present study, 123
lobectomies by VATS and 89 lobectomies by thoracotomy were
performed. No significant differences were identified in age,
gender, comorbidity, forced expiratory volume in the first second
(observed to predicted), tumor size, clinical stage, number of
mediastinoscopies and American Society of Anesthesiologists
Physical Status classification system score (23) between the two groups
(P>0.05).
 | Table IDemographic data. |
Table I
Demographic data.
| Parameter | VATS (n=123) | Thoracotomy
(n=89) | P-value |
|---|
| Age, yearsa | 65.0 (50–70) | 64.0 (46–75) | 0.859 |
| Gender,
male:female | 74:49 | 51:38 | 0.676 |
| Comorbidity, n | | | 0.373 |
| COPD | 2 | 1 | |
| Hypertension | 12 | 8 | |
| Diabetes
Mellitus | 6 | 2 | |
| Smoking | 46 | 32 | |
| Atrial
fibrillation | 1 | 2 | |
| Earlier myocardial
infarction | 1 | 2 | |
| FEV1 (observed to
predicted), %b | 86.0 (76–95) | 86.0 (80–98) | 0.920 |
| Tumor size,
cmb | 1.90 (0.8–3.9) | 1.90 (0.5–3.9) | 0.675 |
| Clinical stage,
n | | | 0.750 |
| IA | 65 | 49 | |
| IB | 58 | 40 | |
| Mediastinoscopy,
n | 2 | 3 | 0.713 |
| ASA score, n | | | 0.546 |
| I | 66 | 42 | |
| II | 59 | 45 | |
| III | 1 | 2 | |
Surgical outcome and pathological
data
The surgical and pathological outcomes are
summarized in Table II. No
conversion to open lobectomy occurred in the cases where VATS was
performed. No intraoperative or in-hospital mortality occurred in
either group. Significantly longer operative times were recorded in
the VATS group as compared with the open surgery group (P<0.05).
No significant differences in pathological stage or residual tumor
status between the two groups was identified (P>0.05). Patients
in the VATS group exhibited significantly faster recovery, with
reduced blood loss (P<0.05), less post-operative analgesia
required (P<0.05) and earlier hospital discharge (P<0.05)
than the patients who had undergone thoracotomy.
 | Table IISurgical and pathological data. |
Table II
Surgical and pathological data.
| Clinical
parameter | VATS (n=123) | Thoracotomy
(n=89) | P-value |
|---|
| Type of resection,
n | | | 0.305 |
| Left upper
lobectomy | 36 | 16 | |
| Left lower
lobectomy | 24 | 20 | |
| Right upper
lobectomy | 21 | 13 | |
| Right middle
lobectomy | 10 | 8 | |
| Right lower
lobectomy | 32 | 32 | |
| Operative time,
mina | 200.0
(120–380) | 160.0
(100–330) | 0.000 |
| Blood loss,
mla | 160.0
(100–320) | 210.0
(110–500) | 0.000 |
| Histological type,
n | | | 0.166 |
|
Adenocarcinoma | 101 | 81 | |
| Squamous cell
carcinoma | 20 | 7 | |
| Other | 2 | 1 | |
| Pathological stage,
n | | | 1.000 |
| IA | 42 | 26 | |
| IB | 64 | 51 | |
| IIA | 10 | 7 | |
| IIB | 5 | 3 | |
| IIIA | 2 | 2 | |
| Residual tumor,
R0/R1/R2, n | 122/1/0 | 87/2/0 | 0.384 |
| Post-operative
analgesia, daysb | 2.0 (1.0–5.0) | 5.0 (1.0–6.0) | 0.000 |
| Duration of chest
drainage, daysb | 6.0 (3–9) | 7.0 (5–13) | 0.000 |
| Hospital stay,
daysb | 8.0 (6–21) | 16.0 (11–21) | 0.000 |
Lymph nodes and stations harvested
The nodes and stations harvested, including N1 and
N2, are summarized in Table III.
