Introduction
Identified in the early 1980s, the transforming
growth factor-β (TGF-β) ligands, TGF-β1, TGF-β2 and TGF-β3,
regulate diverse biological functions (1,2). These
three ligands may bind to a specific receptor through their
interaction with TGF-β receptor I (TGF-βRI), which subsequently
heterodimerizes with the TGF-βRII. This heterodimer complex
phosphorylates the intracellular proteins SMAD2 and SMAD3, which
initiate an activation cascade to induce a number of nuclear
transduction proteins. Through the induction of such proteins, the
TGF-β signaling pathway affects cellular proliferation,
differentiation, motility, survival and apoptosis in tumor cells
and the surrounding microenvironment. In glioblastoma, TGF-β
signaling is important in tumor progression (3,4).
LY2157299 monohydrate (LY2157299) is a TGF-βRI
kinase inhibitor that interrupts the receptor-mediated signaling
cascade (5–7). However, small-molecule TGF-β inhibitors
have been associated with unique cardiovascular toxicities in
animals (8). Therefore, it is
critical to develop a therapeutic window in which LY2157299 may be
safely administered during a first-in-human dose (FHD) study. Based
on preclinical information, a pharmacokinetic and pharmacodynamic
model was developed to prospectively describe a therapeutic window
to facilitate the safe clinical evaluation of LY2157299 (9). In short- and long-term toxicological
studies in rats and dogs, LY2157299 was considered to potentially
have the required therapeutic window in which a dose escalation to
a biologically active dose range may safely be implemented
(10).
Angiogenesis is critical in the development of
glioma; anti-vascular endothelial growth factor agents, including
bevacizumab, have shown activity in recurrent glioblastoma
(11). TGF-β-mediated angiogenesis
has also been described in mice, and postulated for humans. For
example, mutations of components of the TGF-β signaling pathway,
such as Endoglin and SMAD4, have been associated with certain forms
of hereditary hemorrhagic telangiectasia (12). The importance of the TGF-β signaling
pathway is further highlighted by the fact that the deletion of
certain components, including TGF-β1, TGF-βRII, activin
receptor-like kinase 1, Endoglin, SMAD1, SMAD4 and SMAD5, is not
compatible with gestational survival. All these components are
associated with embryonic lethality resulting from severe vascular
abnormalities (13–16). In vitro studies indicate that
the angiogenic effect of TGF-β signaling is differentially
modulated by endothelial migration and proliferation, and depends
upon the different stages of angiogenic development. Overall, this
appears to be dose- and cell-dependent (17).
Magnetic resonance imaging (MRI) of brain tumors may
be utilized to assess anatomical changes in response to treatment,
and is also able to detect changes in vascular permeability, blood
volume, blood flow and mean transit time of blood flow (17). Measures of relative cerebral blood
volume (rCBV) and permeability have been used as antitumor response
markers following the initiation of anti-angiogenic treatment
(18,19). For example, patients who were treated
with cediranib and who exhibited increased perfusion also
experienced prolonged survival (20).
rCBV has also been demonstrated to be sensitive to tumor grade, to
be predictive of survival and to be able to distinguish contrast
enhancement following post-radiation changes (21–26). These
observations indicate that MRI techniques facilitate the assessment
of the anti-vascular activity of novel antitumor agents. In the
present study, the perfusion and permeability of glioblastoma was
assessed in patients who received combination lomustine and
LY2157299 therapy, in order to determine whether this treatment
exerts anti-angiogenic effects.
Patients and methods
Patients
Patients with relapsed and progressive glioblastoma
were eligible to participate in the FHD study, as described
previously (27). All patients were
required to have an Eastern Cooperative Oncology Group performance
status score of ≤2, adequate hematological, hepatic and renal
function, and must have discontinued all previous cancer therapies
>4 weeks prior to study enrollment. The exclusion criteria
included medically uncontrolled cardiovascular illness and
electrocardiogram anomalies.
All patients provided written informed consent to
participate in the study, and the protocol was approved by the
ethics committees of University Hospital 12 October (Madrid, Spain)
and Vall d'Hebron University Hospital (Barcelona, Spain). The study
was conducted in accordance with good clinical practice and the
ethics principles of the Declaration of Helsinki.
