Introduction
Glomus tumors (GTs) are neoplasms arising from the
modified smooth muscle cells surrounding arteriovenous anastomosis
(1,2). GT is an uncommon soft tissue tumor
with an incidence of 1.6%, which is usually located in the dermis
and subcutaneous tissue, with ≤65% occurring in the subungual area.
Due to sparse or absent glomus bodies in the visceral organs,
extracutaneous presentation of GT is rarely observed (3–8).
Previously reported atypical sites of origin include the stomach,
mediastinum, vagina, penis, lung, patella and trachea.
Histologically, GTs have been divided into three subtypes: Classic
glomus tumors, glomangiomas, and glomangiomyomas. Glomangiomas are
an uncommon type, accounting for <20% of GTs (1,2,9–11).
Until now, only 27 cases of GTs, and five reports of glomangioma
subtype arising from the trachea, including the present case, have
been reported (8–32). GTs are usually benign and recurrence
rates are variable, ranging from 10 to 30% (1,2). The
present study reported a primary GT of the trachea, which is a
possible differential diagnostic alternative when a tracheal tumor
is detected by radiographic or endoscopic examination. Written
informed consent was obtained from the patient and the patient’s
family.
Case report
A 44-year-old male, exhibiting a defined tracheal
tumor that was diagnosed by the local hospital (Bazhou People’s
Hospital, Bazhou, China) two months earlier, was admitted to our
hospital (The Third People’s Hospital of Chengdu, Chengdu, China)
due to acute respiratory distress. The patient had suffered from
cough, expectoration and dyspnea without any evident incentive for
>1 year. Six days before admission, the symptoms were aggravated
with hemoptysis. A chest X-ray scan was found to be normal.
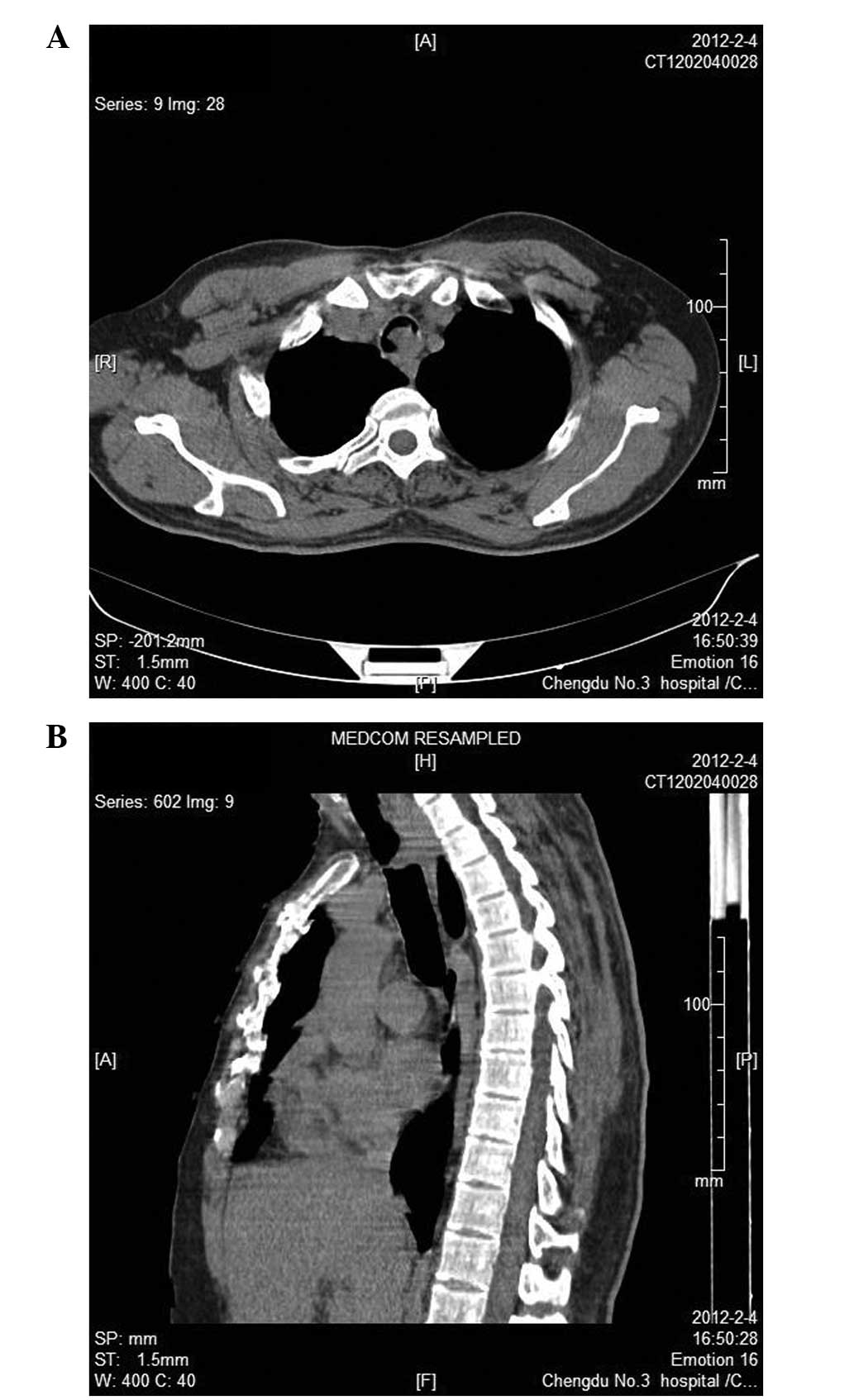
However, a computed tomographic (CT) scan revealed a demarcated