No significant differences between the two groups were detected
when comparing either the number of lymph node stations or the
overall number of lymph nodes dissected (P>0.05) The numbers of
harvested lymph nodes and lymph node stations were also similar in
the two groups (P>0.05). The number of harvested lymph nodes in
each resection was >10 and a minimum of six lymph node stations
from each patient were harvested. The lymphadenectomy results were
comparable with those of other studies (Table IV).
 | Table IIINodes and stations harvested. |
Table III
Nodes and stations harvested.
| Parametera | VATS (n=123) | Thoracotomy
(n=89) | P-value |
|---|
| No. of harvested
lymph node stations | 8.0 (6–8) | 8.0 (6–8) | 0.449 |
| No. of mediastinal
lymph node stations dissected | 5.0 (3–5) | 5.0 (3–5) | 0.344 |
| No. of harvested
lymph nodes | 28.0 (22–36) | 28.0 (22–40) | 0.164 |
| No. of mediastinal
lymph nodes dissected | 17.0 (12–23) | 17.0 (12–28) | 0.110 |
 | Table IVLiterature review of mediastinal
lymph node dissection using VATS vs. thoracotomy. |
Table IV
Literature review of mediastinal
lymph node dissection using VATS vs. thoracotomy.
| Study | No. patients | Lymph nodes | N1 lymph nodes | N2 lymph nodes | Lymph node
stations |
|---|
| Palade et al
(18) (2013, Germany) | VATS: 32 | 25.1 | 10.5 | NR | NR |
| Open: 32 | 25.2 | 8.9 | | |
| Yang et al
(16) (2013, China) | VATS: 31 | 28.2 | 9.5 | 18.6 | 6.8 |
| Open: 31 | 29.8 | 8.4 | 21.4 | 6.7 |
| Ramos et al
(15) (2012, France) | VATS: 96 | 22.6 | NR | 17.7 | 5.1 |
| Open: 200 | 25.4 | | 18.2 | 4.5 |
| Watanabe et
al (17) (2005, Japan) | VATS: 191 | 33.8 | NR | 23.4 | NR |
| Open: 159 | 30.9 | | 21.0 | |
Post-operative complications
The post-operative complications in the VATS and
thoracotomy groups are reviewed in Table V. The overall morbidity within 30
postoperative days was similar in the two groups (P>0.05).
However, when the severity of complications was compared, a
