Introduction
Nasopharyngeal carcinoma (NPC) is a tumor arising
from the epithelial cells covering the nasopharynx surface. The
highest incidence of NPC worldwide has been reported in Southern
China, with an age-standardized incidence rate of 20–50 cases per
100,000 people (1). Viral infections,
genetic alterations and environmental factors are considered to
contribute towards the initiation and development of NPC (2–5).
Currently, radiation therapy is the primary treatment strategy for
NPC (6). However, despite
improvements in radiotherapy equipment and techniques, the
five-year survival rate of NPC patients remains at 50–60%.
Therefore, understanding the key genes involved in the development
of NPC is required to identify new therapeutic targets for the
treatment of this disease (7).
Krüppel-like factor 8 (KLF8) is a member of the KLF
family of proteins, which are essential in cell cycle progression,
epithelial-mesenchymal transition (EMT), invasion, oncogenic
transformation and cancer development (8–10). Several
studies have reported KLF8 overexpression in breast cancer,
hepatocellular carcinoma and renal cancer (11–13);
therefore, KLF8 may function as a predictor of metastasis and
overall survival in certain types of cancer. However, the
expression of KLF8 in NPC cell lines and its effects on NPC cells
have not yet been investigated.
The present study aimed to evaluate the role of KLF8
in the pathogenesis of NPC by assessing its expression in NPC cell
lines. Small hairpin RNA (shRNA) was used to knock down KLF8 in the
NPC cell line, SUNE1-5-8F, which was subsequently transplanted into
severe combined immunodeficiency (SCID) mice to investigate the
tumor formation and invasion.
Materials and methods
Cell culture and sample
collection
The NPC cell lines, CNE1, CNE2, CEN1-LMP1 and
SUNE1-5-8F, were obtained from the Cancer Research Institute of
Southern Medical University (Guangzhou, China). All the cell lines
were maintained in RPMI 1640 medium, supplemented with 10% newborn
calf serum (PAA Laboratories, Inc., Pasching, Austria) and
incubated in a humidified atmosphere of 5% CO2 at
37°C.
Extraction of RNA and quantitative
polymerase chain reaction (qPCR)
Total RNA was extracted using TRIzol reagent
(Invitrogen Life Technologies, Carlsbad, CA, USA), according to the
manufacturer's instructions. Subsequently, RNA (2 µg) was
reverse-transcribed into first-strand cDNA using M-MLV Reverse
Transcriptase (Promega Corporation, Madison, WI, USA) according to
the manufacturer's instructions. KLF8 and GAPDH were amplified by
qPCR using the following primers: KLF8 forward, 5′-GGG TGT TTG GCT
TCT TTGC-3′, and reverse, 5′-GGC TGT GGT CTC ATC TGC-3′; and GAPDH
forward, 5′-CTC CTC CTG TTC GAC AGT CAGC-3′, and reverse, 5′-CCC
AAT ACG ACC AAA TCC GTT-3′. Gene-specific amplification was
performed using an ABI 7900HT real-time PCR system (Applied
Biosystems Life Technologies, Foster City, CA, USA) with a 15 µl
PCR mix containing 0.5 µl cDNA, 7.5 µl 2X SYBR® Green master mix
(Invitrogen Life Technologies) and 200 nM of the appropriate
primers. Next, the mixture was preheated at 95°C for 10 min and
then amplified in 45 cycles of 95°C for 30 sec and 60°C for 1 min.
The resolution curve was measured at 95°C for 15 sec, 60°C for 15
sec and 95°C for 15 sec. Subsequently, the threshold cycle (Ct)
value of each sample was calculated, and the relative expression of
KLF8 mRNA was normalized against the GAPDH value using the
2−ΔCt method.
