Introduction
Acute myeloid leukemia (AML) refers to a highly
heterogeneous group of hematopoietic diseases, which are
characterized by clonal accumulation and the growth of immature
myeloid cells within the bone marrow. At present, the primary
treatment for AML is remission induction therapy with multiple,
combined chemotherapeutic agents, which aims to reduce the total
body leukemic cell population to an undetectable level. This is
usually followed by either consolidation chemotherapy or allogeneic
hematopoietic stem cell transplantation (HSCT), depending on how
well the patient responds to chemotherapy alone and their response
to intensive treatments (1). The
benefits of this treatment approach vary considerably with patient
age and AML subtype, which are two of the most important prognostic
factors. Elderly AML patients, particularly those >65 years old
and with a history of myelodysplastic syndromes (MDS) or antecedent
hematological disorders, exhibit unsatisfactory tolerance profiles
and disappointing complete remission (CR) and overall survival
rates following this conventional approach (2–4). As
AML predominantly occurs in older individuals and the global
population is rapidly aging, novel treatment strategies are
urgently required for elderly patients with AML (5). The present study describes a case of
CR without relapse nine months after the administration of a
combination regimen of decitabine and cytarabine, aclarubicin and
granulocyte colony-stimulating factor (G-CSF) (CAG) in an elderly
patient with AML transformed from chronic myelomonocytic leukemia
(CMML) (6).
Case report
In March 2013, a 70-year-old male presented to The
First Central Hospital of Tianjin (Tianjin, China) with fever,
pruritus and weakness that had been apparent for two weeks, and a
two-year history of monocytosis (22.5–27.0%). The inpatient
laboratory tests revealed a hemoglobin (Hb) level of 120 g/l, a
white blood cell (WBC) count of 9.23×109/l and a
platelet count of 122×109/l, but an abnormal monocyte
percentage of up to 35.5% (normal range, 5–10%). The
anti-inflammatory drug, piperacillin-tazobactam (4.5 g, once every
12 h), was administered for three days, however, no improvement was
noted with regard to the symptoms of pruritus and weakness. The
patient was therefore admitted to The First Central Hospital of
Tianjin. Upon admission, a physical examination revealed rashes on
the extremities, but no sternal pain or tenderness, and no
peripheral lymphadenopathy or hepatosplenomegaly. The routine blood
test identified a Hb level of 106 g/l, a WBC count of
39.52×109/l and a platelet count of 81×109/l.
A diagnosis of AML was suspected. A bone marrow aspirate
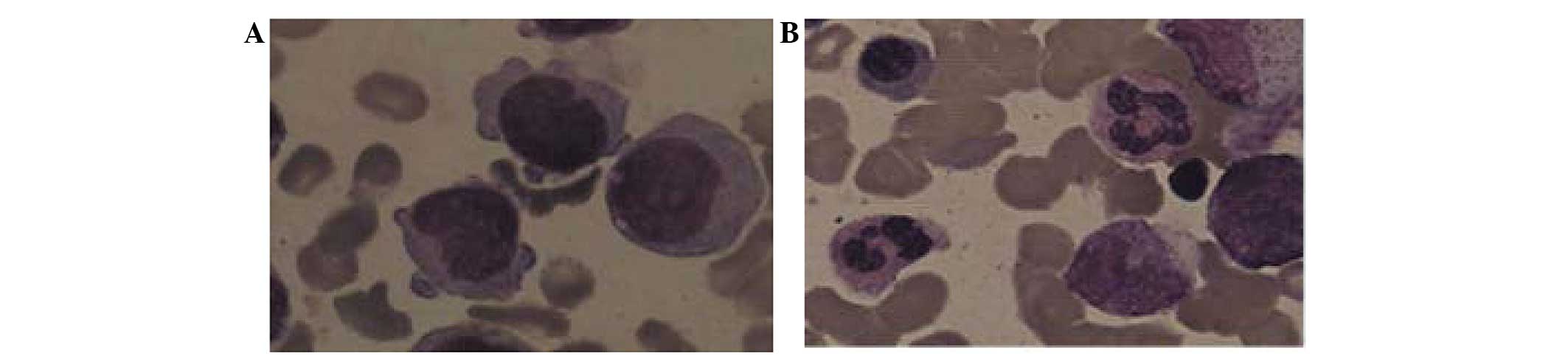
examination revealed a monoblast percentage of 38% and a
promonocyte percentage of 55% (Fig.
