Introduction
Ovarian cancer is one of the most common
gynecological cancers and a leading cause of cancer-related deaths
in females (1). For example, the
estimated number of new ovarian cancer cases and mortalities in the
United States in 2012 is 22,280 and 15,500, respectively (1). The prognosis for advanced-stage
ovarian cancer remains poor. Cytoreductive surgery is the
predominant treatment for the majority of ovarian cancers, and
removing the maximum amount of affected tissue may improve the
efficacy of other subsequent therapies (2). It is vital to distinguish malignant
tumors from nearby normal tissue and/or benign lesions. Various
imaging methods, including ultrasound, X-ray, computed tomography
(CT), magnetic resonance imaging (MRI) and positron emission
tomography (PET) are all used for the detection and preoperative
evaluation of tumors, however, achieving a high contrast over
nearby normal tissues is challenging using conventional imaging
modalities. Therefore, more reliable techniques for assisting with
cytoreductive surgery are required. Fluorescence imaging is
attractive for superior intraoperative tumor detection, and several
clinical studies have explored its applications in clinical
practices (3,4). A recent study utilized folate
conjugated with fluorescein for specific, intraoperative
fluorescence imaging of tumor tissue in patients undergoing an
exploratory laparotomy for suspected ovarian cancer (3); this demonstrated the feasibility and
potential benefit of this unique imaging method.
Fluorescence imaging is advantageous due to its
high-resolution, high sensitivity and low cost (5). The most widely used fluorophores are
fluorescein and its derivatives, which have extremely high quantum
yields and desirable excitation and visible emission wavelengths
(6). However, fluorescein also has
limitations, including high tissue absorption, tissue scattering
and high inference from autofluorescence (7). Near-infrared (NIR) fluorescent dyes,
with an emission wavelength ranging from 650–900 nm, can provide
real-time, dynamic images of tumors in vivo with high
sensitivity (8,9). Tumor-targeted fluorescence imaging for
cancer diagnosis and treatment has attracted significant attention,
and is on the verge of clinical implementation (10). Many investigational clinical studies
involving NIR fluorescence imaging have been reported (11,12).
Troyan et al developed an NIR fluorescence imaging system
for image-guided oncologic surgery (11), and Crane et al used
indocyanin green to detect the sentinel lymph nodes in patients
with cervical cancer (12). Folate
receptor-α, vascular endothelial growth factor, epidermal growth
factor receptor (EGFR), chemokine receptor 4, and matrix
metalloproteinase are the five most prominent targets, which have
relatively high expression rates in ovarian cancer, high
availability of an antibody or substrate, and are promising for
translation to human use (10).
Mucin 1 (MUC1) is a transmembrane mucin whose extra cellular domain
can serve as a ligand for stromal and endothelial cell adhesion
receptor, and its cytoplasmic domain plays a role in the cell
migration, invasion and survival (13). MUC1 is often overexpressed in
metastatic cancers, and its overexpression has been observed in
colon, breast, ovarian, lung and pancreatic cancers. It is also a
reliable epithelial marker for ovarian carcinoma cells.
Additionally, it is a favorable target for immunotherapy, and a
number of therapies targeting MUC1 in patients with advanced
disease are being assessed in preclinical development or clinical
trials (13,14). Monoclonal antibodies have been
widely utilized for targeted therapies and may also be used for
tumor imaging (15). The present
study aimed to evaluate the feasibility of in vivo molecular
imaging using fluorescent labeled antibodies against MUC1 for the
detection of ovarian cancer. The anti-MUC1 antibody (Mouse
Anti-Human CD227 antibody) was labeled with a near-infrared dye
[Cy5.5-N-hydroxysuccinimide (NHS)] and a green dye
(fluorescein-NHS) and evaluated in OVCAR3 tumor-bearing mice.
Materials and methods
Reagents
Cy5.5-NHS was purchased from GE Healthcare
(Piscataway, NJ, USA). Mouse Anti-Human CD227 antibody (cat. no.
6378-0150, 0108) was purchased from RayBiotech, Inc. (Norcross, GA,
USA). Mouse IgG (cat. no. A7208) and fluorescein-NHS were purchased
from Beyotime Institute of Biotechnology (Haimen, China). Other
chemicals were of analytical grade or better and were used as
purchased from Sigma-Aldrich, St. Louis, MO, USA. The human ovarian
carcinoma OVCAR3 cell line was purchased from Shanghai Cell Bank of
the Chinese Academy of Science (Shanghai, China). Ethics approval
was provided by Wenzhou Medical University (Wydw2014-0134).