homogenous intratracheal mass at the layer of the superior border
of the manubrium (Fig. 1). Flexible
bronchoscopy revealed a sessile tumor with a smooth surface arising
from the posterior wall of the trachea, which occluded ~90% of the
trachea lumen, at 3 cm proximal to the carina. A biopsy was
performed to elucidate the nature of the tumor. At 3 h after
referral, the patient exhibited no response to voice and became
signally dyspneic and cyanotic in the face and extremities, which
indicated apnea induced by neoplastic obstruction.
To prevent mortality and restore the airway,
endotracheal intubation was conducted initially using a rigid
tracheoscope under surface anesthesia. Due to the unstable
condition of the patient, comprehensive treatment was administered
to prepare for the operation. A preoperative coagulation profile
revealed prolonged activated partial thromboplastin time (APTT;
68.3 sec) and prothrombin time (PT; 19.1 sec), which indicated an
increased risk for the surgery. With plasma transfusion, after five
days the patient underwent segmental resection of the trachea with
an end-to-end anastomosis and the bilateral pulmonary infection led
to a poor general postoperative condition. Due to being bed-ridden
postoperatively, 13 days following the surgery the patient
exhibited femoral and deep vein thrombosis. Thrombolytic therapy
was challenging, due to the persistent bleeding disorder. To
prevent pulmonary embolus, vena cava filters were placed and
clot-busting therapy was conducted simultaneously. The patient was
discharged without the support of ventilation 26 days after
presentation. Three months after resection, the patient underwent
follow-up with CT and endoscopy to exclude focal recurrence or
suspicious metastasis. The lumen of the trachea remained clear for
20 months after the surgery.