Study design
The MRI-based assessment was conducted as part of a
main protocol evaluating the safety and pharmacokinetics of
LY2157299 (Eli Lilly and Company, Indianapolis, IN, USA), either
alone or in combination with lomustine (Medac GmbH, Wedel, Germany)
(27,28). LY2157299 was evaluated in a
multicenter, open-label, non-randomized, dose-escalation FHD phase
I study. Patients participating in this substudy received two doses
of LY2157299 (160 and 300 mg/day), in combination with lomustine at
standard doses: 100 mg/m2 every 6 weeks in the first
cycle, and 130 mg/m2 in the second and subsequent
cycles, according to the patient's tolerance.
Safety analysis
Safety assessment was based on the summaries of
adverse events, including severity [as defined by the Common
Terminology Criteria for Adverse Events (version 3.0)] (29), and possible associations with the
study drug, dose-limiting toxicities and laboratory changes at each
dose level. Logistic regression analysis for the probability of
experiencing a dose-limiting toxicity was not performed, as there
was only one occurrence of a dose-limiting toxicity. Safety was
also analyzed by evaluation of Eastern Cooperative Oncology Group
performance status (30),
electrocardiogram and echocardiography/Doppler scanning. Standard
laboratory tests, including chemistry, hematology and urinalysis
panels, were also conducted. All concomitant medications were
documented throughout the patient's participation in the study.
Treatment
LY2157299 was administered orally in tablet form at
80- and 150-mg doses twice daily (in the morning and evening;
total, 160 and 300 mg/day) for 14 days, followed by a 14-day
drug-free period, for a 28-day cycle. Lomustine was administered at
a starting dose of 100 mg/m2 in the first cycle and 130
mg/m2 in the second and subsequent cycles; the time
between lomustine doses was 6 weeks. Based on toxicities, doses
were lowered when indicated.
Radiological assessment
All patients underwent a baseline MRI exam <2
weeks prior to the commencement of treatment, and a second MRI exam
prior to the third cycle of treatment. Radiographic changes were
evaluated according to Macdonald criteria (31).
Conventional MRI
Imaging was performed with a 1.5T scanner (Signa
Excite; GE Healthcare, Milwaukee, WI, USA). Anatomic sequences were
conducted as follows: A 3-plane localizer sequence, sagittal
T1-weighted with an inversion recovery (IR) pulse [repetition time
(TR), 2,400 msec; echo time (TE), 24 msec; IR, 750 msec], axial
T2-weighted fast spin-echo (TR, 4,100 msec, TE, 85 msec), and
fluid-attenuated IR [FLAIR; TR, 8,000 msec; TE, 120 msec; inversion
time (TI), 2,000 msec]. Post-contrast axial and coronal T1-weighted
images with an IR pulse (TR, 2,400 msec; TE, 24 msec; IR, 750 msec)
were obtained following the acquisition of the perfusion MRI data.
All data were obtained using 4-mm thick sections with a 1-mm skip,
a 320×256 matrix and a field of view (FOV) of 24×24 cm.
Dynamic contrast-enhanced perfusion
MRI
Dynamic contrast-enhanced T2-weighted gradient-echo
echo-planar images were acquired during the first pass of a bolus
of gadobutrol (Gadavist™, 1 mmol/ml; Berlex Laboratories, Wayne,
NJ, USA) at a dose of 0.1 mmol/kg. For perfusion MRI, 19 sections
were selected to cover the tumor on the basis of T2-weighted and
FLAIR images. Imaging parameters were as follows: TR/TE, 2,000/21.1
msec; FOV, 26×26 cm; section thickness, 4 mm with a 0.4-mm skip, a
matrix of 128×128 and a flip angle of 90°. A series of 40
multisection acquisitions was acquired at 0.2-sec intervals. The
first 8 acquisitions were performed prior to the injection of the
contrast agent in order to establish a precontrast baseline. For
the ninth acquisition, gadobutrol was injected at a rate of 5
ml/sec using a power injector (Medrad® Spectris Solaris® EP MR
Injection System; Medrad Inc., Bayer Healthcare, Indianola, PA,
USA) followed by the administration of a 20-ml bolus of saline at 5
ml/sec. Immediately prior to dynamic imaging, a prebolus dose (2
ml) of gadobutrol was administered to diminish any T1 effects that
may have resulted from agent extravasation.