Labeling of the antibody
Conjugation of the dyes to the antibody was
performed according to the manufacturer’s instructions. The
Cy5.5-NHS and fluorescein-NHS dyes were dissolved in dimethyl
sulfoxide at a concentration of 10 μg/μl. The antibodies were
transferred to 0.1 M sodium bicarbonate buffer (pH 8.5), typically
at a concentration of 1 mg/ml. A molar ratio of 10:1 (dye:antibody)
was used. Following reaction in the dark at 4°C for two hours, the
reaction mixture was purified using Zeba™ Spin Desalting Columns
(7K MWCO; Thermo Fisher Scientific, Waltham, MA, USA) to separate
the low-molecular-weight dyes from the dye-conjugated antibody. The
antibody and the bound dye contents were determined by measuring UV
absorption using a Shimadzu UV-1800 spectrophotometer (Nakagyo-ku,
Kyoto, Japan).
Cell culture
The OVCAR3 cells were cultured in Dulbecco’s
modified Eagle medium (DMEM; GIBCO, Carlsbad, CA, USA) supplemented
with 10% fetal bovine serum and 0.1 μg/ml penicillin-streptomycin.
The cells were expanded in tissue culture dishes and incubated in a
humidified atmosphere of 5% CO2 at 37°C. The medium was
replaced every other day. A confluent monolayer was detached using
0.05% Trypsin-EDTA and 0.01M phosphate buffered saline (PBS; pH
7.4), and dissociated into a single-cell suspension for further
cell culture.
Animals
The animal experiment was approved by Wenzhou
Medical University Animal Care and Use Committee (Wenzhou, China).
Nude mice of six-eight weeks of age and a mean weight of 20 g were
divided randomly into two groups: The Ab-FL-Cy5.5 group (n=3) and
the IgG-Cy5.5 group (n=3).Approximately 3×106 cultured
OVCAR3 cells were suspended in 50 μl PBS and subcutaneously
implanted in the right shoulders of female nude mice. Tumors grew
to a size of 0.6–1 cm in four weeks.
In vivo fluorescence imaging
In vivo fluorescence imaging was performed
using a Kodak In-Vivo FX Pro Imaging System (Kodak, Woodbridge, CT,
USA) and analyzed with Kodak Molecular Imaging Software (Kodak). A
filter set (excitation wavelength, 610 nm; emission wavelength, 700
nm) was used for achieving NIR fluorescence in vivo.
Identical illumination settings were used to obtain all images.
For the experiment, mice were injected via the tail
vein with 0.5 nmol of probe. Mice in the Ab-FL-Cy5.5 group were
injected with Ab-FL-Cy5.5 probe, and mice in the IgG-Cy5.5 group
were injected with IgG-Cy5.5, which was used to evaluate the
non-specific binding effects of antibodies. NIR fluorescence images
were acquired at 4, 12, and 36 h post injection (p.i.). The mice
were subsequently sacrificed at 36 h p.i. The tumor and major
organs were dissected for evaluation with ex vivo
fluorescence imaging.
Statistical analysis
SPSS software, version 17.0 (SPSS Inc., Chicago, IL,
USA) was used for data analysis. Measurement data were analyzed
using an independent samples t-test, and results are expressed as
the mean ± standard deviation. P<0.05 was considered to indicate
a statistically significant difference.