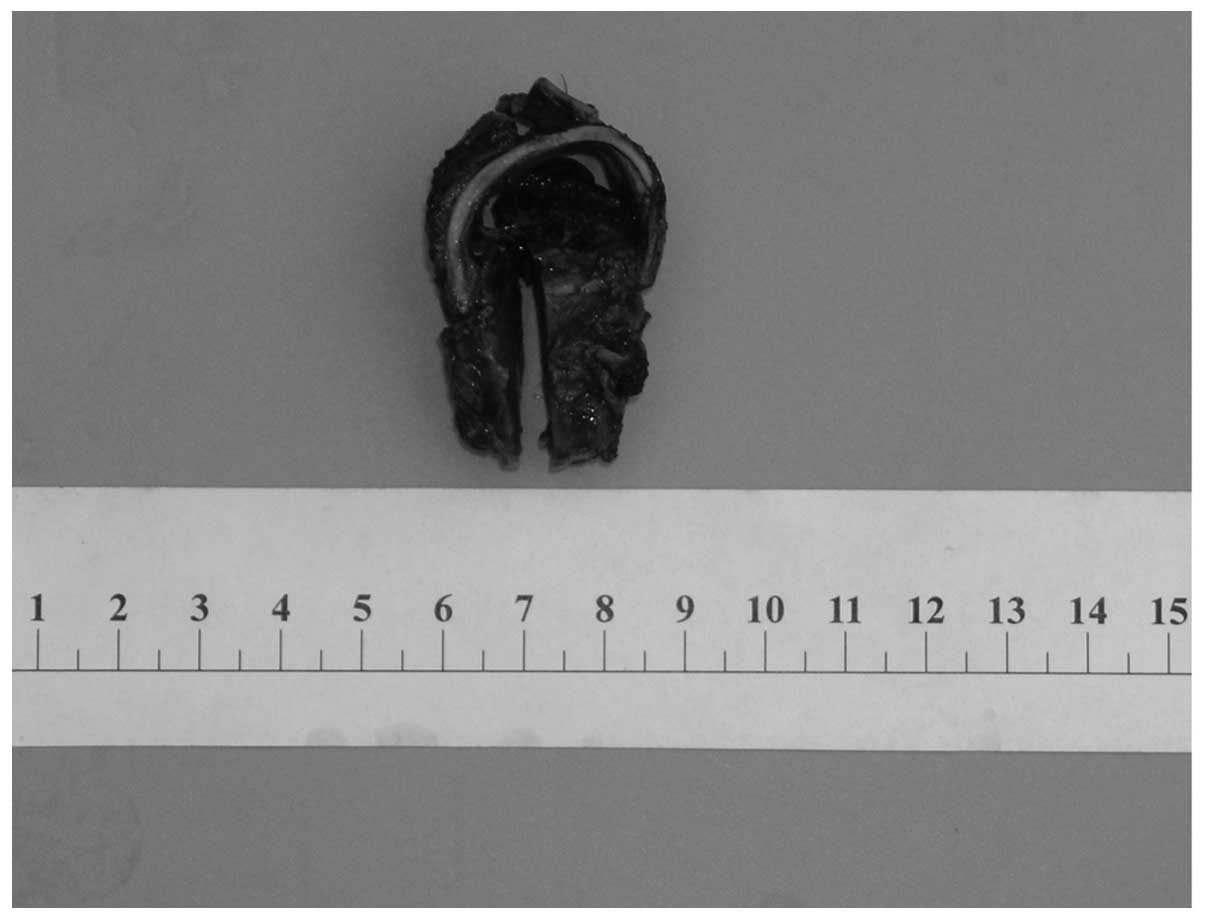
The surgical specimen was ~2 cm in length, with a
luminal diameter of 2.5 cm. The specimen exhibited a 3×2.5×1 cm
red-brown mass sessile in the posterior wall of the trachea, which
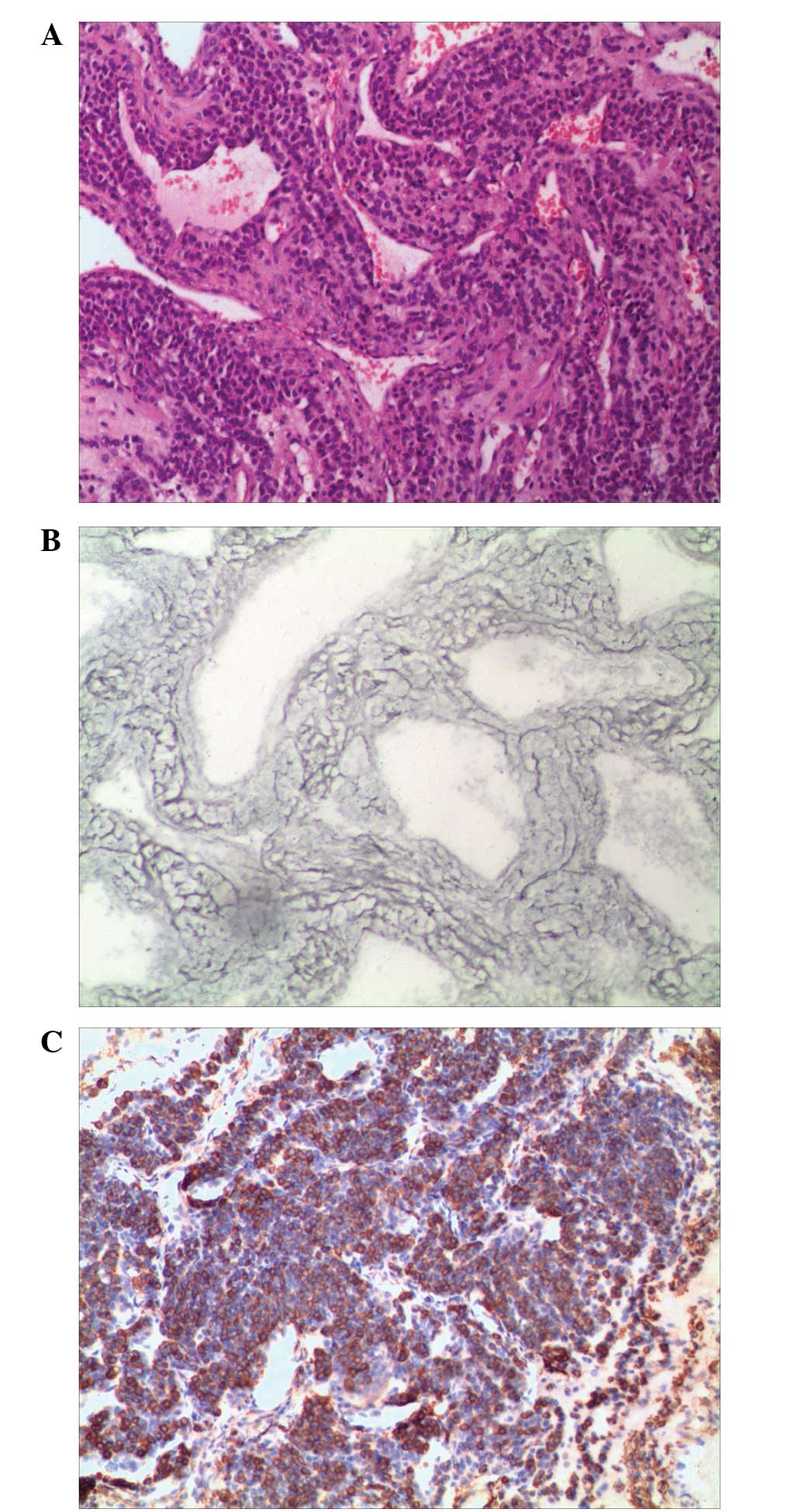
occupied the lumen as a polypoid mass (Fig. 2). Microscopically, the tumor was
composed of epithelioid round to polygonal cells with defined
cellular borders, weakly eosinophilic or clear cytoplasm and
uniform round to ovoid nuclei. These formed solid sheets, small
nests or organoidal structures surrounding dilated and tangled
venous vessels, which were different from the thin-walled and
capillary-like vascular channels normally observed in GT proper
type. Cellular atypia and mitotic figures were absent (Fig. 3A). Gomori’s staining demonstrated a
delicate network of reticulum fibers lying between individual tumor
cells (Fig. 3B). In addition,
evidence of squamous metaplasia was observed in the intact
overlying respiratory epithelium. Immunohistochemical staining
revealed positivity for vimentin and smooth muscle actin antibodies
(Fig. 3C). Pan-cytokeratin, desmin,
chromogranin, synaptophysin, S-100, calfetinin, leukocyte common
antigen, HMB-45 and cluster of differentiation 99 were negative
(Table I). The tumor was diagnosed
as a glomangioma.
 | Table IAntibody panel used in this case
study. |
Table I
Antibody panel used in this case
study.
