Introduction
Mesenchymal stem cells (MSCs) are multipotent cells
that originate from bone marrow or other tissues. MSCs are
distributed almost ubiquitously between the perivascular niches of
a number of human tissues and organs, and are important components
in angiogenesis, local tissue repair and concomitant
immunomodulation (1). In addition,
MSCs can be recruited to a variety of tumors, including breast
(2) and gastric cancer (3). A previous study by El-Haibi et
al (4) reported that MSCs
migrated to the site of tumorigenesis, where the MSCs were
activated by cancer cells, and in turn promoted metastasis.
Chaturvedi et al (2)
revealed that hypoxia-inducible factors mediated the interactions
of MSCs with breast cancer cells to promote metastasis.
Furthermore, a study by Zhang et al (5) identified that MSCs were able to
promote CXC chemokine receptor (CXCR) type 4-mediated osteosarcoma
growth and pulmonary metastasis by upregulating the expression of
vascular epidermal growth factor (VEGF). The CXC chemokine ligand
16 and CXCR6 signaling pathway stimulates the conversion of MSCs
into cancer-associated fibroblasts, which ultimately facilitates
prostate tumor metastasis (6).
However, as lymphatic vessels are the main route of tumor
metastasis, the changes that occur in MSCs to promote tumor
metastasis have yet to be elucidated. Lymph vessels are an
important component in lymph node metastasis, but the association
between MSCs and lymph vessels remains unknown.
Since VEGF receptor (VEGFR)-3 was identified as the
first lymphatic marker almost 20 years ago, the mechanisms
underlying lymphangiogenesis and metastasis have been extensively
investigated (7–9). Although it is known that blood and
lymphatic vessels are the major routes of metastatic spread, cancer
cells were first identified to be disseminated to lymphatic vessels
rather than blood vessels in a number of cancers, including breast,
colon, prostate and lung cancers, and melanoma (10). In addition, the tumor
microenvironment has been demonstrated to induce the expression of
lymphangiogenic factors that promote metastasis (11). Breast cancer metastasis to regional
lymph nodes has been revealed to be associated with lymphatic
vessel density (LVD) rather than tumor size (12). At present, the VEGF-C/VEGFR-3
signaling pathway has been indentified to be involved in lymphatic
metastasis (12–16). VEGF-C is able to promote tumor
lymphangiogenesis and metastasis by binding to its corresponding
receptor, VEGFR-3 (13).
Cancer stem cells (CSCs), also termed
tumor-initiating cells, are important for the initiation of
tumorigenesis and metastasis, a phenotype that can be partly
maintained by altered c-Jun N-terminal kinase signaling. CSCs
subsequently affect tumorigenesis and lymphatic metastasis
(10,17). In the present study, cancer cells
were treated with human bone marrow MSC-conditioned medium
(hBM-MSC-CM) for a period of time to allow the tumor cells to
express the lymphatic vessel-associated markers Prox-1 and
VEGFR-3.
Materials and methods
Cell culture
The primary lymphatic endothelial cells (LECs) were
purchased from ScienCell (Carlsbad, CA, USA). The hMSCs were
isolated, cultured and characterized as previously described
(16). The human gastric carcinoma
SGC-7901 and HGC-27 cell lines were purchased from the Chinese
Academy of Sciences Type Culture Collection Committee cell bank
(Beijing, China). The cell lines were cultured in Dulbecco’s
modified Eagle’s medium (DMEM; Gibco Life Technologies, Carlsbad,
CA) supplemented with 10% fetal bovine serum (FBS; Gibco) at 37°C
in 5% CO2.
Preparation of the hBM-MSC-conditioned
medium and co-culture with tumor cells
The hMSCs were cultured to ~70% confluency, and the
5 ml of medium was refreshed prior to the cells being incubated for
an additional 48 h. In total, 0.22 μm filter-sterilized supernatant
was collected and designated as hBM-MSC-CM. For the pre-treatment
of tumor cells with hBM-MSC-CM, the HGC-27 cells were washed three
times with phosphate-buffered saline, and then incubated with
hBM-MSC-CM at 37°C in 5% CO2 for a further five days,
prior to being collected for use in the subsequent experiments.
Tube formation assay
To perform the tube formation assay, 50 μl (10
mg/ml) growth factor-reduced Matrigel (BD Biosciences, Billerica,
MA, USA) was first used to pre-coat a 96-well plate. Next,
1×104 LECs or 1×104 BM-MSC-CM-treated HGC-27
cells in 150 μl DMEM supplemented with 10% FBS, or one-half DMEM
supplemented with 10% FBS and one-half hBM-MSC-CM medium were
seeded into each well. Following 16-h incubation at 37°C, an
inverted phase-contrast microscope (magnification, ×100; Eclipse
Ti-S; Nikon Corporation, Tokyo, Japan) was used to observe and
capture images of the tube structures. The average of two fields
was taken as the value for each treatment.