Results
Labeling of the antibody
The average numbers of fluorescein and Cy5.5
molecules per antibody molecule were determined as 2.5 and 2.8,
respectively, as calculated by the UV absorptions. In vitro
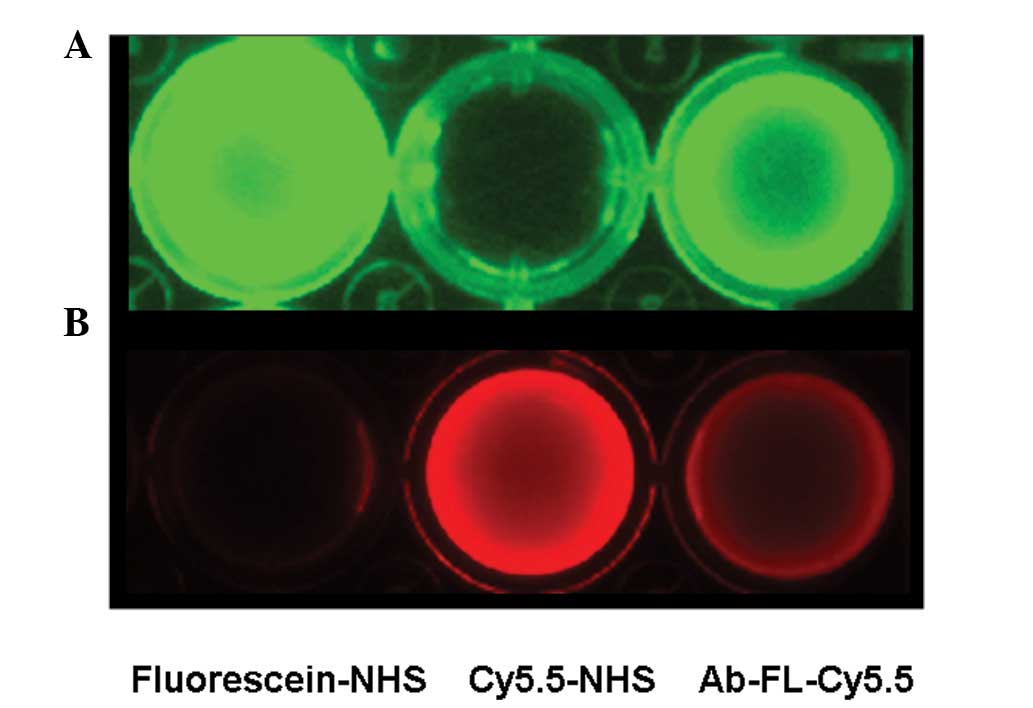
fluorescence images of unconjugated fluorescein-NHS and Cy5.5-NHS
and the dye-conjugated antibody probe, Ab-FL-Cy5.5, were obtained
under different excitation wavelengths (Fig. 1). The dual-labeled antibodies were
visible following excitation at wavelengths of 470 and 610nm.
Fluorescence imaging of OVCAR3
tumor-bearing mice
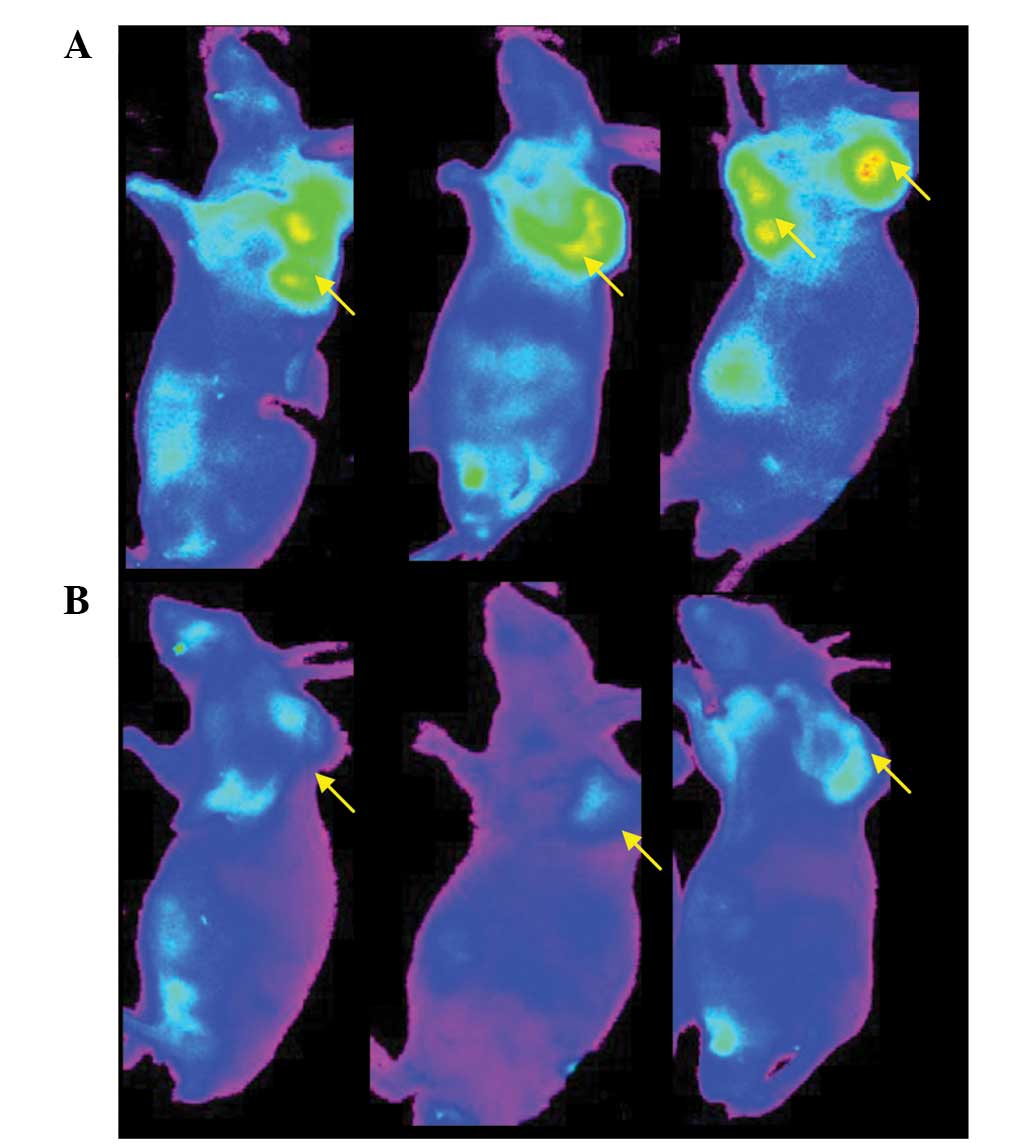
Following injection of Ab-FL-Cy5.5 and IgG-Cy5.5,
tumors were visualized in mice from both groups (Fig. 2). The fluorescence signal
