Introduction
Primary bone lymphoma (PBL) is an uncommon,
malignant, neoplastic disorder of the skeleton, which accounts for
5–7% of primary bone tumors, 4–5% of extranodal non-Hodgkin
lymphomas (NHLs) (1,2) and 1% of all NHLs (3–5). Diffuse
large B-cell lymphoma (DLBCL) is the most common histological type
of PBL, and the majority of patients have limited-stage disease
(stages IE-IIE) at presentation (4,6–9). The most common symptoms at presentation
are pain, swelling and pathologic fractures (PFs), however,
systemic symptoms, such as fever, night sweats and weight loss,
occur less frequently in PBL when compared with other NHLs
(10). Previous studies have reported
the use of radiotherapy, chemotherapy or a combination of the two
for the treatment of PBL, resulting in a generally good prognosis.
The present study reported a case of PBL of the sternum and
reviewed previous relevant cases, suggesting potential future
investigations.
Case report
A 68-year-old male with a four-month history of a
sternal mass was admitted to the Second Affiliated Hospital of
Dalian Medical University (Dalian, China) in March 2014. The
patient complained of bone swelling in the sternum, but did not
present systemic symptoms, including fever, night sweats or weight
loss. A physical examination detected an 8×6-cm painless, firm mass
on the anterior sternum, a 4×1-cm flat mass in the right side of
the anterior inferior rib and an 1×1-cm, enlarged cervical lymph
node. Laboratory tests revealed increased serum levels of lactate
dehydrogenase (LDH) (381 U/l; normal, 10–240 U/l) and erythrocyte
sedimentation rate (64 mm/h; normal, 0–15 mm/h). A computed
tomography (CT) scan identified a soft tissue mass eroding the
sternum, cortical breakthrough and a destructive osteolytic lesion
in the sternum, which was permeative with a ‘moth-eaten’ appearance
and an irregular margin. In addition, bone destruction in his right
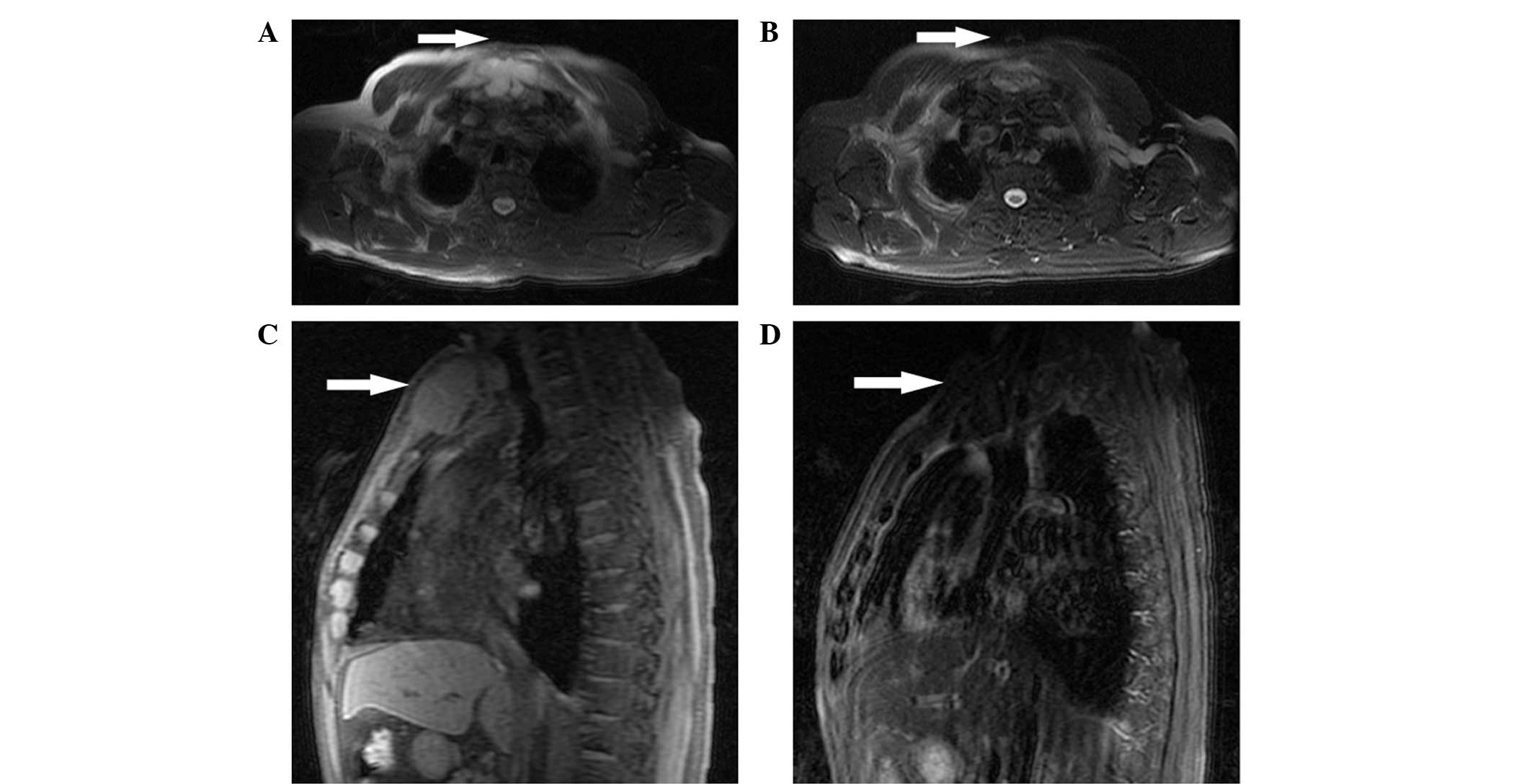
side of the anterior inferior rib was observed (Fig. 1). A magnetic resonance imaging (MRI)
scan revealed a mass with a size of 34×63 mm arising from the
sternum, which presented moderately long T1 and T2 signal
intensities on a T1-weighted image (WI) and T2-WI, respectively,
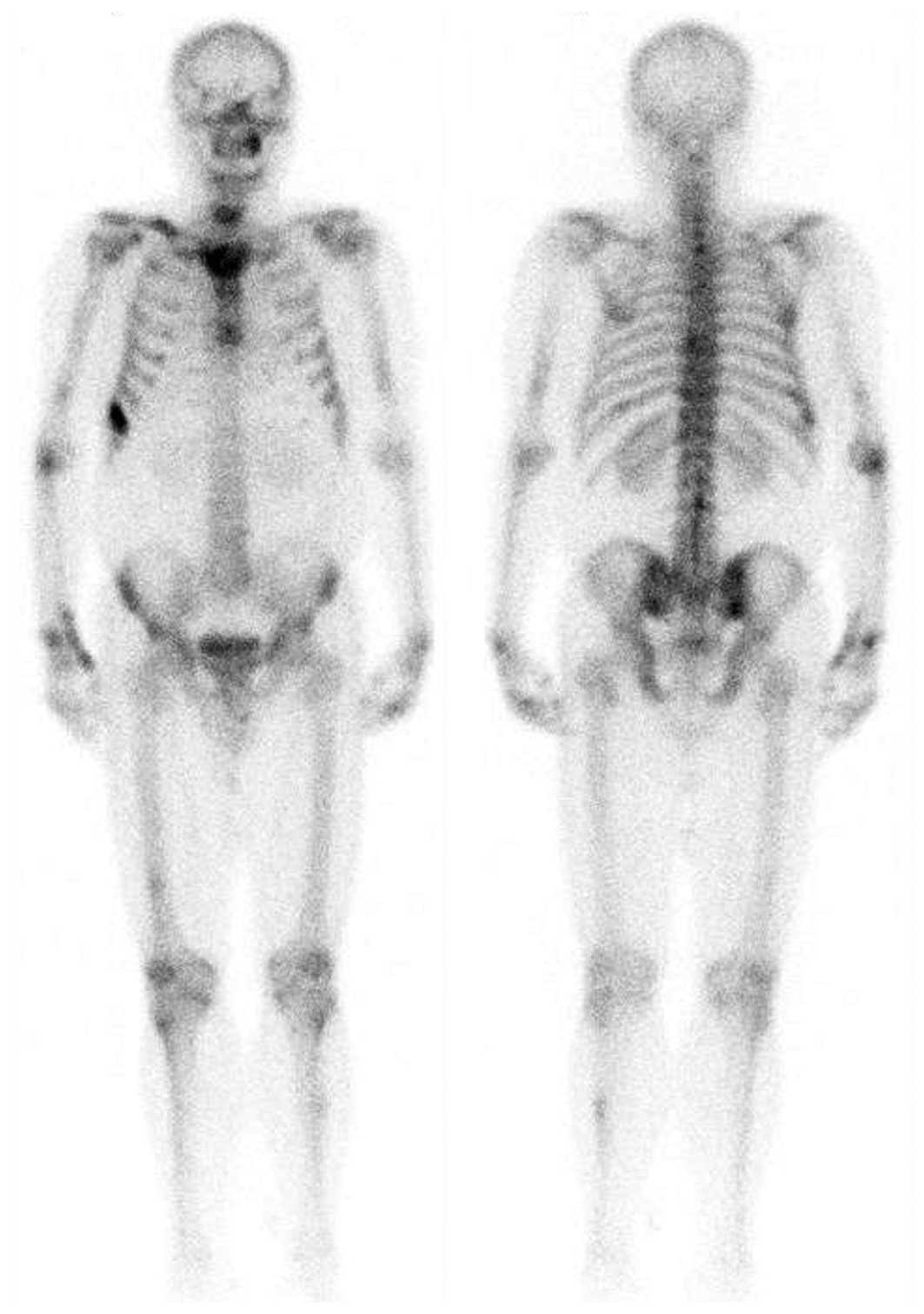
with involvement of the surrounding soft tissue (Fig. 2). Furthermore, a bone emission CT
(ECT) scan demonstrated increased tracer uptake in the two humeri,
right clavicle, spine, right femur and left tibia, which
demonstrated multiple bone metastases (Fig. 3). A bone marrow cytology examination
was normal.