Transwell migration assay
For the Transwell migration assay, 5×104
LECs/well were plated in the upper wells, which were filled with
200 μl DMEM supplemented with 1% FBS. In the lower chamber,
hBM-MSC-CM was used as a chemoattractant to encourage cellular
migration. The cells were incubated for 8 h at 37°C, and 10% FBS
served as the control. The cells that did not migrate were removed
using a cotton swab. The cells that did migrate were stained using
crystal violet stain, and then counted under a microscope (Ti-S;
Nikon Corporation). In total, three views were chosen at random,
and each experiment was repeated independently in triplicate.
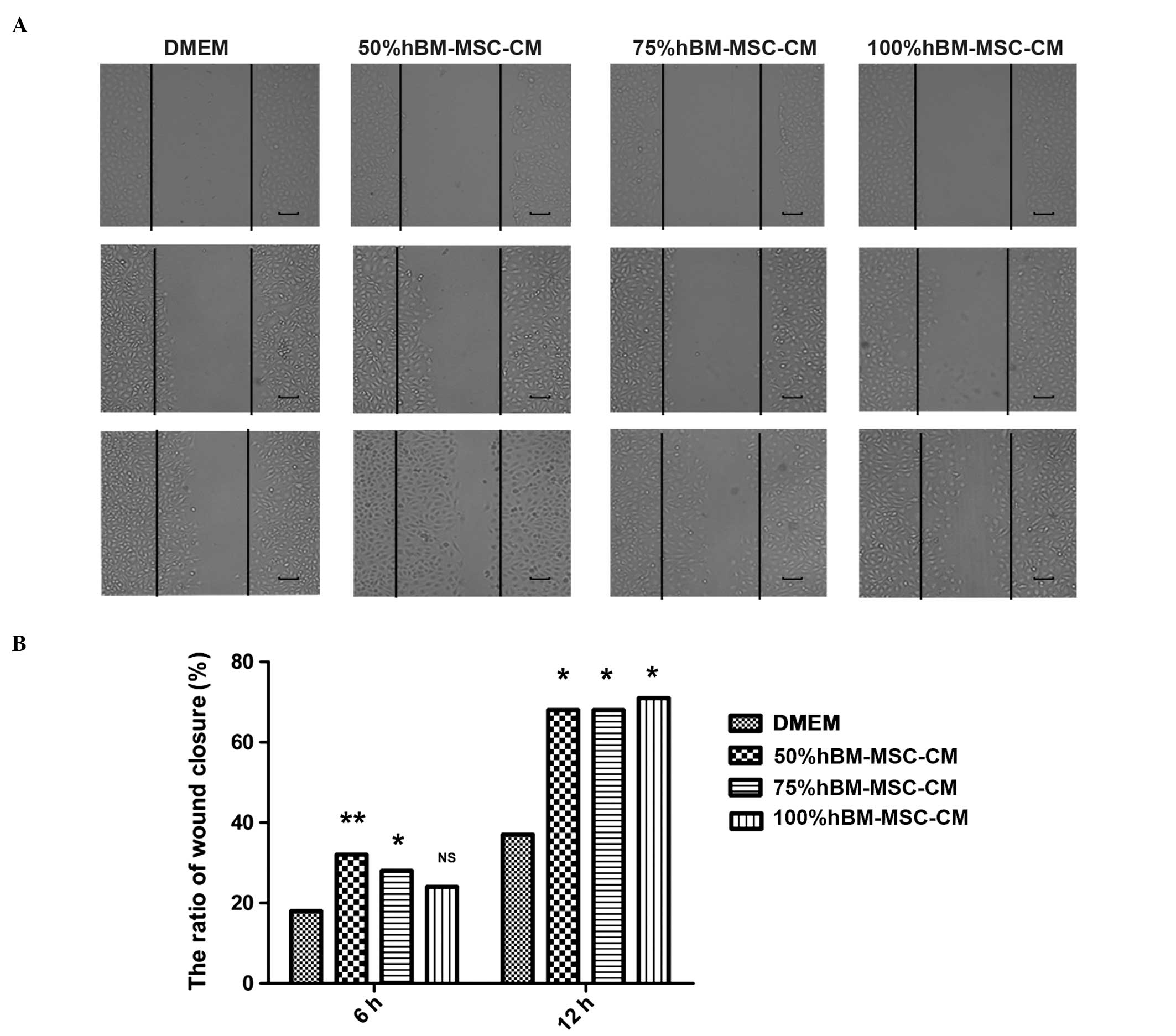
Scratch-wound assay
The cells were seeded a density of 2×105
cells/well into six-well plates (Corning Inc., Corning, NY, USA),
and then cultured for ~48 h, at which time the cells had reached
~80% confluency. Subsequent to the cell monolayer being scratched
with a sterile 200 μl pipette tip, the cells were treated with 0,
50, 75 or 100% hBM-MSC-CM, and then incubated for a further 12 h to
allow time for migration into the cell-free area.