intensities observed in the tumor regions were higher than those in
other regions of the mice.
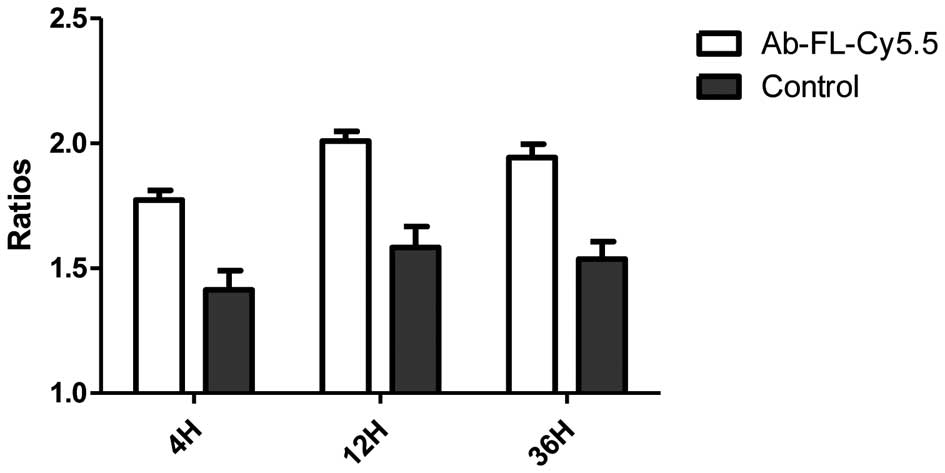
Fluorescence intensity of the mouse’s central back
area was set as the background level, and the ratios of tumor to
background intensity were calculated. A quantitative analysis of
the in vivo fluorescence imaging data is represented in
Fig. 3. At 4, 12 and 36 h, the
tumor:background ratios for Ab-FL-Cy5.5 were 1.77±0.066, 2.01±0.065
and 1.94±0.093, respectively, while the ratios for IgG-Cy5.5 were
1.41±0.13, 1.58±0.14 and 1.53±0.12, respectively. The uptakes of
Ab-FL-Cy5.5 were significantly higher compared with those of
IgG-Cy5.5 at every time point (P<0.05). The fluorescence
intensity reached a plateau at 12 h.