1A), values which were significantly higher than the threshold
of blasts (>20%) required in the bone marrow for a diagnosis of
AML, according to the 2008 World Health Organization (WHO)
(6) AML classification systems.
Immunohistochemical analysis revealed strong staining signals for
myeloperoxidase in the cytoplasm of the blasts, and the myeloid
lineage markers, human leukocyte antigen-DR and cluster of
differentiation (CD)15, CD4, CD13, CD33, CD38, CD64, CD11b, CD14
and CD56, were detected on the surface of the monoblasts by
multiparameter flow cytometry (Fig.
2). There was no evidence of a JAK2V617F mutation or a BCR/ABL
fusion transcript. In addition to the bone marrow aspiration
assessment, immunohistochemical staining and flow cytometric
analyses, single nucleotide polymorphism (SNP) arrays were
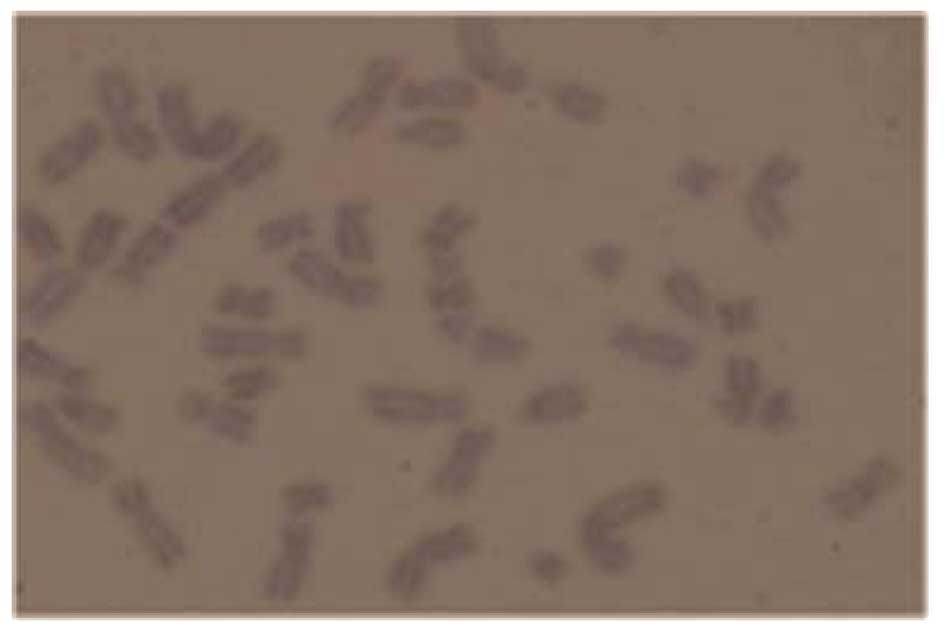
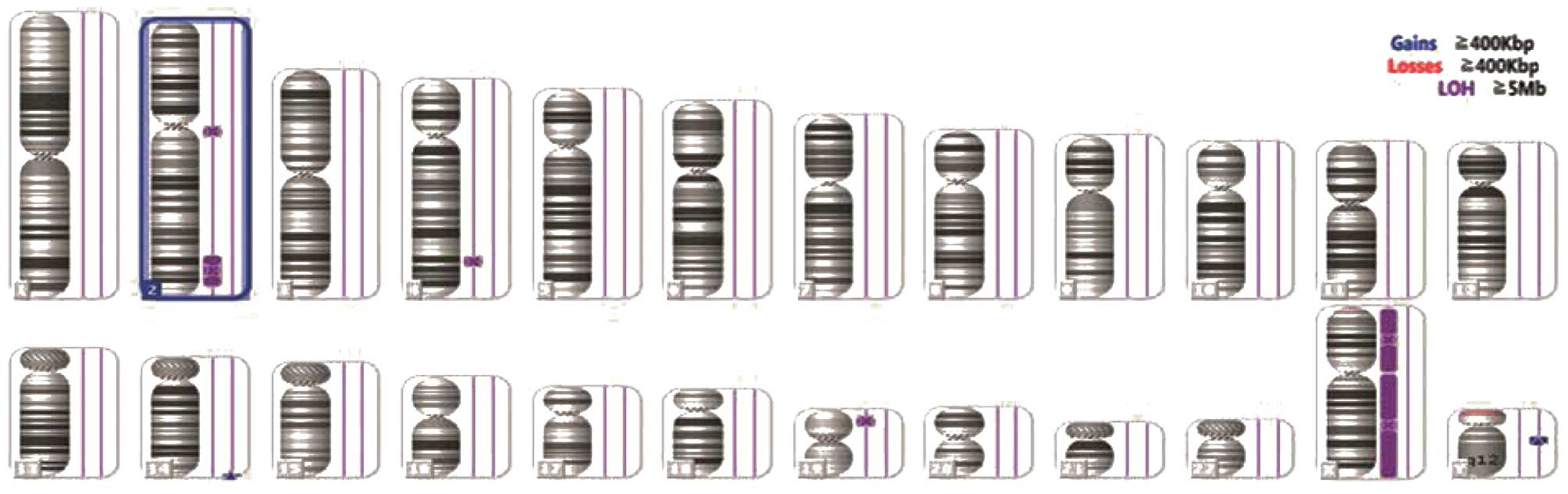
performed. Cytogenetic abnormalities, including Y chromosome loss
(Fig. 3) and uniparental disomy
(UPD) on chromosomes 4q, 2q and 19p (Fig. 4) were detected. Considering the
two-year history of monocytosis, the patient was diagnosed with AML
transformed from CMML together with a rare genetic abnormality
(6).
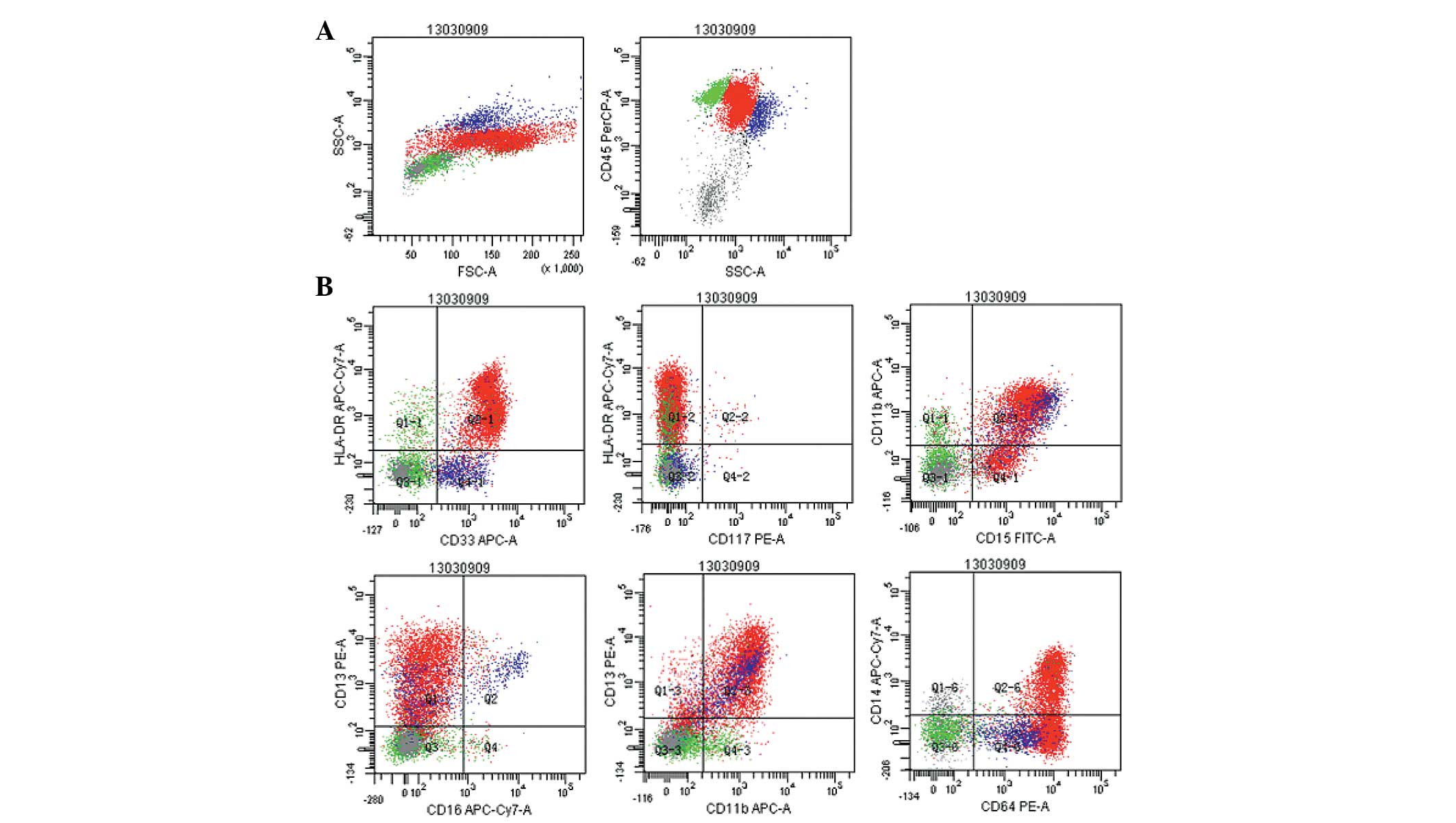 | Figure 2Flow cytometry scatter plots revealing
the gating strategy with (A) cluster of differentiation
(CD)45/side-scattered light and (B) the ratio of monoblasts
(72.3%), and their expression of the myelomonocytic antigens, human
leukocyte antigen-DR, CD15, CD4, CD13, CD33, CD38, CD64, D11b, CD14
and CD56. |
The patient was treated with 1,000 mg imipenem every