significantly greater number of complications were classified as
major in patients who had undergone thoracotomy, as compared with
the VATS patients (P<0.05).
 | Table VPost-operative complications. |
Table V
Post-operative complications.
| Adverse event | VATS (n=123) | Thoracotomy
(n=89) | P-value |
|---|
| Post-operative
complications, n | 31 | 21 | 0.763 |
| Severity of
complications, n | | | 0.002 |
| Major (3b, 4a, 4b
or 5) | 4 | 11 | |
| Minor (1, 2 or
3a) | 27 | 10 | |
| Major, n | | | 1.000 |
| Pulmonary
embolism | 1 | 3 | |
| Acute coronary
syndrome | 1 | 2 | |
| Respiratory
insufficiency | 1 | 4 | |
| DIC | 1 | 2 | |
| Minor, n | | | 1.000 |
| Pneumonia | 6 | 2 | |
| Wound
infection | 3 | 1 | |
| Urinary tract
infection | 4 | 1 | |
| Atrial
fibrillation | 5 | 1 | |
| Chylothorax | 3 | 1 | |
| Recurrent nerve
palsy | 3 | 1 | |
| Prolonged air leak
(>5 days) | 3 | 3 | |
| Mortality within 30
days after surgery, n | 0 | 0 | |
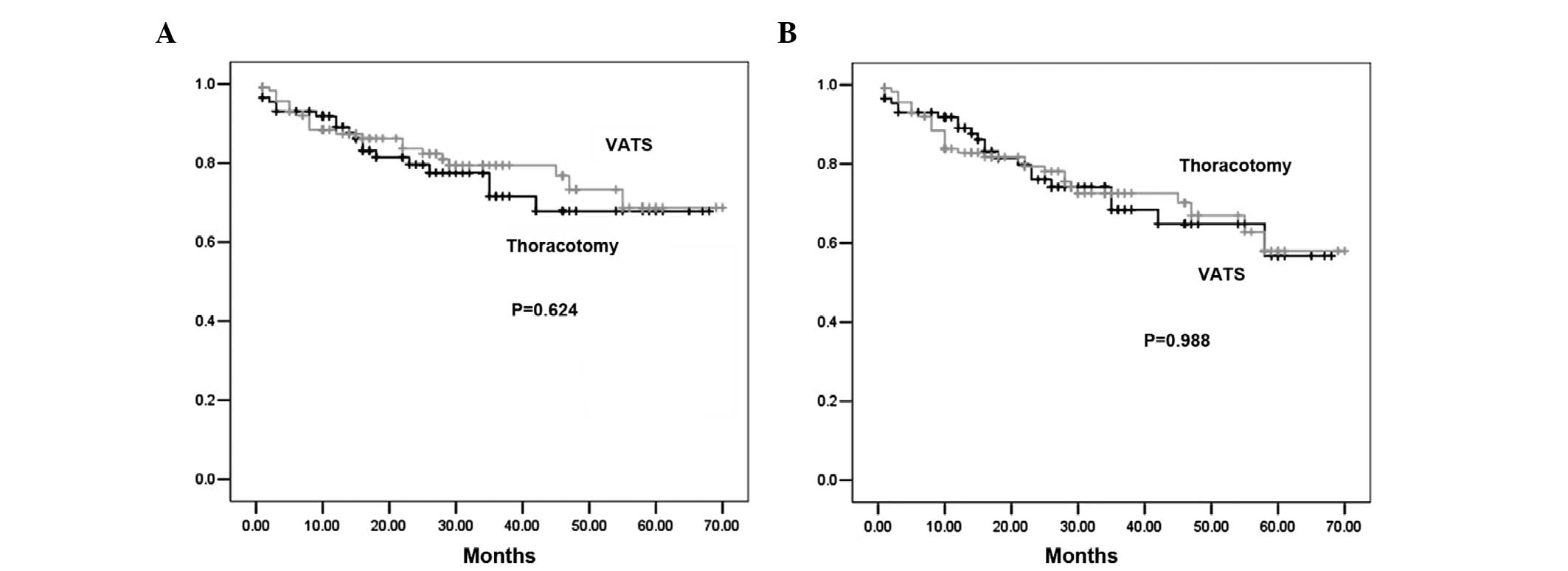
Overall survival
The median follow-up duration was 36 months, with
similar average follow-up times in the two groups. No differences
in overall survival times between the VATS and open surgery groups
were identified (P=0.624; Fig. 1).
The three- and five-year overall survival rates were 79.2 and
71.6%, respectively, in the VATS group as compared with 72.6 and
68.0%, respectively, in the thoracotomy group. Multivariate Cox
regression analysis of the overall survival times of all patients
in the whole cohort was also performed. Significant predictors of
shorter overall survival times were T3 pathological stage
(P=0.001), pathological N1 or N2 disease (P=0.001), and poor tumor
differentiation (P=0.005) (Table
VI). The VATS surgical approach was not found to be a
significant predictor for overall survival time by univariate
analysis.
 | Table VIMultivariate Cox regression analysis
of overall survival times. |
Table VI
Multivariate Cox regression analysis
of overall survival times.