Perfusion MRI data evaluation
Dynamic susceptibility contrast images were
processed on an Advantage Workstation using FuncTool (GE
Healthcare). The beginning and the end of the first-pass bolus were
determined through inspection of the time-signal-intensity curve
and care was taken to exclude any recirculation-related signal
intensity. Cerebral blood volume (CBV) refers to the amount of
blood in a given region of brain tissue at any time, commonly
measured in milliliters per 100 g of brain tissue. As the CBV must
be expressed relative to an internal reference, it was normalized
in the present study by expressing ratios relative to values
measured in the normal white matter of the contralateral lobe;
these values were referred to as rCBV. Color-coded rCBV maps were
generated to target regions of maximum abnormality. Two
neuroradiologists determined 3 regions of interest (ROIs) within
the tumor, on areas exhibiting the highest intratumoral rCBV on the
color-coded maps. The standardized ROI was 2–3 mm2 and
was used for the majority of the tumor and white matter
measurements. Care was taken to avoid the inclusion of large intra-
or peritumoral vessels, as these may confound perfusion
measurements. The maximum rCBV value in the intratumoral ROIs was
selected for quantitative analysis, and correlated with the
corresponding specimen histopathology. This method has been
demonstrated to provide the most optimal interobserver and
intraobserver reproducibility (13).
Statistics
A two-tailed Wilcoxon test for paired samples was
used to establish the significance of any differences in the same
variable, in perfusion and rCBV observed between different time
points, and between baseline MRI and MRI performed after the
completion of two cycles of chemotherapy. Summary statistics were
reported as the median and standard deviations. A two-sided P-value
of <0.05 was considered to indicate a statistically significant
difference. SPSS software version 12 (SPSS, Inc., Chicago, IL, USA)
was used for the statistical analyses.
Results
Patient demographics and disease
characteristics
In this single-institution imaging study, patients
were enrolled between September 2010 and June 2011. All patients
participated in the FHD study of LY2157299 combined with lomustine,
and discontinued all previous therapies for glioblastoma, such as
radiochemotherapy with temozolamide or other investigational
therapy regarded to be effective in glioblastoma, prior to the
commencement of the study. Of the 12 patients enrolled, 8 had been
treated with bevacizumab-based therapies (Table I). Two patients (S11 and S12) did not
receive additional chemotherapies following their first relapse,
but instead underwent re-resection. Two patients were considered to
have secondary glioblastoma based on their previous low-grade
glioma diagnosis (S8 and S10).
 | Table I.Patient demographics and disease
characteristics of all enrolled and treated patients by dose. |
Table I.
Patient demographics and disease
characteristics of all enrolled and treated patients by dose.
| Patient ID | Gender | Age, years | Original/subsequent
diagnosis | Time from initial
diagnosis to study entry | Primary/secondary
GBM | Previous
treatments |
|---|
| S1 | M | 45 | GBM | 3 years, 5
months | Primary | Chemoradiotherapy
with TMZ, adjuvant TMZ (6 cycles), surgery (complete resection) and
bevacizumab + irinotecan (12 cycles) |
| S2 | M | 57 | GBM | 1 year, 4 months | Primary | Chemoradiotherapy
with TMZ, adjuvant TMZ (5 cycles), surgery (complete resection) and
bevacizumab + irinotecan (12 cycles) |
| S3 | F | 57 | GBM | 1 year, 9 months | Primary | Chemoradiotherapy