|
Antibody/molecule | Clone | Dilution | Expression |
|---|
| SMA | 1A4 | 1:70 | (+) |
| Vimentin | SP20 | 1:70 | (+) |
| Desmin | D33 | 1:70 | (−) |
| Pan-cytokeratin | AE1/AE3 | 1:70 | (−) |
| Chromogranin | SP12 | 1:70 | (−) |
| Synaptophysin | SP11 | 1:70 | (−) |
| S-100 | 4C4.9 | 1:70 | (−) |
| Calfetinin | Polyclone | 1:60 | (−) |
| LCA | ZB11+PD7/26 | 1:70 | (−) |
| Melanosome | HMB-45 | 1:70 | (−) |
| CD99 | SP119 | 1:70 | (−) |
Discussion
GTs are uncommon mesenchymal neoplasms. The etiology
of these tumors remains a conundrum, and certain individuals
attribute GTs to trauma, endocrine disorder or autosomal dominant
inheritance (1,2). Murray and Stout (2) indicated that GT often occurred in
subungual hematoma and the fingertips of female patients, while in
the extradigital tissues of male patients. This viewpoint coincides
with previous studies on the tracheal GTs presented in Table II, in
which the majority of cases involved male patients. GTs in the
trachea are scarce. The first GT case was reported in 1950 and, to
the best of our knowledge, since then only 27 additional cases
(including the present study) have been reported (Table II) (8–32).
Based on the literature reviewed (Table III), the majority of cases have
been identified on the posterior wall of the lower two-thirds of
the trachea (82%), where mucus glands and vessels are numerous. The
cases included 16 males and 10 females (1.6:1), with a mean age of
50. The main symptoms included coughing, dyspnea and hemoptysis.
Certain patients also suffered from chest pain, stridor and
hoarseness. The mass diameter had a range of 1.2–4.5 cm. Almost all
the tumors, including that of the present study, were benign and
noninvasive.
 | Table IIReported cases of tracheal glomus
tumor. |
Table II
Reported cases of tracheal glomus
tumor.
| First author, year
(ref) | Patient
age/gender | Symptoms | Size (cm) | Tracheal
location | Main treatment | Follow-up |
|---|
| Hussarek, 1950
(8) | 43/F | Dyspnea | ‘Bean-sized’ | Upper trachea | Tracheal
resection | Not stated |
| Fabich, 1980
(9) | 63/M | Cough | 2.5×2×1 | Lower trachea | Tracheal
resection | Succumbed to
complications, postop day 10 |
| Heard, 1982 (10) | 50/M | Asthma-like
symptoms | 2.5×1.5×1.0 | Lower trachea | Tracheal
resection | Sepsis, died postop
day 15 |
| Ito, 1988 (11) | 51/M | Respiratory
infections, hemoptysis | 1.5×1.2×1.0 | Upper trachea | Tracheal
resection | No recurrence at 2
years |
| Kim, 1989 (12) | 54/F | Cough, dyspnea,
hemoptysis | 1.5×1.2 | Mid-trachea | Tracheal
resection | No recurrence at 13
months |
| Shin, 1990 (13) | 47/F | Intermittent cough
and hemoptysis | 1.5×1.0×1.0 | Lower trachea | Tracheal
resection | Not stated |
| Garcia-Prats, 1991
(14) | 58/M | Dyspnea, cough,
hemoptysis | 2.5×1.8 | Mid-trachea | Tracheal
resection | No recurrence at 8
months |
| Haraguchi, 1991
(15) | 61/M | Asymptomatic | 1.2 | Mid-trachea | Tracheal
resection | Not stated |
| Arapantoni, 1995
(16) | 65/M | Dyspnea,
hemoptysis | 4.5×3 | Lower trachea | Endoscopic resection
and Nd-YAG | No recurrence at 1
year |
| Koskinen, 1998
(17) | 66/M | Asymptomatic | 3×2 | Lower trachea | Endoscopic
resection, Nd-YAG, and external radiotherapy | Not stated |
| Watanabe, 1998
(18) | 43/M | Hoarseness | 2.0×1.6×1.4 | Lower trachea | Tracheal
resection | No recurrence at 20
months |
| Menaissy, 2000
(19) | 34/M | Hemoptysis | 2.4×2.1×1.6 | Mid-trachea | Tracheal
resection | No recurrence at 4
months |