MTT assay
The cell viability was determined using an MTT
assay. First, the LECs were seeded into 96-well plates (Corning
Inc.) at a density of 5×103 cells per well, and then
cultured overnight. Next, various concentrations, comprising
one-half hBM-MSC-CM and one-half DMEM supplemented with 10% FBS,
two-thirds hBM-MSC-CM and one-third DMEM supplemented with 10% FBS
or three-quarters hBM-MSC-CM and one-quarter DMEM supplemented with
10% FBS, were added to the plates. The plates were then incubated
at 37°C in a 5% CO2 atmosphere for 24, 48 or 72 h,
respectively. The untreated SGC-7901 cells served as the control.
MTT dye was added to each well for the final 4 h of treatment. The
reaction was terminated by the addition of dimethyl sulfoxide
(Sigma-Aldrich, St. Louis, MO, USA), and the optical density (OD)
was determined at 490 nm using a multiwell plate reader (FLx800;
BioTek, Winooski, VT, USA). The background absorbance of the medium
in the absence of cells was subtracted. All samples were assayed in
triplicate, and the mean for each experiment was calculated.
Western-blot analysis
The proteins were extracted from the whole cell
lysates using cell extraction buffer (Invitrogen, Carlsbad, CA,
USA) and the protein concentration was determined. In total, 20 μg
of the extracted total cellular protein from each sample was
separated via SDS-PAGE, and subsequently transblotted onto a
polyvinylidene fluoride membrane (Millipore, Billerica, MA, USA).
The blotted nitrocellulose membranes were incubated with a
polyclonal primary rabbit anti-human Prox-1 (cat. no. ab38692;
dilution, 1:800; Abacam, Cambridge, UK) and polyclonal rabbit
anti-human VEGFR-3 (dilution, 1:200, cat. no. 21410-2, Signalway
Antibody, College Park, MD, USA) antibodies, and then with a