| Regression
variable | Adjusted hazard
ratio | 95% CI | P-value |
|---|
| Pathological T
stage |
| T1 | 1.00 | | |
| T2 | 1.23 | 0.51–2.36 | 0.896a |
| T3 | 2.36 | 1.52–5.69 | 0.001a |
| Pathological N
stage |
| N0 | 1.00 | | |
| N1/N2 | 1.23 | 0.65–3.65 | 0.001b |
| Differentiation
grade |
| Good | 1.00 | | |
| Moderate | 1.36 | 0.36–2.36 | 0.259c |
| Poor | 3.25 | 1.23–6.89 | 0.005c |
Disease-free survival
When the disease-free survival rates were examined,
the three- and five-year disease-free survival rates were 75.3 and
59.0%, respectively, in the VATS group as compared with 70.1 and
58.2%, respectively, in the thoracotomy group, with no
statistically significant differences identified between the two
groups (P=0.988; Fig. 1). To
determine whether the patients who underwent VATS exhibited a
higher incidence of recurrent cancer as compared with the open
surgery patients, recurrence patterns and time to recurrence were
examined (Table VII). The
location of the recurrence and the time to recurrence were not
significantly different between the two groups. No port-site
recurrence was noted in the VATS cases. Multivariate Cox regression
analysis of disease-free survival times revealed that significant
predictors of shorter disease-free survival times were advanced
pathological T3 stage (P=0.023), pathological N1 or N2 disease
(P=0.003) and poor tumor differentiation (P=0.020) (Table VIII). The surgical approach was
not found to be a significant predictor for reduced disease-free
survival times. The oncological outcomes were comparable with those
of other large sample size studies (Table IX).
 | Table VIIComparison of recurrence pattern and
site following lobectomy. |
Table VII
Comparison of recurrence pattern and
site following lobectomy.
| Recurrence
parameter | VATS | Thoracotomy | P-value |
|---|
| Overall recurrence,
n (%) | 15 (12.1) | 12 (13.5) | 1.000 |
| Locoregional, n
(%) | 8 (6.5) | 7 (7.9) | 1.000 |
| Mediastinal lymph
node | 2 | 1 | |
| Pleura | 2 | 3 | |
| Ipsilateral
lung | 4 | 3 | |
| Distant, n (%) | 7 (5.6) | 5 (5.6) | 1.000 |
| Brain | 3 | 2 | |
| Liver | 2 | 2 | |
| Bone | 1 | 1 | |
| Median time to
recurrence, months | 18 | 16 | 0.360 |
 | Table VIIIMultivariate Cox regression analysis
of disease-free survival times. |
Table VIII
Multivariate Cox regression analysis
of disease-free survival times.
| Regression
variable | Adjusted hazard
ratio | 95% CI | P-value |
|---|
| Pathological T
stage |
| T1 | 1.00 | | |
| T2 | 1.36 | 0.63–2.69 | 0.450 |
| T3 | 3.20 | 1.56–4.62 | 0.023 |
| Pathological N
stage |
| N0 | 1.00 | | |
| N1/N2 | 2.31 | 0.96–4.21 | 0.003 |
| Differentiation
grade |
| Good | 1.00 | | |
| Moderate | 1.62 | 0.25–3.22 | 0.230 |
| Poor | 3.58 | 1.69–6.32 | 0.020 |
 | Table IXLiterature review of long-term
survival rates following VATS or thoracotomy. |
Table IX
Literature review of long-term
survival rates following VATS or thoracotomy.
| | | | Overall survival
rate (%) | Disease-free
survival rate (%) |
|---|
| | | |
|
|
|---|
| Study | Clinical stage | Approach | No. | Three-year | Five-year | Three-year | Five-year |
|---|
| Lee et al
(23) (2013, USA) | I | VATS | 188 | 87.4 | 76.5 | 77.7 | 61.1 |
| Open | 187 | 81.6 | 77.5 | 76.9 | 72.1 |
| Thomas et al
(24) (2002, France) | I | VATS | 110 | NR | 62.9 | NR | NR |
| Open | 404 | NR | 62.8 | NR | NR |
| Shiraishi et
al (25) (2006, Japan) | I | VATS | 81 | NR | 89.1 | NR | 79.0 |
| Open | 79 | NR | 77.7 | NR | 80.2 |
| Flores et al
(26) (2009, USA) | I | VATS | 398 | NR | 79.0 | NR | NR |
| Open | 343 | NR | 75.0 | NR | NR |
Discussion
Although VATS lobectomy for stage I NSCLC has been
widely used due to proven benefits, the merits of the technique
with regard to oncological outcomes remains controversial (24–27).