Initially, the patient was considered to suffer from
a bone tumor due to the presence of a soft tissue mass eroding the
bone, as determined using imaging. However, PBL was also considered
as a differential diagnosis. Establishing a diagnosis was
challenging and pathological results were required for
verification. A superficial temporal artery biopsy revealed a
diagnosis of DLBCL, not otherwise specified (NOS), germinal center
B-cell-like (GCB; Fig. 4).
Immunohistochemical staining demonstrated that the biopsy specimen
was positive for CD20, CD79a, B-cell lymphoma (BCL)-6, CD10,
multiple myeloma oncogene 1 and epithelial membrane antigen,
revealing a high proliferative index (Ki-67 = 70%). However, the
specimen was negative for CD5, CD3, anaplastic lymphoma kinase,
CD30, BCL-2 and anion exchanger (AE)1/AE3 (Fig. 4). These results led to the diagnosis
of DLBCL (NOS, GCB), stage IVA (Ann Arbor staging system) (6), with an international prognostic index
(IPI) (11) score of 4, which
indicated that the patient belonged to the high-risk group. The
five-year overall survival (OS) of the high-risk group is 26%,
indicating a poor prognosis (11).
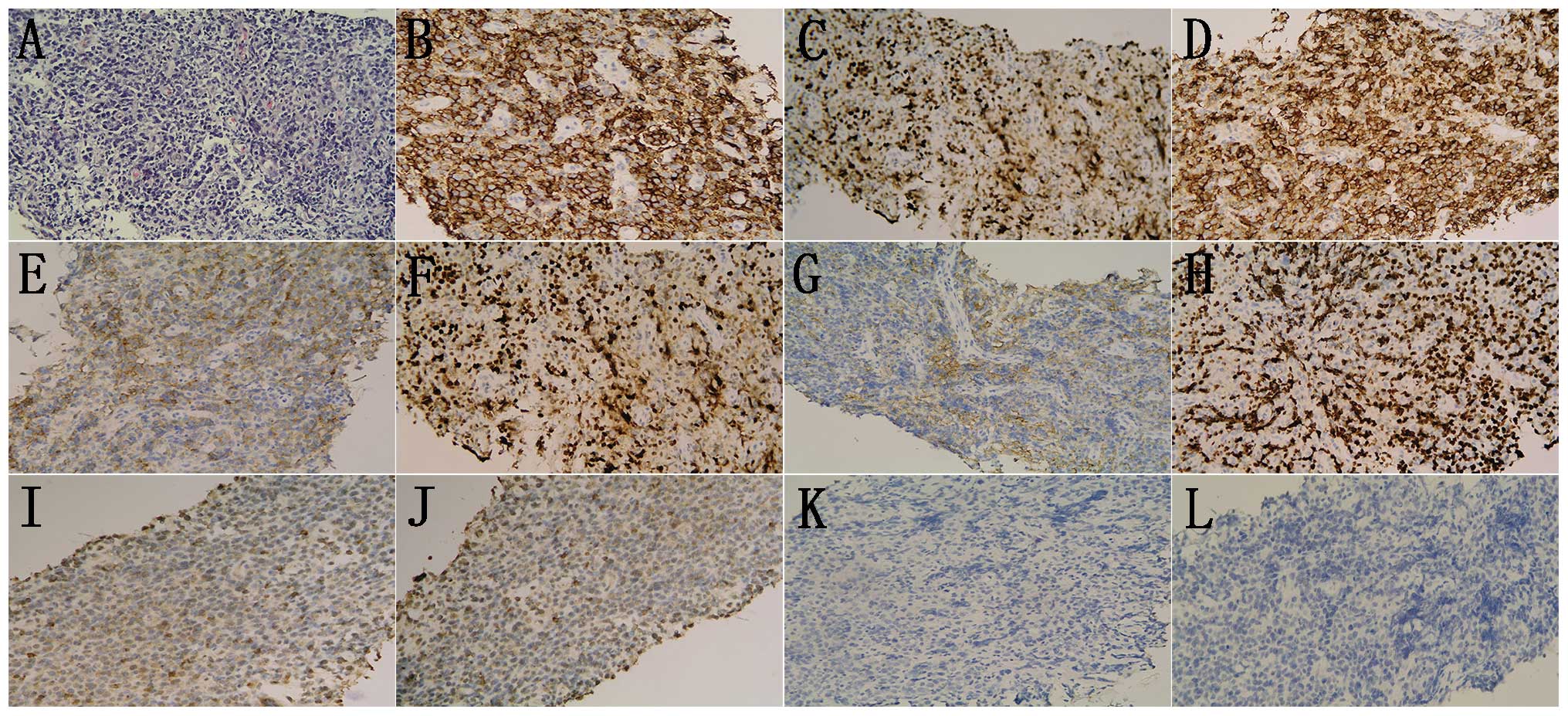 | Figure 4.Diffuse infiltration of large
B-cells. (A) Hematoxylin and eosin stained cells.