8 h for eight days, and 500 mg hydroxycarbamide every 12 h for four
days. Subsequently, a combination treatment regimen consisting of
decitabine and CAG was initiated. In total, 15 mg/m2
decitabine was administered daily on days one to five, 20 mg
aclarubicin daily on days three to six, 20 mg/m2
cytarabine twice daily on days three to 16 and 150 μg G-CSF daily
from day two until the attainment of a normal WBC count. At the end
of the low-dose regimen, the patient achieved a CR and experienced
less severe adverse events, specifically severe infections. The
values of the major test parameters were normal or close to normal,
and included a Hb level of 117 g/l, a WBC count of
4.52×109/l, a platelet count of 370×109/l, a
monocyte percentage of 6.5% and a monoblast percentage of 1.5%
(Fig. 1B). Administration of the
regimen was continued. Subsequent to four cycles of treatment with
decitabine in combination with CAG, the patient achieved a
successful clinical remission and demonstrated no evidence of AML
relapse for nine months. Written informed consent was obtained from
the patient’s family for the publication of the case report and the
accompanying images.
Discussion
CMML, defined by the 2008 WHO classification of
myeloid neoplasms as a clonal hematopoietic stem cell disorder
(6), is currently classified under
a new division of myeloid neoplasms, the MDS/myeloproliferative
neoplasm disorders. These disorders exhibit dysplastic and
proliferative features. Cytogenetics is considered to be one of the
most valuable determinants used during the risk classification and