According to the Society of Thoracic Surgeons database (28), only 20% of lobectomies are performed
via VATS, with 80% conducted using conventional thoracotomy. The
success of VATS can only be definitively measured using the
long-term survival times, as compared with those following
thoracotomy. In the present study, VATS lobectomy and thoracotomy
lobectomy were compared using a consecutive series of patients who
underwent surgery performed by surgeons extensively experienced in
VATS and open lobectomies. The study demonstrated that VATS
lobectomy achieves similar oncological results to conventional
thoracotomy.
Although previous studies have been conducted
regarding the effect of mediastinal lymph node removal and
systematic mediastinal lymph node dissection on long-term survival
times (29,30), there remains controversy with regard
to the impact of lymphadenectomy on oncological outcome (31). The largest prospective randomized
control trial comparing mediastinal lymph node sampling with
dissection, termed the Z0030 trial, was reported by the American
College of Surgery Oncology Group (29). This trial revealed that mediastinal
lymph node dissection achieved similar long-term survival times as
compared with lymph node sampling in early-stage NSCLC patients
without evidence of mediastinal or hilar lymph node metastasis
confirmed by sampling. Therefore, the result of the Z0030 trial is
only suitable for highly selected NSCLC patients and is not
applicable for all operable NSCLC patients. Since producing
intra-operative frozen sections is time-consuming and the results
of the Z0030 trial are only applicable for particular patients,
intra-operative lymph node staging is not performed in China.
However, in a prospective randomized control trial involving 532
patients with clinical stage I–IIIA NSCLC, 268 patients underwent
mediastinal lymph node dissection and 264 patients underwent
mediastinal lymph node sampling performed by Chinese surgeons
(30). The five-year survival rate
in the patients who had undergone mediastinal lymph node dissection
was significantly higher than that in those who had mediastinal
lymph node sampling performed (P<0.05), regardless of the
clinical stage. Thus, systematic mediastinal lymph node dissection
was routinely performed for all operable NSCLC patients in the
present study cohort.
The quality of mediastinal lymph node dissection is
the core component when VATS lobectomy is performed. The majority
of studies previously reported have revealed no differences in the
quality of mediastinal lymph node dissection during VATS as
compared with thoracotomy (16–19).
However, certain studies have reported the quality of lymph node
dissection to be inferior to that of thoracotomy, particularly in
the early stages of VATS lobectomy (31–33).
The CALGB 39802 study (32), a
prospective multi-center study, observed that over half of the
resections in patients undergoing VATS had fewer than two stations
sampled and ~15% resections in patients undergoing VATS had no
lymph nodes harvested. The quality of lymphadenectomy in the CALGB
39802 study was far from the recommendation proposed by IASLC that
a minimum of six lymph node stations be removed or sampled in
lobectomy (31,34). IASLC also recommends that three of
these lymph node stations be mediastinal lymph nodes (31,34).
The results from the present study revealed that the number of
dissected lymph node stations and lymph nodes were similar in VATS
and thoracotomy, and that the quality of lymphadenectomy was
applied with recommendation proposed by IASLC.
An increasing number of studies have reported
similar perioperative outcomes following VATS and open lobectomy
(32,35). The results from the present study
did not differ from the conclusions drawn from other studies; VATS
lobectomy was safe and less trauma occurs, as compared with
thoracotomy (16–19). In the present study, the NSCLC
patients who underwent VATS lobectomy benefited from a quicker
recovery, with less bleeding, reduced post-operative pain, shorter
duration of chest drainage and earlier hospital discharge as
compared with the patients who underwent thoracotomy lobectomy, and
these findings were comparable with those of previous studies
(36,37).