| Gowan, 2001
(20) | 73/M | Cough, hemoptysis,
dyspnea, Chest pain | 1.6×0.6×0.3 | Mid-trachea | Tracheal
resection | No recurrence at 6
years |
| Chien, 2003
(21) | 50/F | Cough, dyspnea,
hemoptysis | 2.5×2.5×2 | Lower trachea | Tracheal
resection | No recurrence at 1
years |
| Nadrous, 2004
(22) | 39/M | Intermittent
hemoptysis | 2.0×1.5×1.5 | Upper trachea | Tracheal
resection | No recurrence at 3
months |
| Haver, 2008
(23) | 10/F | Dyspnea | 1.8×1.3×1.3 | Mid-trachea | Tracheal
resection | No recurrence at 2
years |
| Colaut, 2008
(24) | 70/M | Dyspnea | 2×1×1 | Mid-trachea | Endoscopic
resection and Nd-YAG | No recurrence at 2
years |
| Parker, 2010
(25) | 43/F | Chest pain,
asthma | 2.0×1.6×1.5 | Lower trachea | Tracheal
resection | Not stated |
| Shang, 2010
(26) | 59/M | Chest pain,
dyspnea | 2.0×1×0.5 | Lower trachea | Endoscopic
resection | No recurrence at 12
months |
| 22/F | Cough, dyspnea | 1.8×1.5×1.4 | Lower trachea | Endoscopic
resection | No recurrence at 12
months |
| Sakr, 2011
(27) | 66/M | Dyspnea,
stridor | 2×1.2×0.8 | Upper trachea | Endoscopic
resection and tracheal resection | No recurrence at 21
months |
| Mogi, 2011
(28) | 56/F | Cough, dyspnea | 1.3×1.2×1.1 | Lower trachea | Tracheal sleeve
resection | No recurrence at 9
months |
| Okereke, 2011
(29) | 58/M | Stridor, shortness
of breath. | 1.1 | Mid-trachea | Tracheal
resection | Not stated |
| Norder, 2012
(30) | 49/F | Cough, dyspnea | 1.2×1.1×1.1 | Upper trachea | Endoscopic
resection | Not stated |
| Lang-Lazdunski L,
2012 (31) | 62/F | Cough, dyspnea | 1.6 | Lower trachea | Left upper sleeve
lobectomy | Not stated |
| Fan, 2013 (32) | 15/M | Cough,
hemoptysis | 2.5 | Lower trachea | Tracheal
resection | No recurrence at 12
months |
| Present study | 44/M | Cough, hemoptysis,
dyspnea | 3×2.5×1 | Lower trachea | Tracheal
resection | No recurrence at 20
months |
 | Table IIISummary characteristics of previously
reported patients with glomus tumor of the tracheaa. |
Table III
Summary characteristics of previously
reported patients with glomus tumor of the tracheaa.
| Characteristic | Value |
|---|
| Age, range
(mean) | 10–73 (50) |
| Male:Female | 1.6:1 |
| Symptoms, n
(%)b |
| Cough | 11 (41) |
| Hemoptysis | 11 (41) |
| Dyspnea | 15 (56) |
| Chest pain | 3 (11) |
| Asthma or
stridor | 3 (11) |
| None | 2 (7) |
| Tumor size, range
(mean) | 1.1–4.5 (2.06) |
| Tumor location, n
(%)b |
| Upper trachea | 5 (18%) |
| Mid-trachea | 8 (30%) |
| Lower trachea | 14 (52%) |
| Treatment, n |
| Tracheal
resection | 19 |
| Endoscopic
resection | 7 |
| Other | 1 |
To the best of our knowledge, no malignant GTs have
been described in the trachea thus far. The histological morphology
varies in subtypes according to the relative proportions of the
glomus cells, vascular structures and smooth muscle tissue
(2,33). In addition to the classic three
subtypes, including GT proper, glomangioma and glomangiomyoma, an
oncocytic variant exists that was first described in 1990 (13). GT proper type accounts for ~75% of
all the GTs, which also applies to the tracheal mass, while
glomangioma occupies 20% of all the cases (Table II). The oncocytic tumors are the
least common, comprising 1/20 of all the tracheal GTs reported in
the literature (13). In the
present study, the tumor was characterized by organoid nests and
solid sheets of uniform round or polygonal cells around the venous
vessels, but not thin-walled or capillary-like vascular channels.