with TMZ (no adjuvant TMZ due to thrombocytopenia) and bevacizumab
+ irinotecan (24 cycles) |
| S4 | M | 42 | GBM | 1 year, 4 months | Primary | Chemoradiotherapy
with TMZ, adjuvant TMZ (2 cycles) and bevacizumab + irinotecan (20
cycles) |
| S5 | M | 44 | GBM | 2 years, 2
months | Primary | Chemoradiotherapy
with TMZ, adjuvant TMZ (6 cycles), second chemoradiotherapy and
bevacizumab + irinotecan (2 cycles) |
| S6 | M | 31 | GBM | 1 year | Primary | Chemoradiotherapy
with TMZ and bevacizumab + irinotecan (14 cycles) |
| S7 | M | 51 | GBM | 1 year, 2 months | Primary | Chemoradiotherapy
with TMZ, adjuvant TMZ (2 cycles) and bevacizumab + irinotecan (12
cycles) |
| S8 | F | 35 |
Oligodendroglioma/anaplastic
oligodendroglioma | 6 years, 4
months | Not applicable | Radiotherapy,
adjuvant TMZ (18 cycles), second surgery at progression with
partial resection and adjuvant TMZ (8 cycles) |
| S9 | M | 55 | GBM | 7 months | Primary | Chemoradiotherapy
with TMZ, adjuvant TMZ (6 cycles) and dose intense TMZ +
bevacizumab (5 months) |
| S10 | M | 30 | Anaplastic
astrocytoma/GBM | 5 years, 6
months | Not applicable | Chemoradiotherapy
with TMZ, adjuvant TMZ (1 cycle), dose intense TMZ (4 years) and
surgery (at relapse) |
| S11 | M | 59 | GBM | 11 months | Primary | Chemoradiotherapy
with TMZ, adjuvant TMZ (4 cycles) and second surgery (at
relapse) |
| S12 | M | 58 | GBM | 1 year, 5
months | Primary | Chemoradiotherapy
with TMZ, adjuvant TMZ (11 cycles) and surgery (partial
resection) |
Analysis of changes in rCBV and
perfusion
The details of the effects of the treatment on rCBV
and perfusion are listed in Table
II. rCBV increased significantly during treatment (Table III): The baseline mean and median
values were 4.02 and 2.99, respectively. Following the second cycle
of LY2157299 + lomustine, the mean and median values were 6.63 and
8.20, respectively (Wilcoxon test, P=0.015). Changes in the mean
and median rCBV reflect the tumor progression in the majority of
cases without appreciable normalization of the tumor vessels, as
observed with anti-angiogenic agents.
 | Table II.Overview of the rCBV and perfusion
changes in glioblastoma patients. |
Table II.
Overview of the rCBV and perfusion
changes in glioblastoma patients.
|
| Baseline | After 2 cycles | After 4 cycles | After 6 cycles | After 8 cycles | After 10
cycles |
|
|---|
|
|
|
|
|
|
|
|
|
|---|
| Patient ID | rCBV | Perf | rCBV | Perf | rCBV | Perf | rCBV | Perf | rCBV | Perf | rCBV | Perf | Best response |
|---|
| S1 | 7.60 | 0.80 | 9.45 | 4.72 | – | – | – | – | – | – | – | – | PD |
| S2 | 8.37 | 2.80 | 9.79 | 1.80 | – | – | – | – | – | – | – | – | PD |
| S3 | 2.70 | 1.30 | n.a. | n.a. | – | – | – | – | – | – | – | – | PD |
| S4 | 2.36 | 4.00 | n.a. | n.a. | – | – | – | – | – | – | – | – | PD |
| S5 | 1.96 | 1.50 | n.a. | n.a. | – | – | – | – | – | – | – | – | PD |
| S6 | 4.70 | 1.00 | 9.76 | −0.40 | – | – | – | – | – | – | – | – | PD |
| S7 | 1.12 | 0.40 | 2.74 | 3.10 | – | – | – | – | – | – | – | – | PD |
| S8 | 3.28 | 5.00 | 2.22 | 4.70 | 2.02 | 7.20 | 1.55 | 4.70 | 1.17 | 2.80 | 5.10 | 4.00 | PR |
| S9 | 8.00 | 3.60 | 12.00 | 4.10 | – | – | – | – | – | – | – | – | PD |
| S10 | 1.20 | n.a. | 1.20 | 0.50 | 1.20 | 1.40 | 1.20 | 1.40 | 1.10 | 1.30 | 1.10 | 2.00 | PR |
| S11 | 2.50 | n.a. | 4.30 | n.a. | 4.30 | n.a. | – | – | – | – | – | – | PD |
| S12 | 4.70 | 10.00 | 8.20 | 6.20 | 8.20 | 7.40 | – | – | – | – | – | – | PD |
 | Table III.Description of rCBV prior to
treatment and following two cycles of LY2157299. |
Table III.
Description of rCBV prior to
treatment and following two cycles of LY2157299.