The long-term outcomes, measured by overall survival
and disease-free survival times, were comparable with those of
other studies concerning VATS versus open lobectomy (24–27,37).
As thoracic surgeons became more skillful with VATS and recognized
the benefits, such as shorter recovery times and reduced trauma,
establishing prospective randomized multi-center trials that
compared VATS and thoracotomy was difficult, due to difficulty in
enrolling patients on a long-term basis, as VATS have a short-term
outcome when compared with open resection. Studies regarding the
long-term outcomes of VATS versus open lobectomy are mainly
retrospective and produce prospective non-randomized comparisons
(24–27,36).
These studies have revealed marginally improved overall survival
and disease-free survival times following VATS (24–27,37).
In the present study, the patients who had undergone VATS exhibited
marginally improved survival times and later recurrence than the
patients who had thoracotomy performed. This finding may be
difficult to explain. Chen et al (38) have hypothesized that the reduced
trauma during VATS lobectomy may result in quicker recovery time,
earlier administration of adjuvant chemotherapy and improved
compliance with adjuvant chemotherapy. The other factor may be that
the reduced immunological suppression during VATS as compared with
thoracotomy increases the patient’s ability to scavenge residual
cancer cells shed into the blood or lymphatics at lobectomy
(36,38–40).
However, the underlying mechanisms for this process require further
investigation.
Certain limitations of the present study must be
acknowledged. The study was based at a single center, not at
multiple centers, and the results were produced from retrospective
analysis, not prospective randomized analysis. Therefore, bias in
the selection of patients and the surgical approach, by the
surgeons, cannot be excluded. This limitation needs accounting for
when interpreting the results. Other factors that may affect
long-term outcomes and prognosis, such as adjuvant therapy and
treatment for recurrence and distant metastasis, are not completely
described by this analysis.
In summary, it is reasonable to conclude from the
present study that VATS lobectomy performed by specialist thoracic
surgeons is safe and achieves similar long-term survival times to
the open surgery approach. However, further prospective randomized
multi-center trials are warranted prior to the incorporation of
VATS into clinical routine practice.
Acknowledgements
The authors would like to thank the patients, their
families and hospital colleagues involved in this study. The study
was supported by the Natural Science Foundation of Jiangsu Province
(grant no. BK2011658).
References
|
1
|
Jacobaeus HC: Ueber die Moglichkeit die
Zystoskopie bei Untersuchung seroser Hohlungen anzuwenden. Munchner
Meditinische Wochenschrift. 57:2090–2092. 1910.
|
|
2
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
She J, Yang P, Hong Q and Bai C: Lung
cancer in China: challenges and interventions. Chest.
143:1117–1126. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ettinger DS, Akerley W, Borghaei H, et al;
National comprehensive cancer network. Non-Small Cell Lung Cancer,
Version 2.2013. J Natl Compr Canc Netw. 11:645–653. 2013.PubMed/NCBI
|
|
5
|
Lächelt S, Alber M, Söhn M, et al:
Intensity-modulated stereotactic radiotherapy for the treatment of
medically inoperable patients with NSCLC stage I. Oncol Rep.
28:1309–1314. 2012.PubMed/NCBI
|
|
6
|
Zhang J, Qi J, Chen N, et al: High
expression of a disintegrin and metalloproteinase-9 predicts a
shortened survival time in completely resected stage I non-small
cell lung cancer. Oncol Lett. 5:1461–1466. 2013.PubMed/NCBI
|
|
7
|
Song PP, Zhang W, Zhang B, Liu Q and Du J:
Effects of different sequences of pulmonary artery and vein
ligations during pulmonary lobectomy on blood micrometastasis of
non-small cell lung cancer. Oncol Lett. 5:463–468. 2013.PubMed/NCBI
|
|
8
|
McKenna RJ Jr: Lobectomy by video-assisted
thoracic surgery with mediastinal node sampling for lung cancer. J
Thorac Cardiovasc Surg. 107:879–881; discussion 881–882.