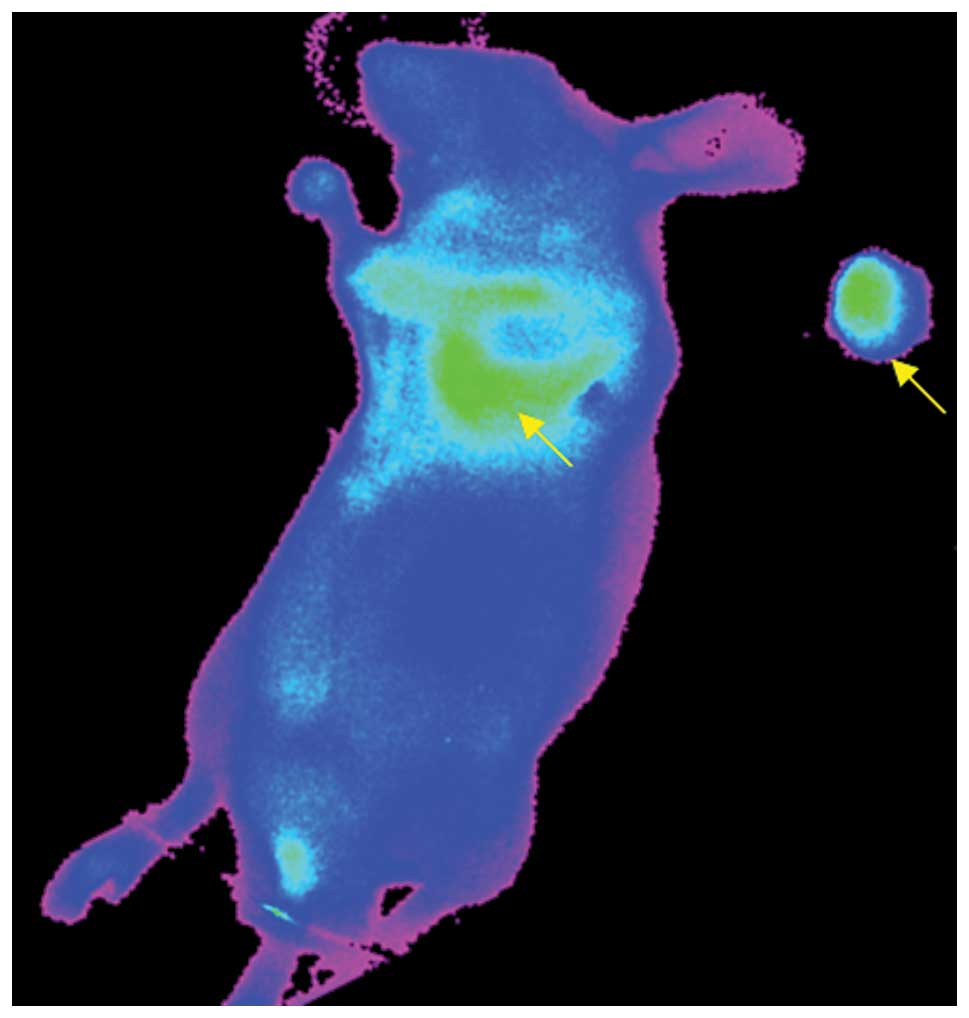
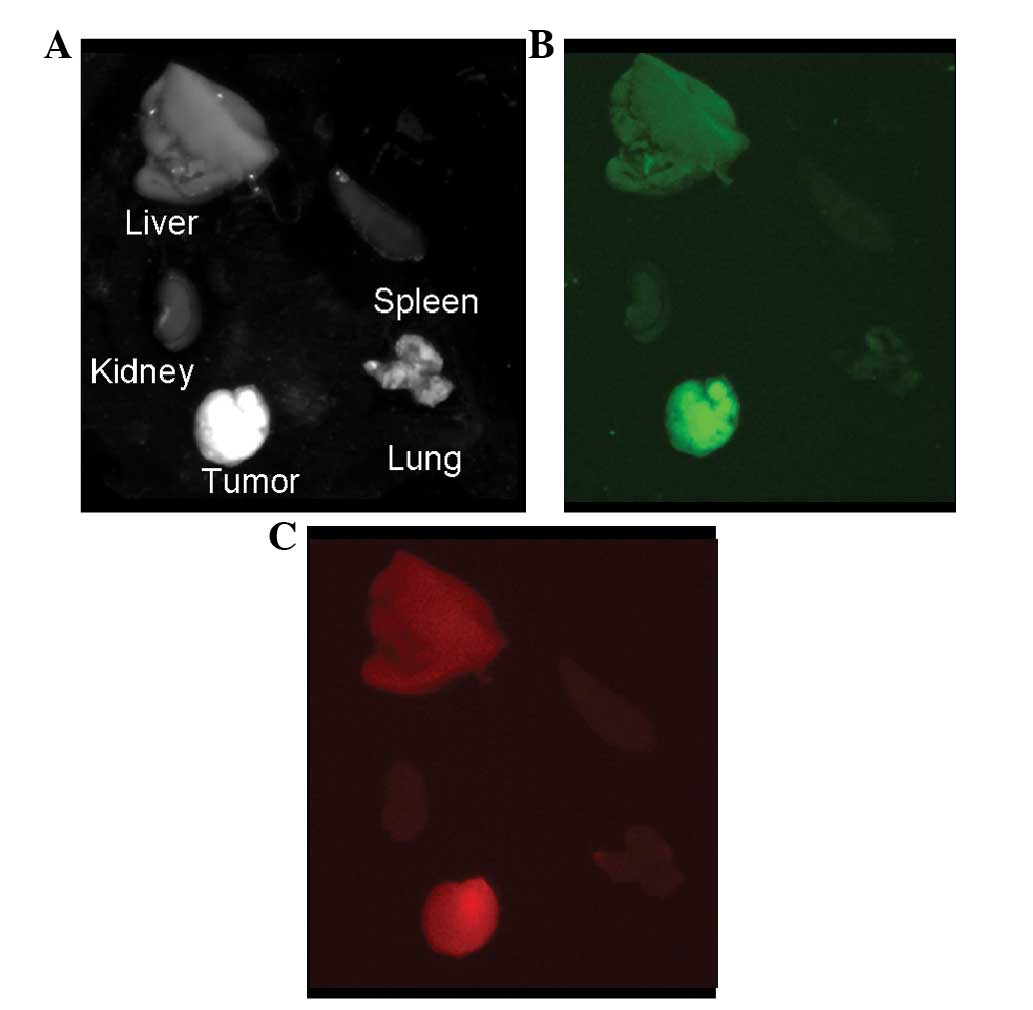
Ex vivo imaging
Sections of tumor tissue were surgically removed
from tumor-bearing mice at 36 h after injection of Ab-FL-Cy5.5. The
dissected tumor tissue and the remaining tissues could be clearly
visualized by NIR fluorescence imaging (Fig. 4). At 36 h following injection,
fluorescence images of the liver, kidney, spleen, lung and tumor
were acquired. As shown in Fig. 5,
the highest intensity of green and NIR fluorescence was observed in
the tumor, and uptake of Ab-FL-Cy5.5 in liver tissue was also
observed. Kidney tissue exhibited the lowest signal intensity,
while lung and spleen tissue showed a slight uptake of the antibody
probe. Signal intensities in the nearby tissues, including healthy
ovarian tissue, were observed to be similar to the background
intensity.
Discussion
This preliminary study showed that the Ab-FL-Cy5.5
probe could accumulate in the tumors, while presenting low
fluorescence in other organs, which proved its specificity and
potential for distinguishing tumor tissue from healthy tissue.
These results demonstrate the feasibility of the anti-MUC1 antibody
in fluorescent imaging of ovarian cancer and its potential
capability to aid surgical procedures. Optimal cytoreductive
surgery is important for the treatment of ovarian cancer, and
imaging techniques that can effectively differentiate tumor tissue
from unaffected tissue are necessary to improve these procedures
(16). However, conventional
imaging modalities, including CT, MRI and PET, have limitations in
the real-time detection of tumor margins during surgery due to the
lack of imaging systems for image-guided surgery (11). Many efforts have focussed on the
development of new intraoperative imaging techniques to overcome
such limitations.