|
|
| rCBV |
|---|
|
|
|
|
|---|
|
|
|
|
|
|
| Percentiles |
|---|
|
|
|
|
|
|
|
|
|---|
| Time-point | n | Meana | SD | Min | Max | 25 | 50 | 75 |
|---|
| Baseline | 12 | 4.02 | 2.66 | 1.12 | 8.37 | 2.06 | 2.99 | 6.8750 |
| After 2 cycles | 9 | 6.63 | 4.01 | 1.20 | 12.00 | 2.48 | 8.20 | 9.7750 |
The mean and median perfusion values showed a
marginal, non-significant increase during treatment (Table IV): The baseline values were 3.04 and
2.15, respectively, and following two cycles of LY2157299 +
lomustine, the mean and median perfusion values were 3.07 and 3.60,
respectively (Wilcoxon test, P=0.93). Although the majority of the
patients exhibited an increase in rCBV and permeability, 1 patient
displayed reduced permeability and rCBV following the tumor
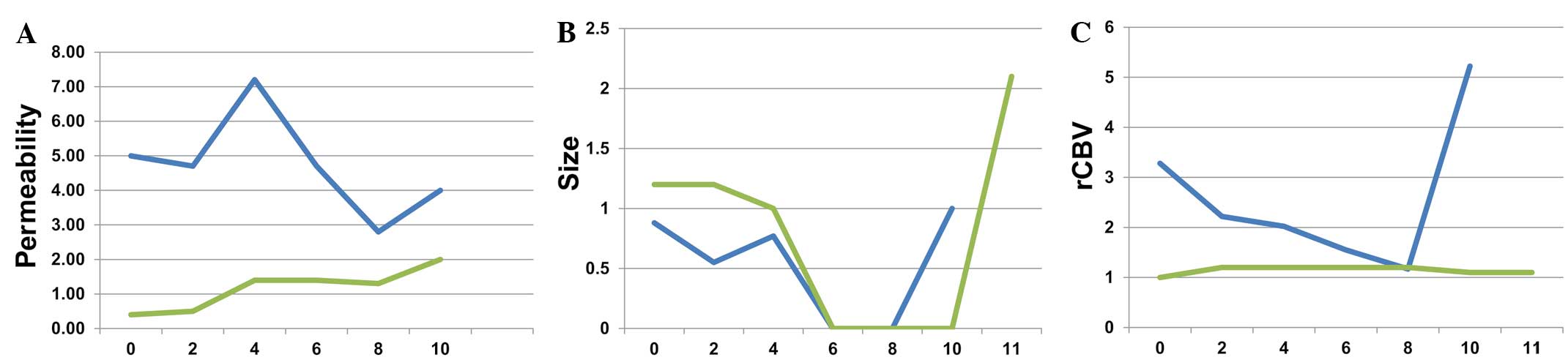
response (Fig. 1A and B).
 | Table IV.Description of perfusion prior to
treatment and following two cycles of LY2157299 and lomustine. |
Table IV.
Description of perfusion prior to
treatment and following two cycles of LY2157299 and lomustine.
|
|
| Perfusion |
|---|
|
|
|
|
|---|
|
|
|
|
|
|
| Percentiles |
|---|
|
|
|
|
|
|
|
|
|---|
| Time-point | n | Meana | SD | Min | Max | 25 | 50 | 75 |
|---|
| Baseline | 10 | 3.04 | 2.88 | 0.40 | 10.00 | 0.95 | 2.15 | 4.25 |
| After 2 cycles | 8 | 3.07 | 2.27 | 0.40 | 6.20 | 0.82 | 3.60 | 4.67 |
Efficacy measures
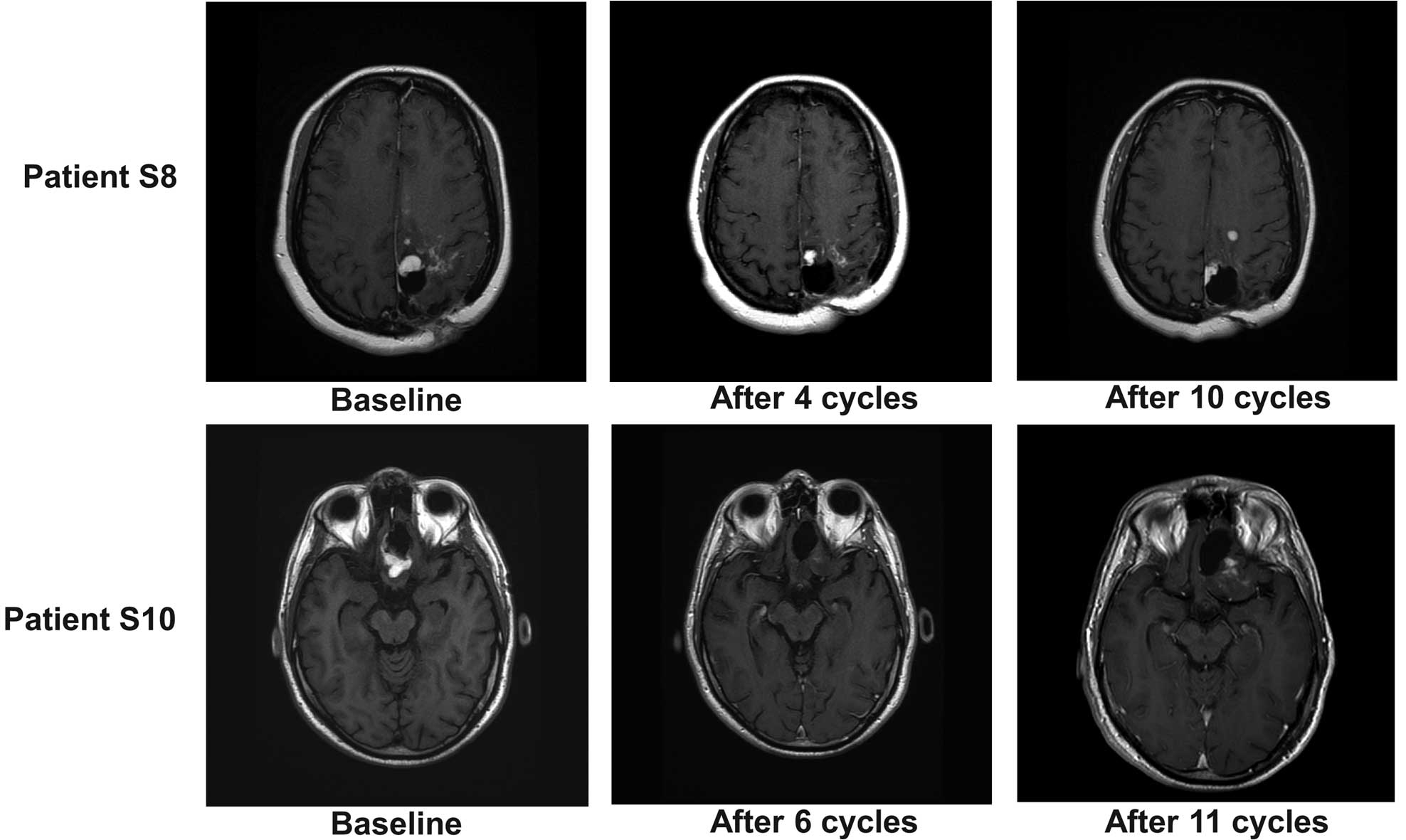
According to Macdonald criteria, 2 patients (S8 and
S10) responded to combined treatment. Responses were noted after 4
cycles (Fig. 1C). Fig. 2 shows that the response was observed
at cycle 4 in patient S8, but at cycle 6 in patient S10.
Discussion
The present study assessed whether LY2157299 has
antitumor effects, as detected by perfusion MRI. The results
indicated that LY2157299 in combination with lomustine does not