1994.PubMed/NCBI
|
|
9
|
Li Z, Liu H and Li L: Video-assisted
thoracoscopic surgery versus open lobectomy for stage I lung
cancer: A meta-analysis of long-term outcomes. Exp Ther Med.
3:886–892. 2012.PubMed/NCBI
|
|
10
|
Li W, Wang Y, He X, et al: Combination of
CT-guided hookwire localization and video-assisted thoracoscopic
surgery for pulmonary nodular lesions: Analysis of 103 patients.
Oncol Lett. 4:824–828. 2012.PubMed/NCBI
|
|
11
|
Yang CF and D’Amico TA: Thoracoscopic
segmentectomy for lung cancer. Ann Thorac Surg. 94:668–681. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhong C, Fang W, Mao T, et al: Comparison
of thoracoscopic segmentectomy and thoracoscopic lobectomy for
small-sized stage IA lung cancer. Ann Thorac Surg. 94:362–367.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee HS and Jang HJ: Thoracoscopic
mediastinal lymph node dissection for lung cancer. Semin Thorac
Cardiovasc Surg. 24:131–141. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li G: Minimally invasive thoracic surgery:
news from the 3rd Asian-Pacific VATS Performance & the 6th
China Lung Cancer MITS Forum. J Thorac Dis. 4:681–687. 2012.
|
|
15
|
Ramos R, Masuet C and Gossot D: Lobectomy
for early-stage lung carcinoma: a cost analysis of full
thoracoscopy versus posterolateral thoracotomy. Surg Endosc.
26:431–437. 2012. View Article : Google Scholar
|
|
16
|
Ramos R, Girard P, Masuet C, Validire P
and Gossot D: Mediastinal lymph node dissection in early-stage
non-small cell lung cancer: totally thoracoscopic vs thoracotomy.
Eur J Cardiothorac Surg. 41:1342–1348. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang H, Li XD, Lai RC, et al: Complete
mediastinal lymph node dissection in video-assisted thoracoscopic
lobectomy versus lobectomy by thoracotomy. Thorac Cardiovasc Surg.
61:116–123. 2013. View Article : Google Scholar
|
|
18
|
Watanabe A, Koyanagi T, Ohsawa H, et al:
Systematic node dissection by VATS is not inferior to that through
an open thoracotomy: a comparative clinicopathologic retrospective
study. Surgery. 138:510–517. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Palade E, Passlick B, Osei-Agyemang T,
Günter J and Wiesemann S: Video-assisted vs open mediastinal
lymphadenectomy for Stage I non-small-cell lung cancer: results of
a prospective randomized trial. Eur J Cardiothorac Surg.
44:244–249. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Reck M, Heigener DF, Mok T, Soria JC and
Rabe KF: Management of non-small-cell lung cancer: recent
developments. Lancet. 382:709–719. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rusch VW, Asamura H, Watanabe H, et al:
Members of IASLC Staging Committee: The IASLC lung cancer staging
project: a proposal for a new international lymph node map in the
forthcoming seventh edition of the TNM classification for lung
cancer. J Thorac Oncol. 4:568–577. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Clavien PA, Barkun J, de Oliveira ML, et
al: The Clavien-Dindo classification of surgical complications:
five-year experience. Ann Surg. 250:187–196. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Froehner M, Koch R, Litz R, Heller A,
Oehlschlaeger S and Wirth MP: Comparison of the American Society of
Anesthesiologists Physical Status classification with the Charlson
score as predictors of survival after radical prostatectomy.