peroxidase-conjugated goat anti-mouse (dilution, 1:2,000; CW0103,
CWBIO, Beijing, China) and goat anti-rabbit (dilution, 1:2,000;
KC-MM-035, Kang Chen Bio-Tech, Shanghai, China) secondary
antibodies. The blots were visualized using the enhanced
chemiluminescent detection system (Amersham plc., Amersham, UK) and
analyzed using Image-Pro Plus version 5.1 (Media Cybernetics, Inc.,
Rockville, MD, USA).
Statistical analysis
The data are expressed as the mean ± standard
deviation. Statistical differences were analyzed using a one-way
analysis of variance, followed by Dunnett’s multiple comparison
tests. P<0.05 was considered to indicate a statistically
significant difference.
Results
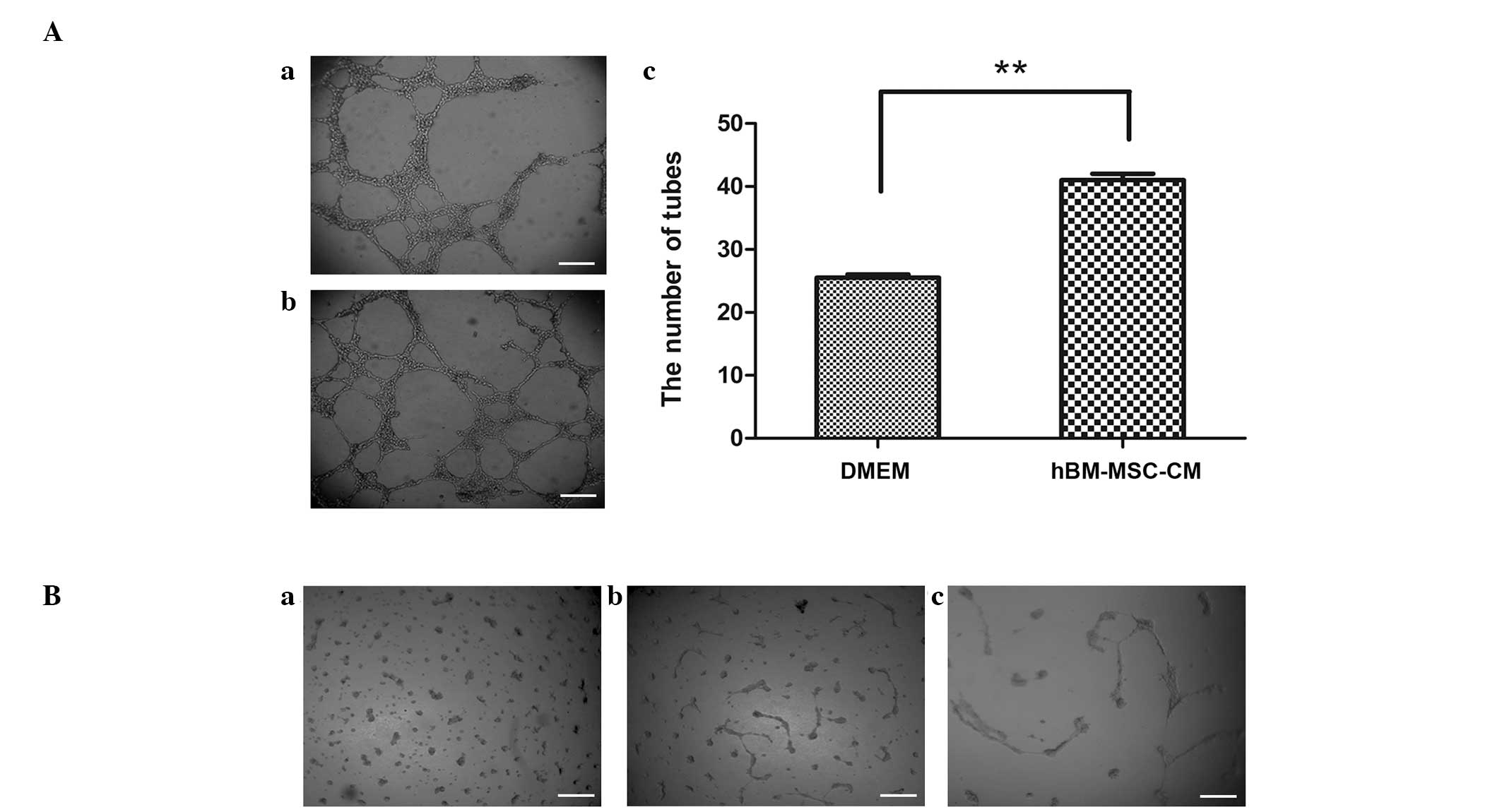
hBM-MSC-CM induces tube formation in LECs
and in the gastric cancer HGC-27 cell line
Lymphangiogenesis is an important factor involved in
neoplastic metastasis. In order to investigate the role of MSCs in
lymphangiogenesis, the present study examined the effect of BM-MSCs
on primary LEC tube formation. As shown in Fig. 1A and B, hBM-MSC-CM significantly
induced LEC tube formation. The number of tubes in
hBM-MSC-CM-treated LECs was significantly increased compared with
the LECs treated with DMEM alone. These data indicate that
hBM-MSC-CM may contribute to lymphangiogenesis. In addition, it was
identified that the gastric cancer HGC-27 cell line treated with
one-half DMEM and one-half hBM-MSC-CM for 20 days exhibited
increased tube formation compared with the HGC-27 cells treated
with DMEM alone (Fig. 1C).
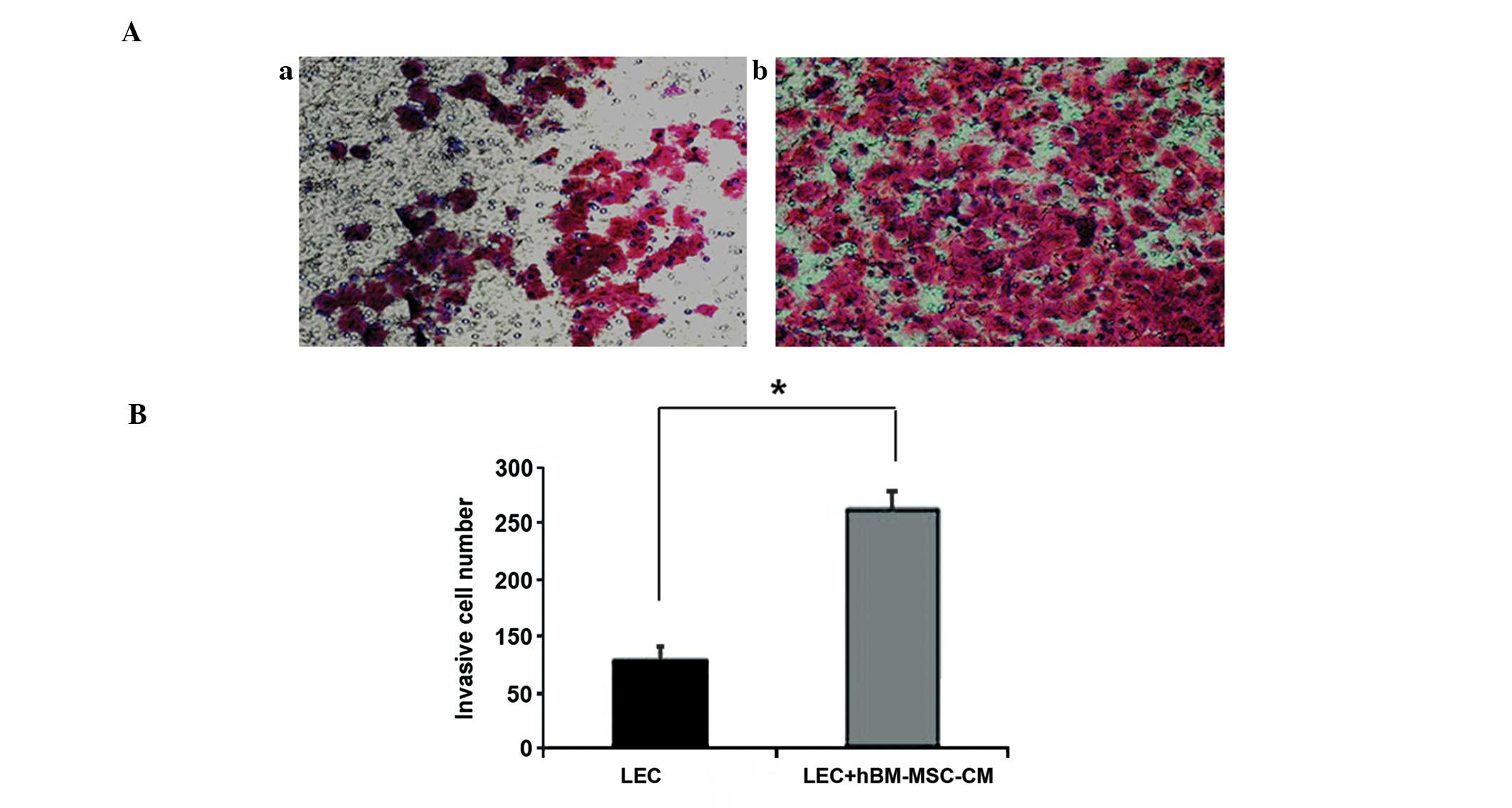
hBM-MSC-CM promotes lymphatic endothelial
cell migration
Cell adhesion is important for tumor
lymphangiogenesis. In order to determine the effect of MSCs on the