prognostication of AML (7,8). Previous studies have revealed that SNP
arrays can identify chromosomal markers that cannot be detected by
conventional cytogenetics (9,10). In
general, specific cytogenetic alterations cannot be identified in
patients with CMML by chromosome banding analysis alone. The most
common single chromosomal abnormalities in cases of CMML are
monosomy 7 (3.9–8.5%), trisomy 8 (4.1–7.8%) and other
abnormalities, which may include complex karyotypes (4.4–6.3%),
isochromosome 17 (1–2%), trisomy 21 (1–2%) and deletion of 5q
(1.5%) (11–13). Abnormal karyotypes have been
reported in 20–40% of CMML cases, and include abnormalities shared
with other myeloid neoplasms (14).
According to a previous study involving 140 patients (10), acquired somatic UPD is not uncommon
in primary (29%) and secondary (35%) AML. Although AML with UPD q4
is rarely reported (15), another
previous study (16) revealed an
association between UPD 4q and CMML. Based on the aforementioned
criteria and studies, the patient in the present study was
diagnosed with AML transformed from CMML.
CMML remains to be a challenging malignancy to
treat, and for this reason, the median survival rate for patients
ranges between 12 and 18 months. If CMML progresses to AML,
patients exhibit poor prognoses, face limited treatment options and
on average, demonstrate survival rates of only a few months
(17). The only potential curative
treatment for patients with CMML is allogeneic HSCT, however, this
particular approach is usually unsuitable for elderly individuals.