reduce rCBV and permeability, as previously described for
anti-angiogenic drugs such as bevacizumab or cediranib (32). A number of anti-angiogenic agents have
been investigated for their potential ability to treat
glioblastoma, and various imaging techniques, such as perfusion MRI
and dynamic susceptibility weighted MRI, have been proposed to
measure responses to treatment (33).
Whilst lomustine monotherapy has not specifically been evaluated as
an anti-angiogenic agent, a recent study observed its
anti-angiogenic effect as part of a combination therapy of
procarbazine, lomustine and vincristine (34). In this study, patients had undergone
fewer prior treatments compared with the present study. The shorter
median treatment of <1 cycle observed in the current study,
compared with that of other trials, is a consequence of the highly
pre-treated study population used for this study.
In the present study, evidence of responses began to
emerge after the patients received 4 cycles of treatment, and
became more clear following 6 cycles. This pattern of responses is
inconsistent with that of previous studies reporting tumor
shrinkage with chemotherapy, in which responses were generally
observed within the first month of treatment (35). A recent phase III study confirmed that
the early responses with lomustine are commonly visible within the
first month of treatment (36). Due
to this lack of early antitumor responses, it is possible that the
changes in the present study are predominantly a result of the
LY2157299 treatment. As demonstrated previously (27), LY2157299 monotherapy in patients with
glioblastoma shows preliminary stabilization of the tumor growth,
followed by a tumor response several months later. In contrast to
the previous studies, the present study shows that these responses
may also be associated with changes in the vasculature of the
glioma.