Fluorescence imaging based on specific probes is one
of the most promising techniques for intraoperative tumor
detection. Visualization of the tumor tissue is achieved via a
fluorescence imaging system and tumor-targeted probe labeled with a
fluorescent dye, therefore the development of a fluorescent probe
with specific targeting ability is vital for imaging-guided
surgery. For ovarian cancer, a number of specific targets have been
identified, most prominently folate receptor-α, vascular
endothelial growth factor, EGFR, chemokine receptor 4, and matrix
metalloproteinase (11). In a study
by van Dam et al (5), folate
molecules were conjugated with fluorescein for the successful
visualization of tumor tissue in ovarian cancer patients using a
real-time intraoperative fluorescence imaging system. Another study
evaluated the in vivo sensitivity, specificity and
diagnostic accuracy of an α(v)β(3)-integrin-targeted NIR fluorescent probe
using an intraoperative fluorescence imaging system (16). These two studies demonstrated the
feasibility of fluorescence imaging for the intraoperative imaging
of ovarian cancer. The development of additional fluorescent probes
to specifically target ovarian cancer tissues is of great
importance to advance this technique.
In recent years, NIR dye-labeled antibodies have
been successfully utilized for the imaging and treatment of tumors,
such as malignant gliomas and breast cancer (17). A Cy5.5-labeled anti-EGFR antibody,
cetuximab, was used to image head and neck squamous cell carcinoma
xenografts in vivo, and the results demonstrated the
capability of a fluorescently labeled anti-EGFR antibody to be
utilized for detecting human tumors in a surgical setting (18). Cy5.5-cetuximab bioconjugate was also
evaluated for its potential utility in the detection and guided
removal of regional and distant micrometastasis (19). It was found that mice bearing
pulmonary metastases displayed remarkable fluorescence across the
lung surface after cetuximab-Cy5.5 injection. Panitumumab
conjugated with IRDye800 (emission wavelength, 800 nm) has also
been used in the imaging of cutaneous head and neck tumors in mice
(20). This study demonstrated that
panitumumab-IRDye800 had potential to be translated to the clinic
for detection and removal of subclinical cutaneous squamous cell
carcinoma using Food and Drug Administration-approved imaging
hardware.
The present study focussed on the MUC1 antigen to
evaluate its potential as a target using an anti-MUC1 antibody
conjugated with two dyes: Cy5.5, which is widely used for in
vivo NIR fluorescence imaging, and a fluorescein derivative,
with an emission wavelength in the range of green light (outside
the visible range). Numerous fluorescence imaging apparatus already
exist for fluorescein derivatives (21). Under the reaction conditions used in
the current study, the resulting Ab-FL-Cy5.5 probe had a molecular
ratio of antibody:fluorescein:Cy5.5 of 1:2.5:2.8 (approximately
five fluorophores per antibody). If too many fluorophores are
conjugated to the antibody molecule, self-quenching of the
fluorescence may occur, particularly for fluorescein (22).
The tumor to background ratio of the probe in the
current study was relatively low, however, the tumor areas could be
visualized clearly by in vivo and ex vivo imaging.
Certain studies have demonstrated that IRDye800 exhibits better
tumor to background contrast than Cy5.5 (20,23).
Use of more specific antibodies may also result in better imaging
outcomes.
In conclusion, the preliminary data of this study
indicate that MUC1 is a suitable target for ovarian cancer imaging,
and anti-MUC1 antibody conjugated with fluorescent dyes is
promising for further imaging applications. Moreover, the
dual-color labeling strategy was successful and provided more
opportunities for the detection of the fluorescence signal.
However, the use of more specific antibodies and other dyes, such
as IRDye800, may result in improved imaging outcomes; further
studies are warranted.
Acknowledgments
This study was supported by the Natural Science
Foundation of Zhejiang Province (grant nos. Y2110467 and Y2110466)
and Wenzhou Science and Technology Bereau Foundation (grant nos.
Y20110024 and S20100048).
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Al Rawahi T, Lopes AD, Bristow RE, et al:
Surgical cytoreduction for recurrent epithelial ovarian cancer.
Cochrane Database Syst Rev. 2:CD0087652013.PubMed/NCBI
|
|
3
|
van Dam GM, Themelis G, Crane LM, et al:
Intraoperative tumor-specific fluorescence imaging in ovarian
cancer by folate receptor-alpha targeting: first in-human results.