Urology. 62:698–701. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee PC, Nasar A, Port JL, et al: Long-term
survival after lobectomy for non-small cell lung cancer by
video-assisted thoracic surgery versus thoracotomy. Ann Thorac
Surg. 96:951–961. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Thomas P, Doddoli C, Yena S, et al: VATS
is an adequate oncological operation for stage I non-small cell
lung cancer. Eur J Cardiothorac Surg. 21:1094–1099. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shiraishi T, Shirakusa T, Hiratsuka M,
Yamamoto S and Iwasaki A: Video-assisted thoracoscopic surgery
lobectomy for c-T1N0M0 primary lung cancer: its impact on
locoregional control. Ann Thorac Surg. 82:1021–1026. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Flores RM, Park BJ, Dycoco J, et al:
Lobectomy by video-assisted thoracic surgery (VATS) versus
thoracotomy for lung cancer. J Thorac Cardiovasc Surg. 138:11–18.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ceppa DP, Kosinski AS, Berry MF, Tong BC,
Harpole DH, Mitchell JD, D’Amico TA and Onaitis MW: Thoracoscopic
lobectomy has increasing benefit in patients with poor pulmonary
function: a Society of Thoracic Surgeons Database analysis. Ann
Surg. 256:487–493. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Scott WJ, Allen MS, Darling G, et al:
Video-assisted thoracic surgery versus open lobectomy for lung
cancer: a secondary analysis of data from the American College of
Surgeons Oncology Group Z0030 randomized clinical trial. J Thorac
Cardiovasc Surg. 139:976–983. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wu Yl, Huang ZF, Wang SY, Yang XN and Ou
W: A randomized trial of systematic nodal dissection in resectable
non-small cell lung cancer. Lung Cancer. 36:1–6. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
D’Andrilli A, Venuta F and Rendina EA: The
role of lymphadenectomy in lung cancer surgery. Thorac Surg Clin.
22:227–237. 2012. View Article : Google Scholar
|
|
32
|
Swanson SJ, Herndon JE II, D’Amico TA, et
al: Video-assisted thoracic surgery lobectomy: report of CALGB
39802 - a prospective, multi-institution feasibility study. J Clin
Oncol. 25:4993–4997. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Okada M, Sakamoto T, Yuki T, et al: Hybrid
surgical approach of video-assisted minithoracotomy for lung
cancer: significance of direct visualization on quality of surgery.
Chest. 128:2696–2701. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Verhagen AF, Schoenmakers MC, Barendregt
W, et al: Completeness of lung cancer surgery: is mediastinal
dissection common practice? Eur J Cardiothorac Surg. 41:834–838.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chin CS and Swanson SJ: Video-assisted
thoracic surgery lobectomy: centers of excellence or excellence of
centers? Thorac Surg Clin. 18:263–268. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Murthy S: Video-assisted thoracoscopic
surgery for the treatment of lung cancer. Cleve Clin J Med.
79:eS23–eS25. 2012.PubMed/NCBI
|
|
37
|
Papiashvilli M, Stav D, Cyjon A, et al:
Lobectomy for non-small cell lung cancer: differences in morbidity
and mortality between thoracotomy and thoracoscopy. Innovations
(Phila). 7:15–22. 2012. View Article : Google Scholar
|
|
38
|
Chen FF, Zhang D, Wang YL and Xiong B:
Video-assisted thoracoscopic surgery lobectomy versus open
lobectomy in patients with clinical stage I non-small cell lung
cancer: a meta-analysis. Eur J Surg Oncol. 39:957–963. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gorenstein LA and Sonett JR: The surgical
management of stage I and stage II lung cancer. Surg Oncol Clin N
Am 2011. 20:701–720. 2011. View Article : Google Scholar
|
|
40
|
Puri V and Meyers BF: Video-assisted
thoracoscopic surgery lobectomy for lung cancer. Surg Oncol Clin N
Am. 22:27–38. 2013. View Article : Google Scholar
|