In the present study, 2 patients with relapsed
glioblastoma responded to LY2157299 and lomustine treatment; one of
these patients also had measurable reduction in rCBV and
permeability. Although there was no control in this study, the late
responses in the 2 patients may be attributable to LY2157299 and
not to lomustine, which is predicted to produce early antitumor
effects. This is the first study evaluating the impact of a TGF-β
inhibitor on vascular changes in patients with glioma.
Acknowledgements
This study was sponsored by Eli Lilly and Company
(Indianapolis, IN, USA). Dr Michael F. Lahn and Dr Marta Garcia de
la Torre are employees of Eli Lilly and Company.
References
|
1
|
Massague J, Blain SW and Lo RS: TGFbeta
signaling in growth control, cancer, and heritable disorders. Cell.
103:295–309. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hughes C, Bauer E and Roberts AP: Spread
of R-plasmids among Escherichia coli causing urinary tract
infections. Antimicrob Agents Chemother. 20:496–502. 1981.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bruna A, Darken RS, Rojo F, et al: High
TGFbeta-Smad activity confers poor prognosis in glioma patients and
promotes cell proliferation depending on the methylation of the
PDGF-B gene. Cancer Cell. 11:147–160. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Penuelas S, Anido J, Prieto-Sanchez RM, et
al: TGF-beta increases glioma-initiating cell self-renewal through
the induction of LIF in human glioblastoma. Cancer Cell.
15:315–327. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sawyer JS, Beight DW, Britt KS, et al:
Synthesis and activity of new aryl- and heteroaryl-substituted
5,6-dihydro-4H-pyrrolo[1,2-b]pyrazole inhibitors of the
transforming growth factor-beta type I receptor kinase domain.
Bioorg Med Chem Lett. 14:3581–3584. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Anido J, Saez-Borderias A, Gonzalez-Junca
A, et al: TGF-β Receptor Inhibitors Target the CD44(high)/Id1(high)
Glioma-Initiating Cell Population in Human Glioblastoma. Cancer
Cell. 18:655–668. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li HY, Wang Y, Yan L, et al: Novel and
potent transforming growth factor beta type I receptor kinase
domain inhibitor: 7-amino
4-(2-pyridin-2-yl-5,6-dihydro-4H-pyrrolo[1,2-b]pyrazol-3-yl)-quinolines.
Bioorg Med Chem Lett. 14:3585–3588. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Anderton MJ, Mellor HR, Bell A, et al:
Induction of heart valve lesions by small-molecule ALK5 inhibitors.
Toxicol Pathol. 39:916–924. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bueno L, de Alwis DP, Pitou C, et al:
Semi-mechanistic modelling of the tumour growth inhibitory effects
of LY2157299, a new type I receptor TGF-beta kinase antagonist, in
mice. Eur J Cancer. 44:142–150. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gueorguieva I, Cleverly AL, Stauber A, et
al: Defining a therapeutic window for the novel TGF-ᵝ inhibitor
LY2157299 monohydrate based on a pharmacokinetic/pharmacodynamic
model. Br J Clin Pharmacol. 77:796–807. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vredenburgh JJ, Desjardins A, Herndon JE
II, et al: Phase II trial of bevacizumab and irinotecan in
recurrent malignant glioma. Clin Cancer Res. 13:1253–1259. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lenato GM and Guanti G: Hereditary
Haemorrhagic Telangiectasia (HHT): Genetic and molecular aspects.
Curr Pharm Des. 12:1173–1193. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lechleider RJ, Ryan JL, Garrett L, et al:
Targeted mutagenesis of Smad1 reveals an essential role in
chorioallantoic fusion. Dev Biol. 240:157–167. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li F, Lan Y, Wang Y, et al: Endothelial
Smad4 maintains cerebrovascular integrity by activating N-cadherin
through cooperation with Notch. Dev Cell. 20:291–302. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dickinson ME, Selleck MA, McMahon AP and
Bronner-Fraser M: Dorsalization of the neural tube by the
non-neural ectoderm. Development. 121:2099–2106. 1995.PubMed/NCBI
|
|
16
|
Oshima M, Oshima H and Taketo MM: TGF-beta
receptor type II deficiency results in defects of yolk sac
hematopoiesis and vasculogenesis. Dev Biol. 179:297–302. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Goumans MJ, Valdimarsdottir G, Itoh S, et
al: Balancing the activation state of the endothelium via two
distinct TGF-beta type I receptors. EMBO J. 21:1743–1753. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gerstner ER, Sorensen AG, Jain RK and
Batchelor TT: Advances in neuroimaging techniques for the
evaluation of tumor growth, vascular permeability, and angiogenesis
in gliomas. Curr Opin Neurol. 21:728–735. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pechman KR, Donohoe DL, Bedekar DP, et al:
Evaluation of combined bevacizumab plus irinotecan therapy in brain
tumors using magnetic resonance imaging measures of relative
cerebral blood volume. Magn Reson Med. 68:1266–1272. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sorensen AG, Emblem KE, Polaskova P, et
al: Increased survival of glioblastoma patients who respond to
antiangiogenic therapy with elevated blood perfusion. Cancer Res.