Treatment with the DNA methyltransferase inhibitors, azacitidine
and decitabine, has been extensively studied for the management of
AML and MDS (18–21). Two mechanisms are believed to
underlie the action of decitabine. First, decitabine incorporates
into the DNA following phosphorylation, without the need for
reduction. Secondly, decitabine does not incorporate into RNA, and
inhibits DNA methyltransferase 2 (22). A Japanese study, which included AML
patients ≥60 years old, revealed that when a 10-day decitabine
regimen was administered and then repeated following an interval of
4–5 days, CR and median survival rates of 47% and 12.7 months,
respectively, were achieved subsequent to an average of three
cycles of therapy (23). The CAG
regimen, which combines G-CSF with low doses of cytarabine and
aclarubicin, was first used by a Japanese study in 1995, and was
reported to be a chemotherapy option for AML (24). In this regimen, aclarubicin is
effective regardless of multi-drug resistance gene status, and
G-CSF acts to enhance the transition of resting G0 phase
AML cells into the cell cycle. The CAG regimen has been used for
relapsed or refractory AML and MDS patients, and also specifically
for elderly patients. The high response rates and good tolerability
of this regimen is observed not only in relapsed or refractory
cases, but also in previously untreated patients (25,26).
Therefore, for these reasons, the combination of decitabine and CAG
was selected to treat the patient in the present study.
Due to the conjecture diagnosis of CMML in the
present study, the regimen of decitabine in combination with CAG
was selected. This regimen consisted of low-dose decitabine (15
mg/m2), aclarubicin (15 mg/m2 for four days)
and low-dose cytarabine (15 mg/m2, every 12 h for 10
days). The patient achieved a CR following only one such low-dose
regimen, and experienced less severe adverse events, specifically
severe infections. Subsequent to a total of four cycles of
treatment with decitabine in combination with CAG, the patient
achieved a successful clinical remission. In conclusion, a
combination of low-dose decitabine and CAG may offer a novel and
potentially effective treatment regimen for cases of AML
transformed from CMML.
Acknowledgments
The present study was supported by grants from the
Tianjin Municipal Health Bureau of Science and Technology Fund 2013
Project (no. 13KG106).
References
|
1
|
Döhner H, Estey EH, Amadori S, et al:
European LeukemiaNet: Diagnosis and management of acute myeloid
leukemia in adults: recommendations from an international expert
panel, on behalf of the European LeukemiaNet. Blood. 115:453–474.
2010. View Article : Google Scholar
|
|
2
|
Dombret H, Raffoux E and Gardin C: New
insights in the management of elderly patients with acute myeloid
leukemia. Curr Opin Oncol. 21:589–593. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lancet JE, Willman CL and Bennett JM:
Acute myelogenous leukemia and aging. Clinical interactions.
Hematol Oncol Clin North Am. 14:251–267. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lin TL and Levy MY: Acute myeloid
leukemia: focus on novel therapeutic strategies. Clin Med Insights
Oncol. 6:205–217. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ungewickell A and Medeiros BC: Novel
agents in acute myeloid leukemia. Int J Hematol. 96:178–185. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vardiman JW, Thiele J, Arber DA, et al:
The 2008 revision of the World Health Organization (WHO)
classification of myeloid neoplasms and acute leukemia: rationale
and important changes. Blood. 114:937–951. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Grimwade D, Walker H, Oliver F, et al: The
importance of diagnostic cytogenetics on outcome in AML: analysis
of 1,612 patients entered into the MRC AML 10 trial. The Medical
Research Council Adult and Children’s Leukaemia Working Parties.
Blood. 92:2322–2333. 1998.PubMed/NCBI
|
|
8
|
Löwenberg B: Diagnosis and prognosis in
acute myeloid leukemia - the art of distinction. N Engl J Med.