Immunohistochemical analysis revealed that the samples were
positive for (B) CD20, (C) CD79a, (D) BCL-6, (E) CD10, (F) MUM-1,
partially positive for (G) EMA and (H) Ki-67 index 70%; and
negative for (I) CD5, (J) CD3, (K) BCL-2 and (L) AE1/AE3.
(hematoxylin and eosin staining; magnification, x200). MUM-1,
multiple myeloma oncogene 1; EMA, epithelial membrane antigen; Bcl,
B-cell lymphoma; AE, anion exchanger. |
Based on the results of the present case, diagnosis
of PLB using imaging is difficult, since the findings are not
pathognomonic, and a prompt histological examination may define the
diagnosis and avoid the delay in treatment. The patient of the
current study was treated with the cyclophosphamide, epirubicin,
vincristine and prednisone (CHOP) regimen, with the absence of
rituximab due to its high cost. Epirubicin was used in place of
doxorubicin, as they are the same type of chemotherapeutic agent
and have a similar mechanism of action. The patient completed four
chemotherapy cycles: Cyclophosphamide, 750 mg/m2 iv d1;
epirubicin, 50 mg/m2 iv d1; vincristine, 1.4
mg/m2 iv d1; prednisone, 100 mg/m2 po d1–5,
21 day per cycle, and the response evaluation indicated partial
remission according to Cheson's criteria (12), with evidence of rapid reduction of all
the lesions. The size of the sternal mass decreased to 2×1 cm,
while the mass on the right side of the anterior inferior rib
disappeared (Figs. 1 and 2). The patient completed eight chemotherapy
cycles; the last follow-up was December 2014 and response
evaluation indicated almost complete remission. At present, the
patient remains in follow-up, and the development of the disease
will continue to be monitored. Written informed consent was
obtained from the patient prior to the publication of the present
study. The study was approved by the ethics committee of The Second
Affiliated Hospital of Dalian Medical University, Dalian,
China.
Discussion
The most common sites of involvement in PBL are the
extremities. For instance, Beal et al reported that the
femur (27%), pelvis (15%) and tibia/fibula (13%) are the three
commonly-affected locations (10). A
case with initial involvement of the sternum was described in the
present study, which is an uncommon phenotype in PBL. To the best
of our knowledge, only six such cases have been previously reported
in the medical literature (13–18). In
addition, the clinical characteristics are nonspecific, making the
diagnosis difficult at the onset.
PBL commonly affects male individuals with an age
range between mid-40 and mid-60 years. The most common
presentations are pain, swelling and PFs, while systemic symptoms,
including fever, night sweats and weight loss, occur less
frequently in PBL compared with other non-Hodgkin lymphomas (NHLs)
(10). The majority of patients in
previous studies presented with localized disease (19–21);
however, with the improvements in imaging techniques, including CT,
MRI, ECT and 18F-fluorodeoxyglucose-positron emission
tomography (PET)/CT, the incidence of multifocal disease has
increased (22). MRI is more accurate
in evaluating PBL, which is particularly useful in evaluating the
extent of soft tissue extension, spine involvement and spinal cord
compression, as well as assisting the reduction of the volume
irradiated (3). In addition, a
previous study suggested that PET/CT is more accurate compared with
bone ECT in assessing bone involvement by lymphoma (23). In addition, PET/CT has been
demonstrated to be superior to CT and MRI, particularly in
multifocal disease, and may be significant in the assessment of
response to treatment (24). However,
the imaging characteristics of PBL are variable, with detectable
abnormalities, and nonspecific to the diagnosis for PBL. Therefore,
adequate biopsy for histological examination and immunophenotyping
remains the gold standard for diagnosis. In the present case,
specific markers were selected, as immunohistochemically, DLBCL is
often observed to be positive for CD10, CD20 and CD79a, while it is
frequently negative for CD3, CD5, CD43, AE1/AE3 and BCL-2.
Furthermore, BCL-6 and MUM-1 are used to distinguish between GCB
and non-GCB types.
The diagnosis of PBL presents a variety of
challenges and, due to the absence of prospective clinical studies,
the majority of PBLs are treated with chemotherapy and/or
radiotherapy. Radiotherapy was established as the standard
treatment in earlier years (19,20).
However, with the development of highly effective chemotherapy
regimens and particularly the advent of novel agents, such as the
anti-CD20 monoclonal antibody rituximab, the role of radiotherapy
has been increasingly questioned by certain researchers (2,25–27). Considering current studies and several
previously-published studies, patients treated with
chemoradiotherapy appeared to present an improved outcome compared
with patients receiving single modality therapy (25–29). These
observations require confirmation by prospective studies with
larger sample groups, since specific studies appear to disprove
them (2,26,27,33,34).
For instance, in the IELSG-14 study, the addition of radiotherapy
following chemotherapy, or the use of larger radiation fields and
doses, were not associated with an improved outcome in patients
treated with chemoradiotherapy (9).