Nat Med. 17:1315–1319. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nguyen QT and Tsien RY:
Fluorescence-guided surgery with live molecular navigation - a new
cutting edge. Nat Rev Cancer. 13:653–662. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Weissleder R and Ntziachristos V: Shedding
light onto live molecular targets. Nat Med. 9:123–128. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu Y, Yu G, Tian M and Zhang H: Optical
probes and the applications in multimodality imaging. Contrast
Media Mol Imaging. 6:169–177. 2011.PubMed/NCBI
|
|
7
|
Licha K: Contrast agents for optical
imaging. Topics in Current Chemistry - Contrast Agents II. Krause
W: 222. Springer; Heidelberg: pp. 1–29. 2002, View Article : Google Scholar
|
|
8
|
Hawrysz DJ and Sevick-Muraca EM:
Developments toward diagnostic breast cancer imaging using
near-infrared optical measurements and fluorescent contrast agents.
Neoplasia. 2:388–417. 2000. View Article : Google Scholar
|
|
9
|
Hilderbrand SA and Weissleder R:
Near-infrared fluorescence: application to in vivo molecular
imaging. Curr Opin Chem Biol. 14:71–79. 2010. View Article : Google Scholar
|
|
10
|
Crane LM, van Oosten M, Pleijhuis RG, et
al: Intraoperative imaging in ovarian cancer: fact or fiction? Mol
Imaging. 10:248–257. 2011.PubMed/NCBI
|
|
11
|
Troyan SL, Kianzad V, Gibbs-Strauss SL, et
al: The FLARE intraoperative near-infrared fluorescence imaging
system: a first-in-human clinical trial in breast cancer sentinel
lymph node mapping. Ann Surg Oncol. 16:2943–2952. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Crane LM, Themelis G, Pleijhuis RG, et al:
Intraoperative multispectral fluorescence imaging for the detection
of the sentinel lymph node in cervical cancer: a novel concept. Mol
Imaging Biol. 13:1043–1049. 2011. View Article : Google Scholar :
|
|
13
|
Horm TM and Schroeder JA: MUC1 and
metastatic cancer: expression, function and therapeutic targeting.
Cell Adh Migr. 7:187–198. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kovjazin R, Horn G, Smorodinsky NI,
Shapira MY and Carmon L: Cell surface-associated anti-MUC1-derived
signal peptide antibodies: implications for cancer diagnostics and
therapy. PloS One. 9:e854002014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Adams GP and Weiner LM: Monoclonal
antibody therapy of cancer. Nat Biotechnol. 23:1147–1157. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Harlaar NJ, Kelder W, Sarantopoulos A, et
al: Real-time near infrared fluorescence (NIRF) intra-operative
imaging in ovarian cancer using an α(v)β(3-)integrin targeted
agent. Gynecol Oncol. 128:590–595. 2013. View Article : Google Scholar
|
|
17
|
Yuan A, Wu J, Tang X, Zhao L, Xu F and Hu
Y: Application of near-infrared dyes for tumor imaging,
photothermal, and photodynamic therapies. J Pharm Sci. 102:6–28.
2013. View Article : Google Scholar
|
|
18
|
Rosenthal EL, Kulbersh BD, King T,
Chaudhuri TR and Zinn KR: Use of fluorescent labeled anti-epidermal
growth factor receptor antibody to image head and neck squamous
cell carcinoma xenografts. Mol Cancer Ther. 6:1230–1238. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gleysteen JP, Newman JR, Chhieng D, Frost
A, Zinn KR and Rosenthal EL: Fluorescent labeled anti-EGFR antibody
for identification of regional and distant metastasis in a
preclinical xenograft model. Head Neck. 30:782–789. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Heath CH, Deep NL, Beck LN, et al: Use of
panitumumab-IRDye800 to image cutaneous head and neck cancer in
mice. Otolaryngol Head Neck Surg. 148:982–990. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nagano T: Development and biological
applications of various bioimaging probes. Yakugaku Zasshi.
126:901–913. 2006.(In Japanese). View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mahmoudian J, Hadavi R, Jeddi-Tehrani M,
et al: Comparison of the photobleaching and photostability traits
of Alexa Fluor 568- and fluorescein isothiocyanate-conjugated
antibody. Cell J. 13:169–172. 2011.PubMed/NCBI
|
|
23
|
Adams KE, Ke S, Kwon S, et al: Comparison
of visible and near-infrared wavelength-excitable fluorescent dyes
for molecular imaging of cancer. J Biomed Opt. 12:0240172007.
View Article : Google Scholar : PubMed/NCBI
|