358:1960–1962. 2008. View Article : Google Scholar
|
|
9
|
Mohamedali A, Gäken J, Twine NA, et al:
Prevalence and prognostic significance of allelic imbalance by
single-nucleotide polymorphism analysis in low-risk myelodysplastic
syndromes. Blood. 110:3365–3373. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tiu RV, Gondek LP, O’Keefe CL, et al: New
lesions detected by single nucleotide polymorphism array-based
chromosomal analysis have important clinical impact in acute
myeloid leukemia. J Clin Oncol. 27:5219–5226. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bacher U, Haferlach T, Kern W, et al:
Conventional cytogenetics of myeloproliferative diseases other than
CML contribute valid information. Ann Hematol. 84:250–257. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Haase D, Germing U, Schanz J, et al: New
insights into the prognostic impact of the karyotype in MDS and
correlation with subtypes: Evidence from a core dataset of 2124
patients. Blood. 110:4385–4395. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kohlmann A, Grossmann V, Klein HU, et al:
Next-generation sequencing technology reveals a characteristic
pattern of molecular mutations in 72.8% of chronic myelomonocytic
leukemia by detecting frequent alterations in TET2, CBL, RAS, and
RUNX1. J Clin Oncol. 28:3858–3865. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Orazi A and Germing U: The
myelodysplastic/myeloproliferative neoplasms: myeloproliferative
diseases with dysplastic features. Leukemia. 22:1308–1319. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Raghavan M, Smith LL, Lillington DM, et
al: Segmental uniparental disomy is a commonly acquired genetic
event in relapsed acute myeloid leukemia. Blood. 112:814–821. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gondek LP, Tiu R, O’Keefe CL, et al:
Chromosomal lesions and uniparental disomy detected by SNP arrays
in MDS, MDS/MPD, and MDS-derived AML. Blood. 111:1534–1542. 2008.
View Article : Google Scholar
|
|
17
|
Parikh SA and Tefferi A: Chronic
myelomonocytic leukemia: 2012 update on diagnosis, risk
stratification, and management. Am J Hematol. 87:610–619. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kantarjian H, Oki Y, Garcia-Manero G, et
al: Results of a randomized study of 3 schedules of low-dose
decitabine in higher-risk myelodysplastic syndrome and chronic
myelomonocytic leukemia. Blood. 109:52–57. 2007. View Article : Google Scholar
|
|
19
|
Steensma DP, Baer MR, Slack JL, et al:
Multicenter study of decitabine administered daily for 5 days every
4 weeks to adults with myelodysplastic syndromes: the alternative
dosing for outpatient treatment (ADOPT) trial. J Clin Oncol.
27:3842–3848. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
de Lima M, Giralt S, Thall PF, et al:
Maintenance therapy with low-dose azacitidine after allogeneic
hematopoietic stem cell transplantation for recurrent acute
myelogenous leukemia or myelodysplastic syndrome: a dose and
schedule finding study. Cancer. 116:5420–5431. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Baer MR and Gojo I: Novel agents for the
treatment of acute myeloid leukemia in the older patient. J Natl
Compr Canc Netw. 9:331–335. 2011.PubMed/NCBI
|
|
22
|
Oki Y, Kondo Y, Yamamoto K, et al: Phase
I/II study of decitabine in patients with myelodysplastic syndrome:
a multi-center study in Japan. Cancer Sci. 103:1839–1847. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Blum W, Garzon R, Klisovic RB, et al:
Clinical response and miR-29b predictive significance in older AML
patients treated with a 10-day schedule of decitabine. Proc Natl
Acad Sci USA. 107:7473–7478. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yamada K, Furusawa S, Saito K, et al:
Concurrent use of granulocyte colony-stimulating factor with
low-dose cytosine arabinoside and aclarubicin for previously
treated acute myelogenous leukemia: a pilot study. Leukemia.
9:10–14. 1995.PubMed/NCBI
|
|
25
|
Saito K, Nakamura Y, Aoyagi M, et al:
Low-dose cytarabine and aclarubicin in combination with granulocyte
colony-stimulating factor (CAG regimen) for previously treated
patients with relapsed or primary resistant acute myelogenous
leukemia (AML) and previously untreated elderly patients with AML,
secondary AML, and refractory anemia with excess blasts in
transformation. Int J Hematol. 71:238–244. 2000.PubMed/NCBI
|
|
26
|
Suzushima H, Wada N, Yamasaki H, et al:
Low-dose cytarabine and aclarubicin in combination with granulocyte
colony-stimulating factor for elderly patients with previously
untreated acute myeloid leukemia. Leuk Res. 34:610–614. 2010.
View Article : Google Scholar
|