migration of LECs, the present study examined the extent of cell
adhesion by performing a cell migration assay in a Transwell
system. The hBM-MSC-CM-pretreated LECs exhibited a 3–4-fold
increase in migration compared with those incubated with DMEM
supplemented with 10% FBS alone (control group) (Fig. 2A and B), which indicated that
hBM-MSC-CM promotes LEC migration. In addition, the scratch-wound
assay also demonstrated that treatment with one-half DMEM and
one-half hBM-MSC-CM, one-third DMEM and two-thirds hBM-MSC-CM, or
hBM-MSC-CM alone demonstrates the ability to promote enhanced LEC
migration compared with DMEM supplemented with 10% FBS alone
(Fig. 3A and B).
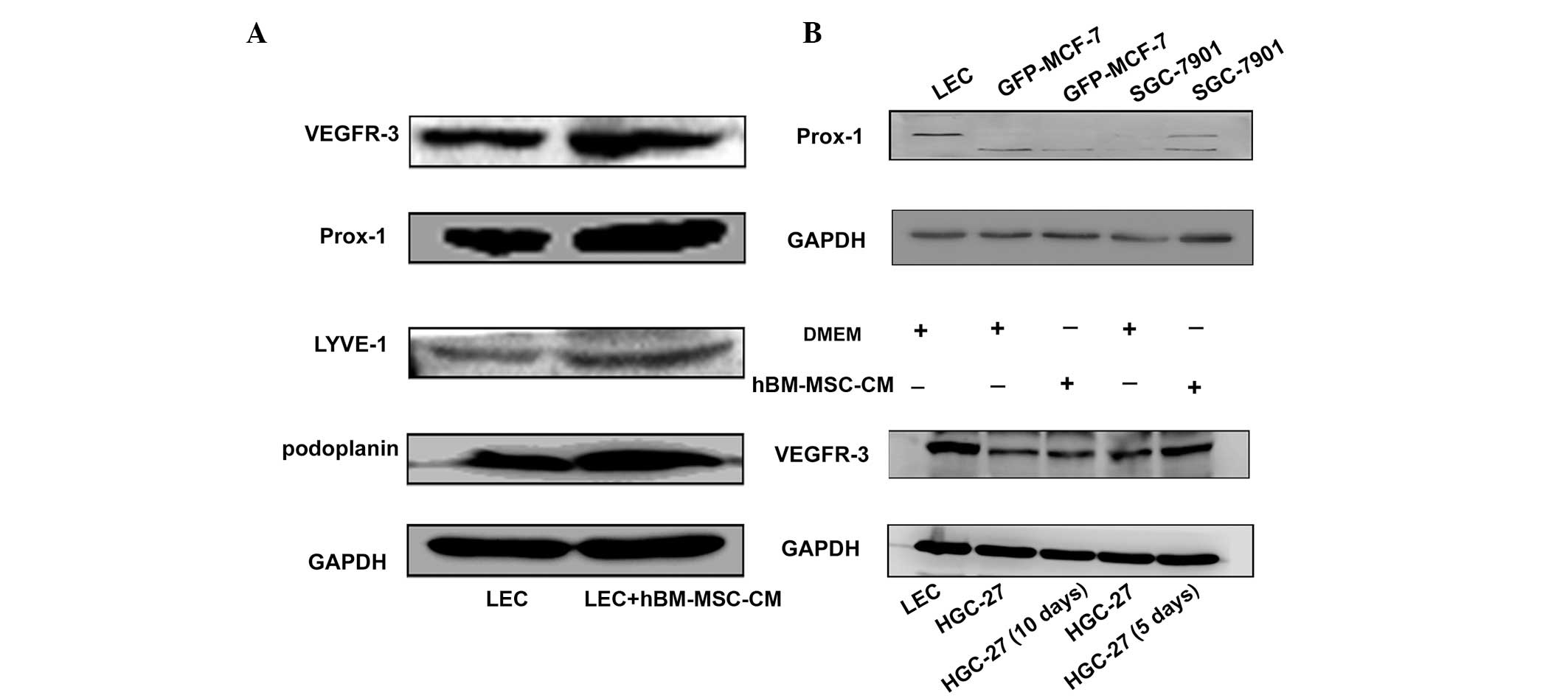
hBM-MSC-CM induces the expression of
lymphatic markers in LECs, and SGC-7901 and HGC27 cells
In order to further verify the role of hBM-MSC-CM in
lymphangiogenesis, the levels of the lymphatic markers podoplanin,
Prox-1, VEGFR-3 and lymphatic vessel endothelial hyaluronic acid
receptor-1 (LYVE-1) were analyzed in hBM-MSC-CM-treated LECs, and
in SGC-7901 and HGC-27 cells. As shown in Fig. 4, high levels of podoplanin, Prox-1,
VEGFR-3 and LYVE-1 were expressed in LECs following treatment with
one-half hBM-MSC-CM and one-half DMEM for 48 h (Fig. 4A). Furthermore, the expression of
Prox-1 in the gastric cancer SGC-7901 cell line increased following
40 days of treatment with hBM-MSC-CM (Fig. 4B). Finally, the expression of
VEGFR-3 was analyzed in hBM-MSC-CM-treated HGC-27 cancer cells. The
results revealed that VEGFR-3 was also upregulated in HGC-27 cells
on days five and 10 after hBM-MSC-CM treatment (Fig. 4B). These results indicate that
hBM-MSC-CM may promote the transition of tumor cells to LECs, and