This is the fifth description of a glomangioma arising from the
trachea. In the study by Shin et al, the round glomus cells
represented the largest component of tumor cells surrounding the
thin-walled vascular spaces, leading to the hypothesis that this
may be a subtype of glomangioma (13). Based on the Pathology and Genetics
of Tumors of Soft Tissue and Bone (World Health Organization
Classification of Tumors), the main histological characteristic of
glomangioma is that the glomus cell clusters are arranged around
dilated venous vessels (33). The
immunohistochemical features have contributed to differential
diagnosis with carcinoid and hemangiopericytoma (28,32,33).
GTs are positive for vimentin and smooth musle actin, but negative
for neuroendocrine and epithelial markers, including S-100 protein,
chromogranin, desmin, cytokeratins and factor VIII.
To avoid local recurrence, tracheal resection is
widely used and exhibits favorable prognosis (Table II). Endoscopic intervention alone
is also applied in a limited number of cases, which has rigorous
indications if: The lesion is strictly confined without extension;
histology confirms the tumor is benign; as a temporary measure for
surgery preparation; or the patient is not fit or willing to
undergo surgical resection. In addition, interventional
bronchoscopy is a first-line treatment to immediately restore the
airway patency in urgent situations (14,16,17,
25–27,30).
Furthermore, as an uncommon site, treating the
complications and accompanying diseases during the perioperative
period is crucial in order to increase survival rates. The main
cause of mortality reported in the literature following surgery was
not the primary tumor or the procedure but the complications
(9,10). The present patient exhibited a
severe clinical condition and the symptoms were caused by tumor
obstruction. Therefore, endotracheal intubation was performed to
aid the recovery of the stabilization, ensuring that the patient
fit for the procedure. Due to his severe coagulopathy, preoperative
correction of coagulation disturbance was performed to prepare for
the surgery. The filters of the vena cava and warfarin
administration were arranged simultaneously following surgery to
treat femoral and deep vein thrombosis caused by a long time period
in bed. Active sputum excretion, prevention of infections and early
mobilization is beneficial for recovery.
In conclusion, previous studies and the present
report reveal that the incidence of GTs in the trachea is low and
these tumors primarily occur in males, with unknown etiology and
concealed onset. Patients with GTs usually resort to medical
attention for symptoms of airway obstruction. CT imaging, magnetic
resonance imaging and flexible bronchoscopy of GTs reveal a
lumen-occupying lesion. Surgical management is generally the
primary option and histopathological examination may determine the
diagnosis. The long-term prognosis of the disease is promising.
References
|
1
|
Enzinger FM, Weiss SW and Chandrasekhar B:
Soft tissue tumors. PRS Journal. 76:153–155. 1985.
|
|
2
|
Murray MR and Stout AP: The glomus tumor:
Investigation of its distribution and behavior, and the identity of
its ‘epithelioid’ cell. Am J Pathol. 18:183–203. 1942.PubMed/NCBI
|
|
3
|
Kanwar YS and Manaligod JR: Glomus tumor
of the stomach. An ultrastructural study. Arch Pathol. 99:392–397.
1975.PubMed/NCBI
|
|
4
|
Brindley GV Jr: Glomus tumor ofthe
mediastinum. J Thorac Surg. 18:417–420. 1949.PubMed/NCBI
|
|
5
|
Banner EA and Winkelmann RK: Glomus tumor
of the vagina; report of a case. Obstet Gynecol. 9:326–328.
1957.PubMed/NCBI
|
|
6
|
Graner BC and Burt JC: Unusual location of
glomus tumor: report of two cases. JAMA. 112:1806–1810. 1939.
View Article : Google Scholar
|
|
7
|
Tang CK, Toker C, Foris NP and Trump BF:
Glomangioma of the lung. Am J Surg Pathol. 2:103–109. 1978.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hussarek M and Rieder W: Glomus tumor of
the trachea. Krebsarzt. 5:208–212. 1950.(In Undetermined Language).
PubMed/NCBI
|
|
9
|
Fabich DR and Hafez GR: Glomangioma of the
trachea. Cancer. 45:2337–2341. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Heard B E, Dewar A, Firmin R K, et al: One
very rare and one new tracheal tumour found by electron microscopy:
glomus tumour and acinic cell tumour resembling carcinoid tumours
by light microscopy. Thorax. 37:97–103. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ito H, Motohiro K, Nomura S, et al: Glomus
tumor of the trachea: immunohistochemical and electron microscopic
studies. Pathology-Research and Practice. 183:778–782. 1988.