Although previous studies have obtained contradicting results,
chemoradiotherapy is likely to be superior to single modality
therapy, which may be advantageous for localized unifocal bulky
sites of disease, which allows a shorter chemotherapy course
(1,30,35), or
for multifocal disease in relapsing cases (36). Anthracycline-containing chemotherapy
has become the standard therapy of DLBCL, while numerous studies
have demonstrated increased long term remission rates with the
incorporation of rituximab (33,37–42). Based
on the aforementioned findings, chemotherapy plus rituximab
(immunochemotherapy) is currently considered to be the first-line
treatment for CD20-positive DLBCLs. Furthermore, a number of
studies pointed out that the positive effect of administering
rituximab may be evident in certain subgroups of DLBCLs. For
instance, previous results support that only BCL-2-positive
(43,44) or BCL-6-negative (45) patients significantly benefited from
rituximab. In addition, patients with activated B-cell-like-DLBCL
appear to benefit more compared with GCB-DLBCL patients (45). Considering these observations, the
patient of the present study may not benefit sufficiently from
rituximab treatment.
Table I lists a number
of representative studies reported in the English literature
identified by searching PubMed and Mediline databases with the key
words ‘primary bone lymphoma’ (1,2,9,19–21,27–34,47–59),
which investigated the response of patients to chemotherapy and
radiotherapy, regardless of the effect of surgery and molecular
targeted therapy. From these studies (1,2,9,19–21,27–34,47–59),
certain observations can be summarized and emphasized. First, an
increasing number of patients are diagnosed with progressive stage
PBL (patients were staged according to the Ann Arbor Staging
System), due to the improvement in examination techniques. In
addition, with the increase in the diagnostic rate of multifocal
disease that is no longer suitable for radiotherapy and with the
development of novel chemotherapy regimens, chemotherapy has
gradually become the main treatment method. Furthermore, local
stage was associated with the use of radiotherapy and advanced
stage was significantly associated with chemotherapy. Therefore,
the survival analysis of PBL patients revealed that
chemoradiotherapy was associated with improved survival of local
stage in previous studies (21,28,31,49);
however, no difference in the survival rates was observed compared
with chemotherapy of advanced stage patients in recent studies
(2,9,54).
Finally, the transformation of treatment mode was inevitable, which
depends on the stage.
 | Table I.Representative series describing
patients with PBL. |
Table I.
Representative series describing
patients with PBL.
| Study period | Number | Stage I and II
(%) | Stage IV (%) | CXT only (%) | RT only (%) | CXRT (%) | Five-year OS rate
(%) | Reference |
|---|
| 1907–1982 | 422 | 62 | 38 | 8 | 57 | 24 | NA | (46) |
| 1943–1996 | 60 | 62 | 16 | NA | 8 | 58 | 61 | (47) |
| 1961–1999 | 94 | 74 | 26 | 7 | 3 | 53 | 88 | (48) |
| 1963–2003 | 82 | 81 | 0 | 30 | 13 | 57 | 88 | (27) |
| 1967–1992 | 45 | 100 | 0 | 8 | 11 | 80 | 68 | (20) |
| 1970–1978 | 37 | 100 | 0 | 5 | 0 | 95 | 91 | (21) |
| 1970–2003 | 37 | 100 | 0 | 46 | 41 | 8 | 65 | (49) |
| 1973–2005 | 1500 | 69 | 31 | NA | 68 | NA | 58 | (50) |
| 1979–2007 | 19 | 58 | 42 | 58 | 0 | 42 | NA | (51) |
| 1980–2003 | 100 | 0 | 100 | 36 | 0 | 64 | NA | (36) |
| 1980–2005 | 295 | 59 | 41 | 21 | 8 | 71 | 54 | (52) |
| 1980–2005 | 161 | 100 | 0 | 8 | 14 | 78 | 75 | (34) |
| 1982–1998 | 52 | 60 | 40 | 15 | 21 | 63 | 68 | (30) |
| 1983–2001 | 77 | 100 | 0 | NA | 13 | 87 | 88 | (29) |
| 1983–2005 | 131 | 46 | 54 | 44 | NA | 48 | 62 | (23) |
| 1985–2003 | 22 | 77 | 23 | NA | NA | 68 | 85 | (37) |
| 1987–2008 | 116 | 100 | 0 | 12 | 13 | 75 | 76 | (28) |
| 1989–2005 | 30 | 70 | 30 | 17 | 10 | 70 | 73 | (31) |
| 1992–2010 | 33 | 39 | 61 | 52 | 0 | 48 | 75 | (53) |
| 1992–2012 | 22 | 86 | 14 | 23 | 4 | 73 | 86 | (54) |
| 1995–2004 | 28 | 32 | 68 | NA | NA | 50 | 84 | (55) |
| 1999–2009 | 21 | 9 | 91 | 48 | 0 | 52 | 95 | (33) |
| 1999–2011 | 21 | 62 | 38 | 19 | 0 | 81 | 74 | (44) |
| 2000–2007 | 53 | 77 | 23 | 21 | 12 | 62 | 100 | (32) |
| 2000–2011 | 24 | 67 | 33 | 62 | 0 | 38 | 67 | (56) |
| NA | 28 | 100 | 0 | 0 | 32 | 68 | 60 | (57) |
| NA | 31 | 68 | 32 | 0 | 0 | 100 | 90 | (58) |
No conclusive view exists on the response of PBL to
various radiotherapy doses, while the majority of studies used
doses that are commonly used to treat NHL (35–45 Gy in 1.8–2 Gy
fractions) (1,30,31).