that the increased expression of Prox-1 and VEGFR-3 in cancer cells
is also able to promote lymphangiogenesis. The transition to LECs
induces lymphangiogenesis and tumor metastasis.
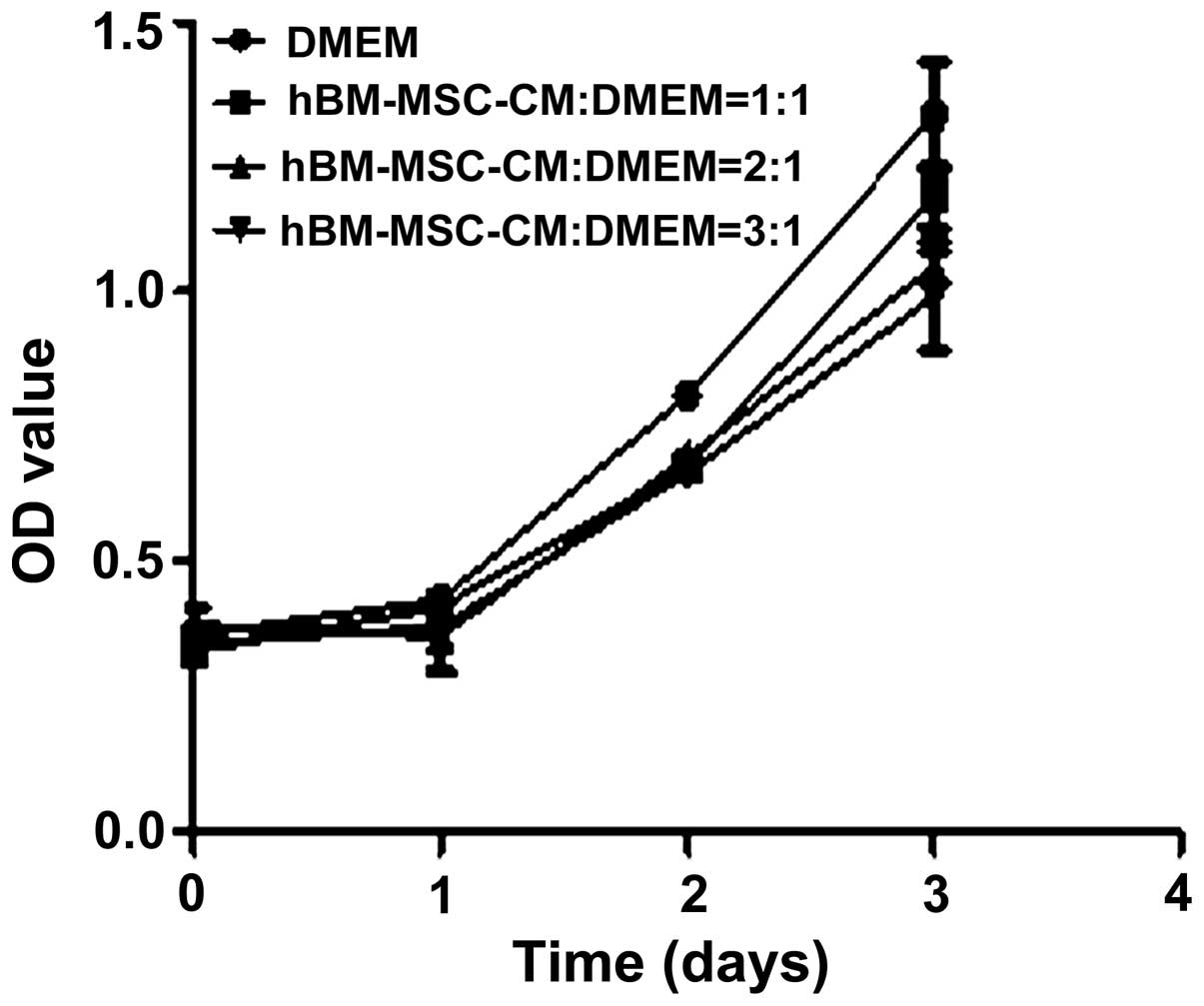
hBM-MSC-CM demonstrated no effect on the
proliferation of primary LECs
In addition to cell migration, cell proliferation
also performs a significant role in lymphangiogenesis. Therefore,
the effect of hBM-MSC-CM on LEC proliferation was investigated. The
primary LECs were treated with hBM-MSC-CM at various
concentrations. However, no significant difference in the rate of
LEC proliferation was identified between the hBM-MSC-CM and DMEM
treatment groups (Fig. 5).
Discussion
Previous studies have reported that hBM-MSC-CM
demonstrates a positive effect on tumor growth. It is hypothesized
that hBM-MSC-CM may induce the expression of VEGF in tumor cells,
and cause the activation of the ras homolog gene family, member
A-guanosine triphosphate and extracellular signal-regulated kinase
1/2 signaling pathways (18). Other
studies have also revealed that MSCs are able to promote tumor
growth and metastasis, including in breast cancer (19–22),
and that MSC-like cells isolated from human colon cancer tissues
can increase tumor growth and metastasis (23). However, another previous study
revealed that MSCs induced tumor growth in models of hepatocellular
carcinoma in vivo, but significantly decreased the presence
of lung metastases (24).
Therefore, it is essential to illustrate the role of MSCs in the
metastasis of SGC-7901 cells in nude mice models. In order to
establish whether MSCs perform an important role in tumor
metastasis, the present study treated MCF-7 and SGC-7901 cells with