View Article : Google Scholar
|
|
12
|
Kim Y I, Kim J H, Suh J S, et al: Glomus
tumor of the trachea. Report of a case with ultrastructural
observation. Cancer. 64:881–886. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shin DH, Park SS, Lee JH, et al: Oncocytic
glomus tumor of the trachea. Chest. 98:1021–1023. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
García-Prats MD, Sotelo-Rodríguez MT,
Ballestín C, et al: Glomus tumour of the trachea: report of a case
with microscopic, ultrastructural and immunohistochemical
examination and review of the literature. Histopathology.
19:459–464. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Haraguchi S, Yamamoto M and Nishimura H: A
glomus tumor of the trachea - a case report. Nihon Kyōbu Geka
Gakkai. 39:214–218. 1991.
|
|
16
|
Arapantoni-Dadioti P, Panayiotides J,
Fatsis M and Antypas G: Tracheal glomus tumour. Respiration.
62:160–162. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Koskinen SK, Niemi PT, Ekfors TO, et al:
Glomus tumor of the trachea. Eur J Radiol. 8:364–366. 1998.
View Article : Google Scholar
|
|
18
|
Watanabe M, Takagi K, Ono K, et al:
Successful resection of a glomus tumor arising from the lower
trachea: report of a case. Surg Today. 28:332–334. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Menaissy YM, Gal AA and Mansour KA: Glomus
tumor of the trachea. Ann Thorac Surg. 70:295–297. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gowan RT, Shamji FM, Perkins DG and Mazaik
DE: Glomus tumor of the trachea. Ann Thorac Surg. 72:598–600. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chien ST, Lee TM, Hsu JY, et al: Glomus
tumor of the trachea. J Chin Med Assoc. 66:551–554. 2003.PubMed/NCBI
|
|
22
|
Nadrous HF, Allen MS, Bartholmai BJ, et
al: Glomus tumor of the trachea: value of multidetector computed
tomographic virtual bronchoscopy. Mayo Clin Proc. 79:237–240. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Haver KE, Hartnick CJ, Ryan DP, et al:
Case records of the Massachusetts General Hospital. Case 10-2008 A
10-year-old girl with dyspnea on exertion. N Engl J Med.
358:1382–1390. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Colaut F, Toniolo L, Scapinello A and
Pozzobon M: Tracheal glomus tumor successfully resected with rigid
bronchoscopy: a case report. J Thorac Oncol. 3:1065–1067. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Parker KL, Zervos MD, Donington JS, et al:
Tracheal glomangioma in a patient with asthma and chest pain. J
Clin Oncol. 28:e9–e10. 2010. View Article : Google Scholar
|
|
26
|
Shang Y, Huang Y, Huang HD, et al: Removal
of glomus tumor in the lower tracheal segment with a flexible
bronchoscope: report of two cases. Intern Med. 49:865–869. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sakr L, Palaniappan R, Payan MJ, et al:
Tracheal glomus tumor: a multidisciplinary approach to management.
Respir Care. 56:342–346. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mogi A, Kosaka T, Yamaki E, et al:
Successful resection of a glomus tumor of the trachea. Gen Thorac
Cardiovasc Surg. 59:815–818. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Okereke IC, Sheski FD and Cummings OW:
Glomus tumor of the trachea. J Thorac Oncol. 6:1290–1291. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Norder E, Kynyk J, Schmitt AC, et al:
Glomus tumor of the trachea. J Bronchology Interv Pulmonol.
19:220–223. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lang-Lazdunski L, Bille A, Cane P and
Congleton J: Glomus tumour: a rare differential diagnosis of
bronchial obstruction in a smoker. Gen Thorac Cardiovasc Surg.
60:774–776. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fan M, Liu C, Mei J, et al: A rare large
tracheal glomus tumor with postoperative haematemesis. J Thorac
Dis. 5:E185–E188. 2013.PubMed/NCBI
|
|
33
|
Kleihues P and Sobin LH: World Health
Organization classification of tumors. Cancer. 88:28872000.
View Article : Google Scholar : PubMed/NCBI
|