Western blot analysis
Homogenized tissues were lysed in
radioimmunoprecipitation assay lysis buffer, and the lysates were
harvested by centrifugation (8,000 × g) at 4°C for 30 min. Next, 8
µg protein samples were separated by electrophoresis on a 15%
sodium dodecyl sulfate-polyacrylamide gel and were transferred onto
a polyvinylidene fluoride membrane. Subsequently, nonspecific
binding sites were blocked by placing the membrane in 5% nonfat
milk for 1 h, followed by incubation with a polyclonal rabbit
anti-human KLF8 antibody (dilution, 1:1,000; cat. no. 252109;
Abbiotec, San Diego, CA, USA) at 4°C overnight. After washing four
times in Tris-buffered saline with Tween-20, the membrane was
probed with a horseradish peroxidase (HRP)-conjugated rabbit
anti-rat immunoglobulin G antibody (dilution, 1:5,000; cat. no.
KC-5G5, Proteintech Group, Inc., Chicago, IL, USA) at 37°C for 60
min. Next, the samples were washed four times and the bands were
detected using Pierce ECL Western Blotting Substrate (Thermo Fisher
Scientific, Waltham, MA, USA). Band density was measured using
ImageJ software [version 1.43b; National Institutes of Health
(NIH), Bethesda, MA, USA] and was standardized against mouse
anti-human GAPDH monoclonal antibody (Shanghai Kangcheng
Biotechnology Co., Ltd., Shanghai, China).
Lentiviral vector construction and
transfection
In order to knockdown the expression of human KLF8,
a KLF8-RNA interference lentiviral vector [pGCSIL-green fluorescent
protein (GFP)-KLF8 shRNA] was constructed by annealing the
KLF8-shRNA (5′-CCG GCT AGC ATG CTA CAA GCT CCA ATT CAA GAG ATT GGA
GCT TGT AGC ATG CTA GTT TTT G-3′) and inserting it into the shRNA
expression vector, pGCSIL-GFP. A scrambled shRNA was used as a
negative control (12). The
recombinant virus was packed using the Lentivector Expression
system (Shanghai GeneChem Co., Ltd., Shanghai, China) and termed as
shRNA-KLF8 lentivirus or negative shRNA lentivirus. Subsequently,
SUNE1-5-8F cells were transfected with the recombinant lentivirus
using Lipofectamine® 2000 (Invitrogen Life Technologies). The
transfected cells were screened under 800 µg/ml G418 (Calbiochen,
Darmstadt, Germany) for four weeks to generate two stable
monoclonal cell lines (including a KLF8 stable downregulated cell
line, SUNE1-5-8F/Sh-KLF8, and a control stable cell line,
SUNE1-5-8F/Mock). Western blot analysis was used to determine the
expression levels of KLF8 in these cell lines.
Cell invasion assays
A cell invasion assay was performed using a
precoated Cell Invasion Assay kit (Chemicon International, Inc.,
Temecula, CA, USA), according to the manufacturer's instructions.
Cells transported from the extracellular matrix layer to the lower
surface of the membrane were fixed with methanol and stained with
crystal violet (Guangzhou Chemical Reagent Factory, Guangzhou,
China). Images of three randomly selected fields of the fixed cells
were captured and the cells were counted using an inverted
microscope (XDS-1; Olympus Corporation, Tokyo, Japan). The
experiments were conducted in triplicate.
In vivo assay for the determination of
tumor growth
All the animal experiments were approved by the
Ethics Committee on Animal Experimentation of the Huazhong
University of Science and Technology (Wuhan, China), and the
procedures complied with the NIH Guide for the Care and Use of
Laboratory Animals (8th edition, 2011). BALB/c nude mice (Nu/Nu;
female; age, 4–6 weeks) were purchased from the Center of
Experimental Animal of Huazhong University of Science and
Technology and maintained under pathogen-free conditions (n=11 per
group). SUNE1-5-8F/Mock and SUNE1-5-8F/Sh-KLF8 cells were injected
into the left and right flanks, respectively, of the mice. Tumor
growth was monitored by measuring tumor volume, which was
calculated using the following formula: Tumor volume
(mm3) = width2 × length (mm) / 2. At the end
of the experiment, the tumor was harvested. Differences in tumor
growth were analyzed for statistical significance.