hBM-MSC-CM for 40 days, and then analyzed the expression of the
lymphatic vessel-associated marker Prox-1 using western blot
analysis. The results revealed that the SGC-7901 cells treated with
hBM-MSC-CM exhibited a high expression of Prox-1. In addition, the
HGC-27 cells were treated with hBM-MSC-CM, and protein samples were
collected every five days. The results demonstrated that the
expression of VEGFR-3 increased over 10 days. Therefore, it was
hypothesized that hBM-MSC-CM contains cytokines that induce the
transition of cancer cells to cells with a lymphatic phenotype,
which in turn promotes tumor metastasis. Furthermore, the data
revealed that hBM-MSC-CM promotes tube formation and the migration
of LECs, but exerts no positive effect upon the proliferation of
LECs. In future studies, it may be of interest to identify the
mechanism by which MSCs promote tumor lymph vessel formation.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (grant nos. 81270214, 81472334
and 31340040), the Major Research Plan of the National Natural
Science Foundation of China (grant no. 91129718), the Jiangsu
Province for Outstanding Sci-tech Innovation Team in Colleges and
Universities (grant no. SJK2013-10) and the Jiangsu Province
Outstanding Medical Academic Leader and Sci-tech Innovation Team
Program (grant no. LJ201117).
References
|
1
|
Lin SY, Dolfi SC, Amiri S, et al: P53
regulates the migration of mesenchymal stromal cells in response to
the tumor microenvironment through both CXCL12-dependent and
-independent mechanisms. Int J Oncol. 43:1817–1823. 2013.PubMed/NCBI
|
|
2
|
Chaturvedi P, Gilkes DM, Wong CC, et al:
Hypoxia-inducible factor-dependent breast cancer-mesenchymal stem
cell bidirectional signaling promotes metastasis. J Clin Invest.