However, a randomized trial conducted in the British population
revealed that radiotherapy at a dose of 30 Gy following
chemotherapy was adequate for the treatment of NHL, including
extra-nodal sites (60), while higher
doses may be reserved for cases with a suboptimal response to
chemotherapy. Another issue is to determine whether surgery is
required. At present, due to the development of effective
oncological treatments, surgery is not routinely used in the
management of PBL, with the exception of the initial biopsy
performed to establish a diagnosis and the treatment of PFs
(1,22). A recent study reported that, although
PFs occurred, the initial surgical stabilization of the PFs did not
change the therapeutic outcome, and patients receiving chemotherapy
prior to irradiation of the fractured bone exhibited an improved
outcome (53).
The prognosis of PBL is relatively good and equal to
that of same-stage systemic DLBCL (55). The five-year OS rates vary greatly, as
demonstrated in clinical trials and retrospective population-based
studies (2,9,20,28,29,51,53).
In addition, various studies have suggested different prognostic
factors, including age (29,50,51,56),
gender (10), stage (2), LDH levels (10), lesion range (51) and IPI score (28,56).
Considering these factors, the case reported in the present study
may have a poor prognosis. Notably, several recent studies
confirmed that the only positive prognostic factor was complete
remission (CR) following chemotherapy. Once a CR is achieved, even
elderly patients may exhibit long-term survival, possibly obviating
the requirement for surgery for less severe bone lesions (57,61).
In conclusion, the case described in the current
study improved the understanding on the characteristics of PBL. The
present authors recommend that unusual and rare conditions should
be considered as a differential diagnosis when dealing with bone
tumors and highlight the paramount importance of histological
analysis for a clarified diagnosis. Although the currently
available data support the use of chemoradiotherapy for the
treatment of PBL, further randomized controlled trials are required
to evaluate the efficacy of this treatment strategy.
References
|
1
|
Ford DR, Wilson D, Sothi S, Grimer R and
Spooner D: Primary bone lymphoma - treatment and outcome. Clin
Oncol (R Coll Radiol). 19:50–55. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ramadan KM, Shenkier T, Sehn LH, Gascoyne
RD and Connors JM: A clinicopathological retrospective study of 131
patients with primary bone lymphoma: a population-based study of
successively treated cohorts from the British Columbia Cancer
Agency. Ann Oncol. 18:129–135. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Krishnan A, Shirkhoda A, Tehranzadeh J, et
al: Primary bone lymphoma: Radiographic-MR imaging correlation.
Radiographics. 23:1371–1387; discussion 1384–1387. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mengiardi B, Honegger H, Hodler J, et al:
Primary lymphoma of bone: MRI and CT characteristics during and
after successful treatment. Am J Roentgenol. 184:185–192. 2005.
View Article : Google Scholar
|
|
5
|
Glotzbecker MP, Kersun LS, Choi JK, et al:
Primary non-Hodgkin's lymphoma of bone in children. J Bone Joint
Surg Am. 88:583–594. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lister TA, Crowther D, Sutcliffe SB, et
al: Report of a committee convened to discuss the evaluation and
staging of patients with Hodgkin's disease: Cotswolds meeting. J
Clin Oncol. 7:1630–1636. 1989.PubMed/NCBI
|
|
7
|
Reddy N and Greer JP: Primary bone
lymphoma: A set of unique problems in management. Leuk Lymphoma.
51:1–2. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhao XF, Young KH, Frank D, et al:
Pediatric primary bone lymphoma - diffuse large B-cell lymphoma:
Morphologic and immunohistochemical characteristics of 10 cases. Am
J Clin Pathol. 127:47–54. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bruno Ventre M, Ferreri AJ, Gospodarowicz
M, et al: International Extranodal Lymphoma Study Group: Clinical
features, management, and prognosis of an international series of
161 patients with limited-stage diffuse large B-cell lymphoma of
the bone (the IELSG-14 study). Oncologist. 19:291–298. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Beal K, Allen L and Yahalom J: Primary
bone lymphoma: treatment results and prognostic factors with
long-term follow-up of 82 patients. Cancer. 106:2652–2656. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
No authors listed: A predictive model for
aggressive non-Hodgkin's lymphoma. The International Non-Hodgkin's
Lymphoma Prognostic Factors Project. N Engl J Med. 329:987–994.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cheson BD, Horning SJ, Coiffier B, et al:
Report of an international workshop to standardize response
criteria for non-Hodgkin's lymphoma. NCI Sponsored International
Working Group. J Clin Oncol. 17:12441999.PubMed/NCBI
|
|
13
|
Langley CR, Garrett SJ, Urand J, Kohler J
and Clarke NM: Primary multifocal osseous Hodgkin's lymphoma. World
J Surg Oncol. 6:342008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gupta A, Kataria SP, Kumar K and
Subramanian S: Primary non Hodgkin's lymphoma of sternum. Indian J
Med Paediatr Oncol. 27:282006.
|
|
15
|
Levitt LJ, Aisenberg AC, Harris NL, et al:
Primary non-Hodgkin's lymphoma of the mediastinum. Cancer.