Statistical analysis
All the experimental data are presented as the mean
± standard deviation. The mean values of different groups were
compared using one-way analysis of variance or Student's t-test. A
two-sided P<0.05 was considered to indicate a statistically
significant difference. All statistical analyses were performed
with the SPSS software (version 16.0; SPSS, Inc., Chicago, IL,
USA).
Results
mRNA and protein KLF8 expression
levels in four NPC cell lines
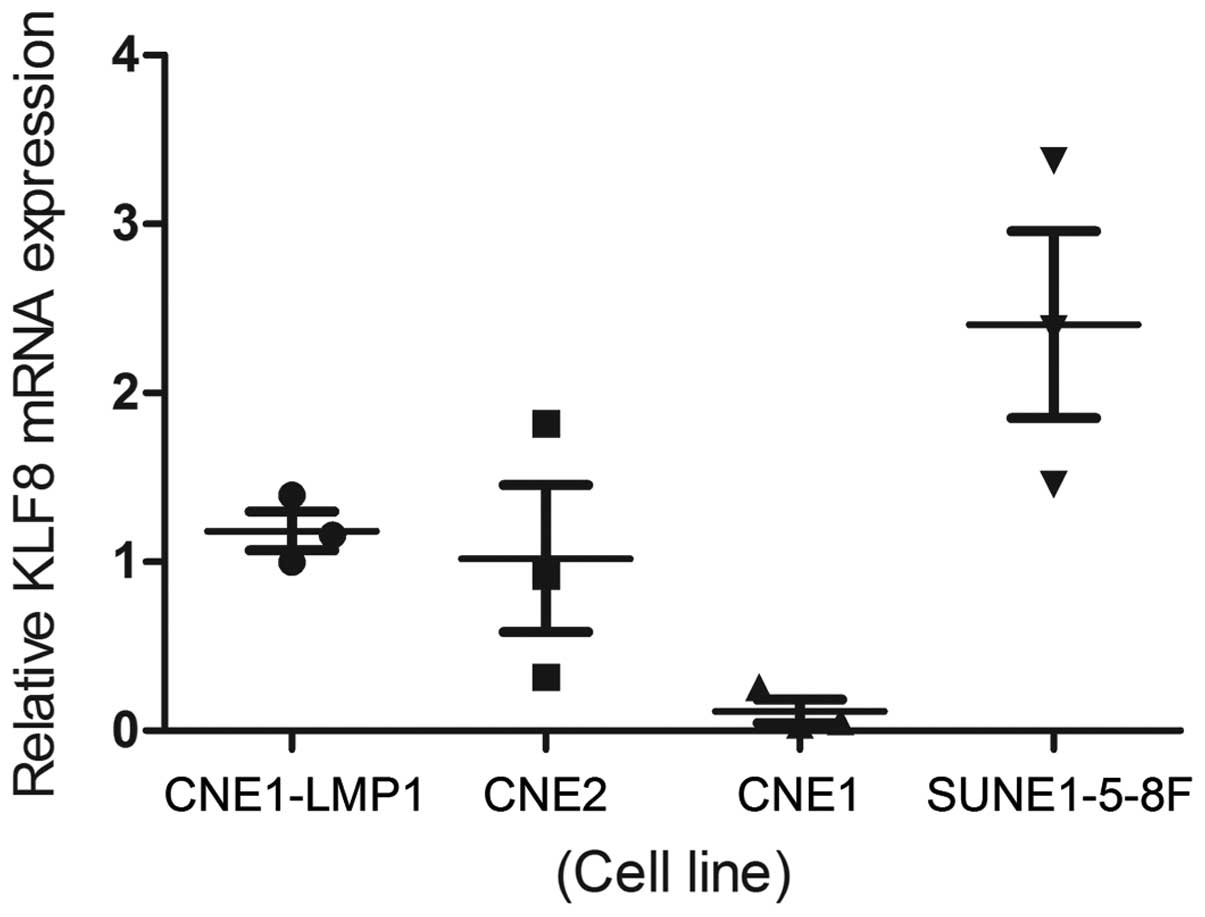
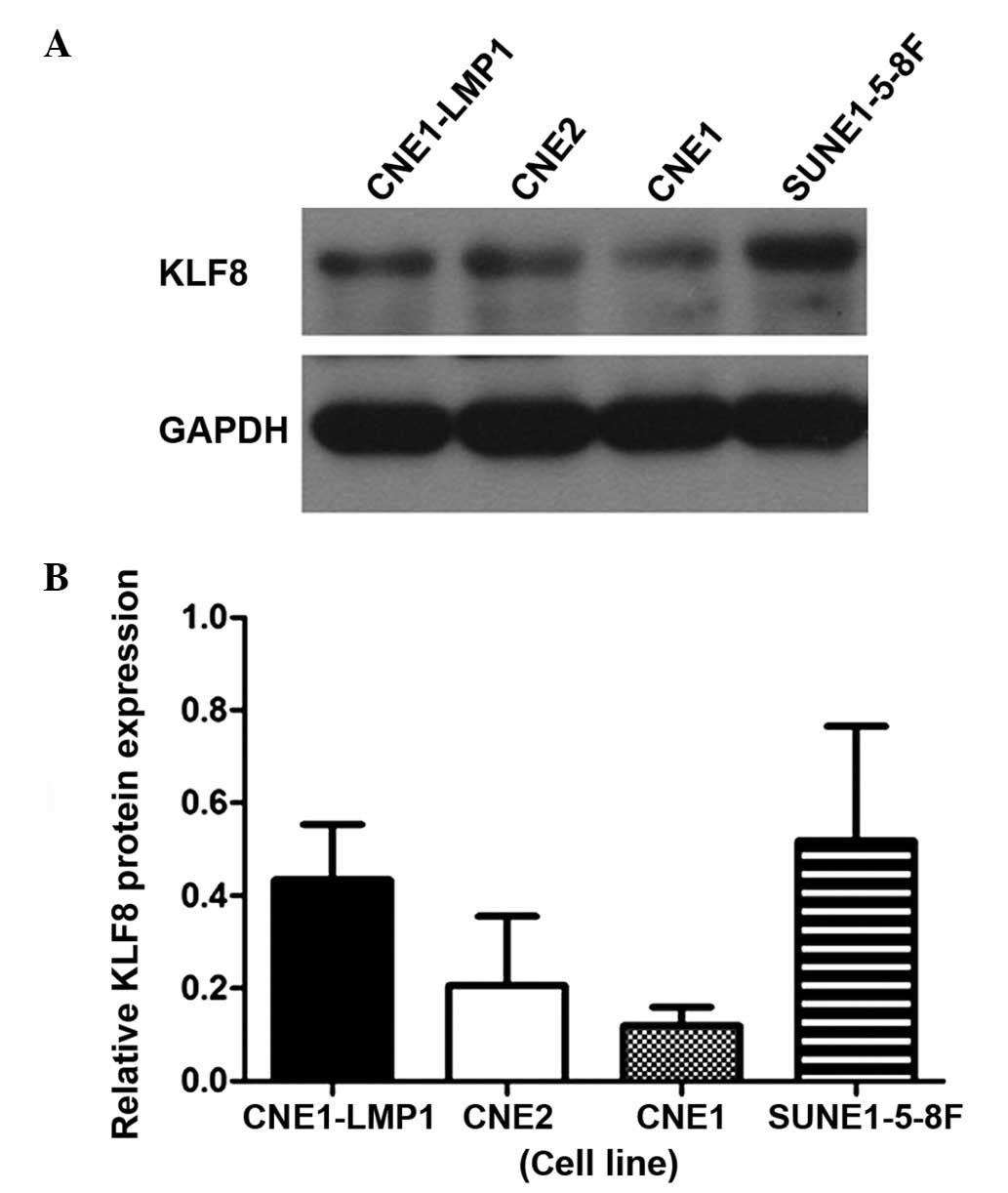
The relative transcriptional level of KLF8 was
determined by qPCR and western blot analysis in the four NPC cell
lines. KLF8 expression was found to be lowest in the CNE-1 cell
line and highest in the SUNE1-5-8F cell line. The mRNA expression
levels of KLF8 were consistent with the protein expression levels
in all the cell lines (Figs. 1,
2A and 2B).