72:402–407. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hilario A, Ramos A, Perez-Nunez A, et al:
The added value of apparent diffusion coefficient to cerebral blood
volume in the preoperative grading of diffuse gliomas. AJNR Am J
Neuroradiol. 33:701–707. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Maeda M, Itoh S, Kimura H, et al: Tumor
vascularity in the brain: Evaluation with dynamic
susceptibility-contrast MR imaging. Radiology. 189:233–238. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Aronen HJ, Gazit IE, Louis DN, et al:
Cerebral blood volume maps of gliomas: Comparison with tumor grade
and histologic findings. Radiology. 191:41–51. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Donahue KM, Krouwer HG, Rand SD, et al:
Utility of simultaneously acquired gradient-echo and spin-echo
cerebral blood volume and morphology maps in brain tumor patients.
Magn Reson Med. 43:845–853. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Law M, Oh S, Babb JS, et al: Low-grade
gliomas: Dynamic susceptibility-weighted contrast-enhanced
perfusion MR imaging - prediction of patient clinical response.
Radiology. 238:658–667. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sugahara T, Korogi Y, Tomiguchi S, et al:
Posttherapeutic intraaxial brain tumor: The value of
perfusion-sensitive contrast-enhanced MR imaging for
differentiating tumor recurrence from nonneoplastic
contrast-enhancing tissue. AJNR Am J Neuroradiol. 21:901–909.
2000.PubMed/NCBI
|
|
27
|
Rodon Ahnert J, Baselga J, Calvo E, et al:
First human dose (FHD) study of the oral transforming growth
factor-beta receptor I kinase inhibitor LY2157299 in patients with
treatment-bractory malignant glioma. J Clin Oncol. 29:abstract
3011. 2011.
|
|
28
|
Azaro A, Baselga J, Sepulveda JM, et al:
The oral transforming growth factor-beta (TGF-β) receptor I kinase
inhibitor LY2157299 plus lomustine in patients with
treatment-bractory malignant glioma: The first human dose study. J
Clin Oncol. 30:abstract 2042. 2012.
|
|
29
|
Trotti A, Colevas AD, Setser A, et al:
CTCAE v3.0: development of a comprenhensive grading system for the
adverse effects of cancer treatment. Semin Radiat Oncol.
13:176–181. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Oken MM, Creech RH, Tormey DC, et al:
Toxicity and response criteria of the Eastern Cooperative Oncology
Group. Am J Clin Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Macdonald DR, Cascino TL, Schold SC Jr and
Cairncross JG: Response criteria for phase II studies of
supratentorial malignant glioma. J Clin Oncol. 8:1277–1280.
1990.PubMed/NCBI
|
|
32
|
Vidiri A, Pace A, Fabi A, et al: Early
perfusion changes in patients with recurrent high-grade brain tumor
treated with Bevacizumab: Preliminary results by a quantitative
evaluation. J Exp Clin Cancer Res. 31:332012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Thompson G, Mills SJ, Coope DJ, et al:
Imaging biomarkers of angiogenesis and the microvascular
environment in cerebral tumours. Br J Radiol. 84:S127–S144. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jenkinson MD, Smith TS, Joyce KA, et al:
Cerebral blood volume, genotype and chemosensitivity in
oligodendroglial tumours. Neuroradiology. 48:703–713. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kappelle AC, Postma TJ, Taphoorn MJ, et
al: PCV chemotherapy for recurrent glioblastoma multiforme.
Neurology. 56:118–120. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wick W, Puduvalli VK, Chamberlain MC, et
al: Phase III study of enzastaurin compared with lomustine in the
treatment of recurrent intracranial glioblastoma. J Clin Oncol.
28:1168–1174. 2010. View Article : Google Scholar : PubMed/NCBI
|