50:2486–2492. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kabiri el H, Kabiri M and Doghmi K:
Primary non-Hodgkin lymphoma of the sternum. Arch Bronconeumol.
45:259–260. 2009.(In Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Maslovsky I and Gefel D: Hodgkin's
disease. Haematologica. 90 (12 Suppl):EIM022005.PubMed/NCBI
|
|
18
|
Graziadio M, Medina N, Amato M, et al:
Primary bone lymphoma with multicentric involvement. Medicina (B
Aires). 72:4284302012.(In Spanish). PubMed/NCBI
|
|
19
|
Heyning FH, Hogendoorn PC, Kramer MH, et
al: Primary non-Hodgkin's lymphoma of bone: a clinicopathological
investigation of 60 cases. Leukemia. 13:2094–2098. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Demircay E, Hornicek FJ Jr, Mankin HJ and
Degroot H III: Malignant lymphoma of bone: a review of 119
patients. Clin Orthop Relat Res. 471:2684–2690. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dubey P, Ha CS, Besa PC, et al: Localized
primary malignant lymphoma of bone. Int J Radiat Oncol Biol Phys.
37:1087–1093. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mikhaeel NG: Primary bone lymphoma. Clin
Oncol (R Coll Radiol). 24:366–370. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Moog F, Kotzerke J and Reske SN: FDG PET
can replace bone scintigraphy in primary staging of malignant
lymphoma. J Nucl Med. 40:1407–1413. 1999.PubMed/NCBI
|
|
24
|
Park YH, Kim S, Choi SJ, et al: Clinical
impact of whole-body FDG-PET for evaluation of response and
therapeutic decision-making of primary lymphoma of bone. Ann Oncol.
16:1401–1402. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ng AK and Mauch PM: Role of radiation
therapy in localized aggressive lymphoma. J Clin Oncol. 25:757–759.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bonnet C, Fillet G, Mounier N, et al:
Groupe d'Etude des Lymphomes de l'Adulte: CHOP alone compared with
CHOP plus radiotherapy for localized aggressive lymphoma in elderly
patients: A study by the Groupe d'Etude des Lymphomes de l'Adulte.
J Clin Oncol. 25:787–792. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Reyes F, Lepage E, Ganem G, et al: Groupe
d'Etude des Lymphomes de l'Adulte (GELA): ACVBP versus CHOP plus
radiotherapy for localized aggressive lymphoma. N Engl J Med.
352:1197–1205. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Beal K, Allen L and Yahalom J: Excellent
long-term experience with primary bone lymphoma: Analysis of
prognostic factors. J Clin Oncol. 22:65872004.
|
|
29
|
Cai L, Stauder MC, Zhang YJ, et al:
Early-stage primary bone lymphoma: a retrospective, multicenter
rare cancer network (RCN) study. Int J Radiat Oncol Biol Phys.
83:284–291. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Barbieri E, Cammelli S, Mauro F, et al:
Primary non-Hodgkin's lymphoma of the bone: treatment and analysis
of prognostic factors for Stage I and Stage II. Int J Radiat Oncol
Biol Phys. 59:760–764. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zinzani PL, Carrillo G, Ascani S, et al:
Primary bone lymphoma: experience with 52 patients. Haematologica.
88:280–285. 2003.PubMed/NCBI
|
|
32
|
Catlett JP, Williams SA, O'Connor SC,
Krishnan J and Malkovska V: Primary lymphoma of bone: an
institutional experience. Leuk Lymphoma. 49:2125–2132. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Alencar A, Pitcher D, Byrne G and Lossos
IS: Primary bone lymphoma - the university of miami experience.
Leuk Lymphoma. 51:39–49. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pellegrini C, Gandolfi L, Quirini F, et
al: Primary bone lymphoma: evaluation of chemoimmunotherapy as
front-line treatment in 21 patients. Clin Lymphoma Myeloma Leuk.