Expression of KLF8 in the SUNE1-5-8F
cell line is significantly downregulated by the pGCSIL-GFP-KLF8
lentiviral vector
pGCSIL-GFP-KLF8 shRNA was transfected into the
SUNE1-5-8F cells in order to knockdown the expression of KLF8 in
the cell line. All the untransfected NPC cells were nonviable
following G418 (800 µg/ml) selection for one week. Cells
transfected with pGCSIL-GFP-KLF8 were continuously selected using
G418 for four weeks until clones were observed using fluorescence
microscopy.
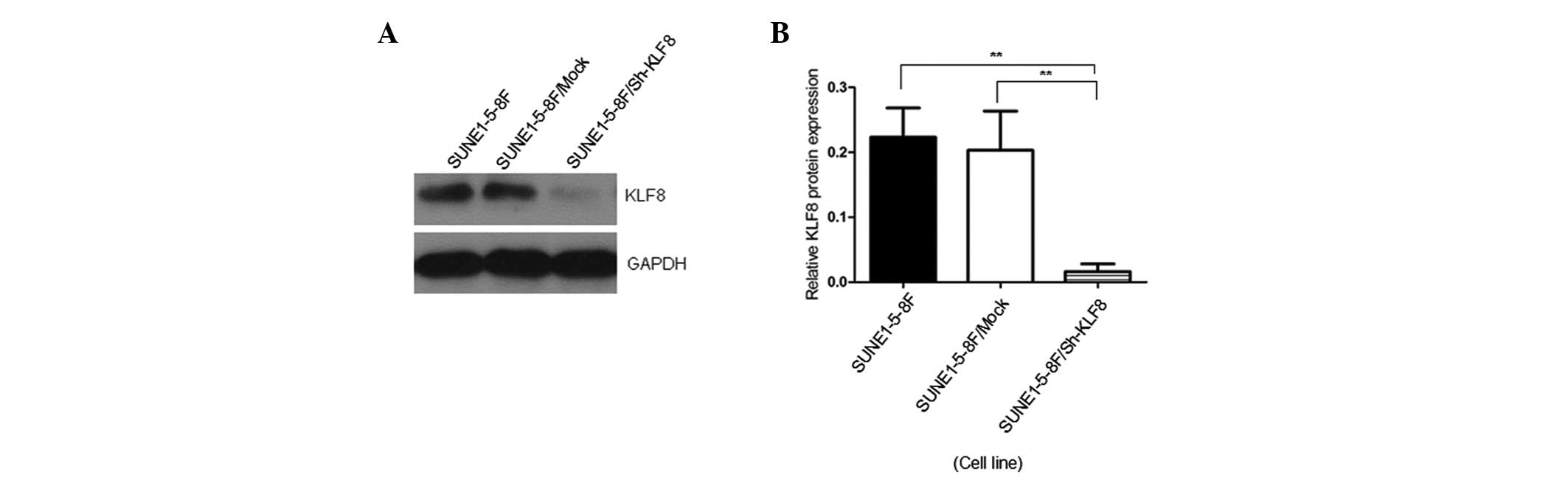
The knockdown efficiency in SUNE1-5-8F cells was
examined by western blot analysis of KLF8 protein expression. KLF8
expression was significantly reduced in cells transfected with
pGCSIL-GFP-KLF8 compared with the SUNE1-5-8F and SUNE1-5-8F/Mock
cells (P<0.05; Fig. 3).