123:189–205. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lin R, Ma H, Ding Z, Shi W, Qian W, Song J
and Hou X: Bone marrow-derived mesenchymal stem cells favor the
immunosuppressive T cells skewing in a Helicobacter pylori model of
gastric cancer. Stem Cells Dev. 22:2836–2848. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
El-Haibi CP, Bell GW, Zhang J, et al:
Critical role for lysyl oxidase in mesenchymal stem cell-driven
breast cancer malignancy. Proc Natl Acad Sci U S A.
109:17460–17465. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang P, Dong L, Yan K, et al:
CXCR4-mediated osteosarcoma growth and pulmonary metastasis is
promoted by mesenchymal stem cells through VEGF. Oncol Rep.
30:1753–1761. 2013.PubMed/NCBI
|
|
6
|
Jung Y, Kim JK, Shiozawa Y, et al:
Recruitment of mesenchymal stem cells into prostate tumors promotes
metastasis. Nat Commun. 4:17952013. View Article : Google Scholar :
|
|
7
|
Zheng W, Aspelund A and Alitalo K:
Lymphangiogenic factors, mechanisms, and applications. J Clin
Invest. 124:878–887. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Karaman S and Detmar M: Mechanisms of
lymphatic metastasis. J Clin Invest. 124:922–928. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Stacker SA, Williams SP, Karnezis T,
Shayan R, Fox SB and Achen MG: Lymphangiogenesis and lymphatic
vessel remodelling in cancer. Nat Rev Cancer. 14:159–172. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jin Y, Mao J, Wang H, et al: Enhanced
tumorigenesis and lymphatic metastasis of CD133+
hepatocarcinoma ascites syngeneic cell lines mediated by JNK
signaling pathway in vitro and in vivo. Biomed Pharmacother.
67:337–345. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Eklund L, Bry M and Alitalo K: Mouse
models for studying angiogenesis and lymphangiogenesis in cancer.
Mol Oncol. 7:259–282. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Matsumoto M, Roufail S, Inder R, et al:
Signaling for lymphangiogenesis via VEGFR-3 is required for the
early events of metastasis. Clin Exp Metastasis. 30:819–832. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yu JW, Wu SH, Lu RQ, et al: Expression and
significances of contactin-1 in human gastric cancer. Gastroenterol
Res Pract. 2013:2102052013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xu ZY, Yu QM, Du YA, et al: Knockdown of
long non-coding RNA HOTAIR supresses tumor invasion and reverses
epithelial-mesenchymal transition in gastric cancer. Int J Biol
Sci. 9:587–597. 2013. View Article : Google Scholar
|
|
15
|
Singh N, Tiem M, Watkins R, et al: Soluble
vascular endothelial growth factor receptor 3 is essential for
corneal alymphaticity. Blood. 121:4242–4249. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Su JL, Yang PC, Shih JY, et al: The
VEGF-C/Flt-4 axis promotes invasion and metastasis of cancer cells.
Cancer Cell. 9:209–223. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen D, Zhang Y, Wang J, et al:
MicroRNA-200c overexpression inhibits tmorigenicity and metastasis
of CD117+CD44+ ovarian cancer stem cells by
regulating epithelial-mesenchymal transition. J Ovarian Res.
6:502013. View Article : Google Scholar
|
|
18
|
Zhu W, Huang L, Li Y, et al: Exosomes
derived from human bone marrow mesenchymal stem cells promote tumor
growth in vivo. Cancer Lett. 315:28–37. 2012. View Article : Google Scholar
|
|
19
|
Zhu W, Huang L, Li Y, et al: Mesenchymal
stem cell-secreted soluble signaling molecules potentiate tumor
growth. Cell Cycle. 10:3198–3207. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Goldstein RH, Reagan MR, Anderson K,
Kaplan DL and Rosenblatt M: Human bone marrow-derived MSCs can home
to orthotopic breast cancer tumors and promoes bone metastasis.
Cancer Res. 70:10044–10050. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Karnoub AE, Dash AB, Vo AP, et al:
Mesenchymal stem cells within tumour stroma promote breast cancer
metastasis. Nature. 449:557–563. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Muehlberg FL, Song YH, Krohn A, et al:
Tissue-resident stem cells promote breast cancer growth and
metastasis. Carcinogenesis. 30:589–597. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lin JT, Wang JY, Chen MK, Chen HC, Chang
TH, Su BW and Chang PJ: Colon cancer mesenchymal stem cells
modulate the tumorigenicity of colon cancer through interleukin 6.
Exp Cell Res. 319:2216–2229. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li GC, Ye QH, Xue YH, et al: Human
mesenchymal stem cells inhibit metastasis of a hepatocellular
carcinoma model using the MHCC97-H cell line. Cancer Sci.
101:2546–2553. 2010. View Article : Google Scholar : PubMed/NCBI
|