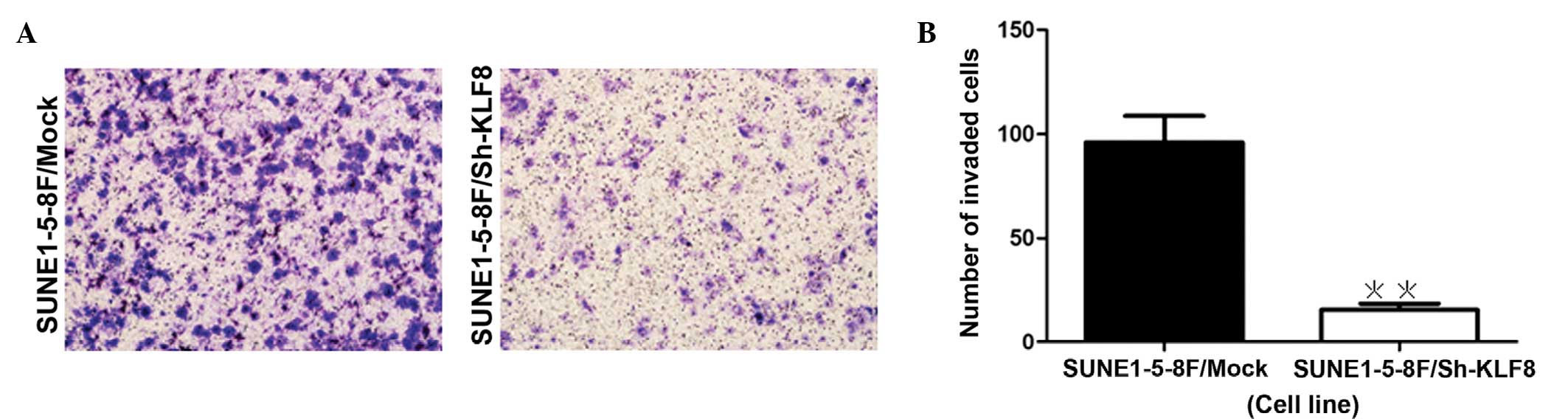
KLF8 knockdown reduces cell invasion
ability
The transwell invasion assay revealed that knockdown
of KLF8 resulted in a reduction of the invasiveness of NPC cells,
as indicated by a significant decrease in the number of invaded
cells (P=0.0005; Fig. 4).
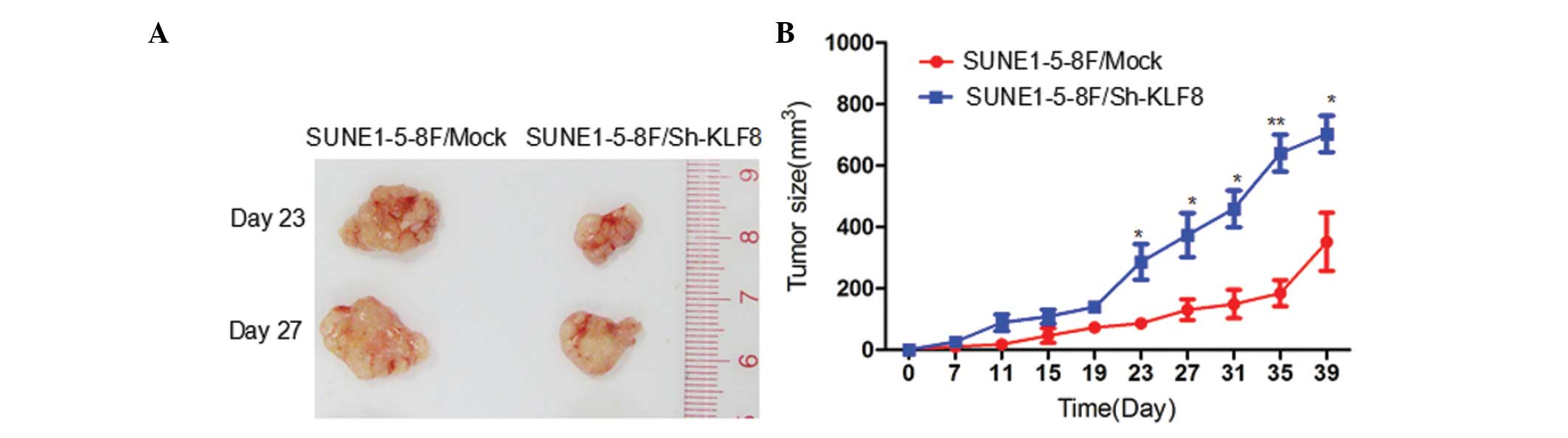
Downregulation of KLF8 expression
suppresses tumor growth in vivo
The effect of reduced KLF8 expression on tumor
growth was investigated in NPC xenografts. Two experimental groups
were examined: i) SUNE1-5-8F/Mock cells; and ii) SUNE1-5-8F/Sh-KLF8
cells. Tumor growth curves were plotted to compare the difference
in tumor formation throughout the experiment. Palpable tumors were
initially detected in all the mice at day 11 following injection.
The tumor volume differed significantly at day 23 (Fig. 5A). The mean tumor volume produced by
injection with SUNE1-5-8F/Sh-KLF8 was >50% lower (P<0.05;
Fig. 5B) compared with the control
group. The mean tumor weight was also significantly reduced in
SUNE1-5-8F/Sh-KLF8 tumors (P<0.05; Fig. 5B).
Discussion
NPC is a serious healthcare problem (14), more frequently occurring in Southern
China, which presents early metastasis features. At present,
radiation therapy is the main NPC treatment. The five-year survival
rate remains at 50–60%, in spite of advances in radiotherapy
devices and techniques. Therefore, comprehensive research into new
therapies for the treatment of NPC are required. A greater
understanding of the key molecules involved in tumor development
may provide new therapeutic targets to develop more effective
treatments for the NPC.
KLF8 is a GT-box (CACCC) binding dual-transcription
factor, which is widely expressed in adult tissues, particularly in
the kidney, heart and placenta. KLF8 plays an important role in the
regulation of cell cycle progression (8), transformation (15), EMT and invasion (10) in various types of cancer. However, the
expression of KLF8 and its role in NPC cells have not been
previously investigated.
To the best of our knowledge, the present study is
the first to characterize the expression of KLF8 in NPC cell lines
and determine the tumor formation and invasion ability in these
cells following KLF8 gene knockdown by shRNA. The mRNA and protein
expression levels of KLF8 in four NPC cell lines were assessed by
qPCR and western blot analysis, revealing that KLF8 expression was
lowest in the CNE-1 cells and highest in the SUNE1-5-8F cells. The
mRNA expression of KLF8 was consistent with its protein expression
in the four cell lines. Notably, the expression of KLF8 was
consistent with the malignancy degree of the NPC cell lines, which
indicated that KLF8 played an important role in the transformation
from normal phenotype to malignant phenotype. A study by Fu et
al (12) demonstrated that the
expression of KLF8 protein and mRNA is higher in tumorous tissue
compared with normal tissue. Another study (13) revealed that the expression of KLF8 was
higher in hepatocellular carcinoma with high metastatic ability.
The aforementioned observations indicate that KLF8 promotes tumor
development and is important in the processes of invasion and
metastasis.
In order to assess the effect of KLF8 on tumor
growth and invasion in NPC cells, in the current study, a
lentiviral vector was constructed and transfected into the
SUNE1-5-8F cell line. The findings revealed that the protein
expression levels of KLF8 in pGCSIL-GFP-KLF8-transfected SUNE1-5-8F
cells were significantly lower compared with SUNE1-5-8F/Mock and
SUNE1-5-8F/Sh-KLF8 cells.
The effect of KLF8 downregulation on tumor growth
was also evaluated using an NPC xenograft, which revealed that the
average tumor volume of the SUNE1-5-8F/Sh-KLF group was
significantly lower compared with the SUNE1-5-8F/Mock group.
In conclusion, the results demonstrated that, of
four NPC cell lines, KLF8 expression was lowest in the CNE-1 cell
line and highest in the SUNE1-5-8F cell line. The mRNA expression
levels of KLF8 were consistent with the protein expression levels
in the four cell lines. Compared with the control group,
downregulation of KLF8 expression is able to reduce the tumor
growth in nasopharyngeal carcinoma xenografts. The present study
indicated that KLF8 is important in NPC development. Further
elucidation of the underlying molecular mechanisms of KLF8 in NPC
may allow for the development of a novel molecular targeted therapy
for the treatment of this disease.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant no. 81160327, awarded to Ruo
Zheng Wang), the Union Hospital Key Laboratory Foundation of
Biological Target Therapy (grant no. 02.03.2013-80, awarded to Pin
Dong Li) and the Independent Innovation Research Foundation of
Huazhong University of Science and Technology (grant no.
01-08-530059, awarded to Pin Dong Li).
References
|
1
|
Yao KT: The application and prospect of
nasopharyngeal carcinoma etiology. China Cancer. 6:3–4. 1997.[(In
Chinese)].
|
|
2
|
Fang W, Li X, Jiang Q, et al:
Transcriptional patterns, biomarkers and pathways characterizing
nasopharyngeal carcinoma of Southern China. J Transl Med. 6:322008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Alajez NM, Shi W, Hui AB, et al: Enhancer
of Zeste homolog 2 (EZH2) is overexpressed in recurrent
nasopharyngeal carcinoma and is regulated by miR-26a, miR-101, and
miR-98. Cell Death Dis. 1:e852010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu Z, Luo W, Zhou Y, et al: Potential
tumor suppressor NESG1 as an unfavorable prognosis factor in
nasopharyngeal carcinoma. PLoS One. 6:e278872011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xiong S, Wang Q, Zheng L, Gao F and Li J:
Identification of candidate molecular markers of nasopharyngeal
carcinoma by tissue microarray and in situ hybridization. Med
Oncol. 28:S341–S348. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee AW, Ng WT, Chan YH, et al: The battle
against nasopharyngeal cancer. Radiother Oncol. 104:272–278. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhen Y, Ye Y, Yu X, et al: Reduced CTGF
expression promotes cell growth, migration, and invasion in
nasopharyngeal carcinoma. PLoS One. 8:e649762013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Urvalek AM, Wang X, Lu H and Zhao J: KLF8
recruits the p300 and PCAF co-activators to its amino terminal
activation domain to activate transcription. Cell Cycle. 9:601–611.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang X, Zheng M, Liu G, et al:
Krüppel-like factor 8 induces epithelial to mesenchymal transition
and epithelial cell invasion. Cancer Res. 67:7184–7193. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mehta TS, Lu H, Wang X, et al: A unique
sequence in the N-terminal regulatory region controls the nuclear
localization of KLF8 by cooperating with the C-terminal
zinc-fingers. Cell Res. 19:1098–1109. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang X, Lu H, Urvalek AM, et al: KLF8
promotes human breast cancer cell invasion and metastasis by
transcriptional activation of MMP9. Oncogene. 30:1901–1911. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fu WJ, Li JC, Wu XY, et al: Small
interference RNA targeting Krüppel-like factor 8 inhibits the renal
carcinoma 786-0 cells growth in vitro and in vivo. J Cancer Res
Clin Oncol. 136:1255–1265. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li JC, Yang XR, Sun HX, et al:
Up-regulation of Krüppel-like factor 8 promotes tumor invasion and
indicates poor prognosis for hepatocellular carcinoma.
Gastroenterology. 139:2146–2157. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen MK, Chen TH, Liu JP, et al: Better
prediction of prognosis for patients with nasopharyngeal carcinoma
using primary tumor volume. Cancer. 100:2160–2166. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao J, Bian ZC, Yee K, et al:
Identification of transcription factor KLF8 as a downstream target
of focal adhesion kinase in its regulation of cyclin D1 and cell
cycle progression. Mol Cell. 11:1503–1515. 2003. View Article : Google Scholar : PubMed/NCBI
|