11:321–325. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Horning SJ, Weller E, Kim K, et al:
Chemotherapy with or without radiotherapy in limited-stage diffuse
aggressive non-Hodgkin's lymphoma: Eastern cooperative oncology
group study 1484. J Clin Oncol. 22:3032–3038. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Messina C, Ferreri AJ, Govi S, et al:
International Extranodal Lymphoma Study Group (I.E.L.S.G.):
Clinical features, management and prognosis of multifocal primary
bone lymphoma: a retrospective study of the international
extranodal lymphoma study group (the IELSG 14 study). Br J
Haematol. 164:834–840. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gutierrez-Garcia G, Colomo L, Villamor N,
et al: Clinico-biological characterization and outcome of primary
nodal and extranodal diffuse large B-cell lymphoma in the rituximab
era. Leuk Lymphoma. 51:1225–1232. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Rieger M, Osterborg A, Pettengell R, et
al: MabThera International Trial (MInT) Group: Primary mediastinal
B-cell lymphoma treated with CHOP-like chemotherapy with or without
rituximab: results of the Mabthera International Trial Group study.
Ann Oncol. 22:664–670. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Feugier P, Van Hoof A, Sebban C, et al:
Long-term results of the R-CHOP study in the treatment of elderly
patients with diffuse large B-cell lymphoma: a study by the Groupe
d'Etude des Lymphomes de l'Adulte. J Clin Oncol. 23:4117–4126.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Pfreundschuh M, Trumper L, Osterborg A, et
al: MabThera International Trial Group: CHOP-like chemotherapy plus
rituximab versus CHOP-like chemotherapy alone in young patients
with good-prognosis diffuse large-B-cell lymphoma: a randomised
controlled trial by the MabThera international trial (MInT) group.
Lancet Oncol. 7:379–391. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sehn LH, Donaldson J, Chhanabhai M, et al:
Introduction of combined CHOP plus rituximab therapy dramatically
improved outcome of diffuse large B-cell lymphoma in British
Columbia. J Clin Oncol. 23:5027–5033. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Qian L, Zhang Z, Shen J and Liu Y: Primary
bone marrow B-cell non-Hodgkin's lymphoma successfully treated with
R-CHOP. West Indian Med J. 62:89–91. 2013.PubMed/NCBI
|
|
43
|
Shivakumar L and Armitage JO: Bcl-2 gene
expression as a predictor of outcome in diffuse large B-cell
lymphoma. Clin Lymphoma Myeloma. 6:455–457. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Mounier N, Briere J, Gisselbrecht C, et
al: Groupe d'Etude des Lymphomes de l'Adulte: Estimating the impact
of rituximab on bcl-2-associated resistance to CHOP in elderly
patients with diffuse large B-cell lymphoma. Haematologica.
91:715–716. 2006.PubMed/NCBI
|
|
45
|
Winter JN, Weller EA, Horning SJ, et al:
Prognostic significance of Bcl-6 protein expression in DLBCL
treated with CHOP or R-CHOP: a prospective correlative study.
Blood. 107:4207–4213. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Nyman H, Adde M, Karjalainen-Lindsberg ML,
et al: Prognostic impact of immunohistochemically defined germinal
center phenotype in diffuse large B-cell lymphoma patients treated
with immunochemotherapy. Blood. 109:4930–4935. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Rigacci L, Scoccianti G, Puccini B, et al:
Primary lymphoma of bone: single center experience. Drugs Cell Ther
Hematol. 2:147–156. 2013. View Article : Google Scholar
|
|
48
|
Ostrowski ML, Unni KK, Banks PM, et al:
Malignant lymphoma of bone. Cancer. 58:2646–2655. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Fidias P, Spiro I, Sobczak ML, et al:
Long-term results of combined modality therapy in primary bone
lymphomas. Int J Radiat Oncol Biol Phys. 45:1213–1218. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Horsman JM, Thomas J, Hough R and Hancock
BW: Primary bone lymphoma: a retrospective analysis. Int J Oncol.
28:1571–1575. 2006.PubMed/NCBI
|
|
51
|
Jawad MU, Schneiderbauer MM, Min ES, et
al: Primary lymphoma of bone in adult patients. Cancer.
116:871–879. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Stein ME, Kuten A, Gez E, et al: Primary
lymphoma of bone - a retrospective study. Experience at the
Northern Israel Oncology Center (1979–2000). Oncology. 64:322–327.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Govi S, Christie D, Messina C, et al:
International Extranodal Lymphoma Study Group (I.E.L.S.G.): The
clinical features, management and prognostic effects of
pathological fractures in a multicenter series of 373 patients with
diffuse large B-cell lymphoma of the bone. Ann Oncol. 25:176–181.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Kim SY, Shin DY, Lee SS, et al: Clinical
characteristics and outcomes of primary bone lymphoma in Korea.
Korean J Hematol. 47:213–218. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Matikas A, Briasoulis A, Tzannou I, et al:
Primary bone lymphoma: a retrospective analysis of 22 patients
treated in a single tertiary center. Acta Haematol. 130:291–296.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Maruyama D, Watanabe T, Beppu Y, et al:
Primary bone lymphoma: a new and detailed characterization of 28
patients in a single-institution study. Jpn J Clin Oncol.
37:216–223. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Liu YC, Gau JP, Yu YB, et al: Prognostic
factors and treatment efficacy in patients with primary diffuse
large B-cell lymphoma of the bone: single institute experience over
11 years. Intern Med. 53:95–101. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Marshall DT, Amdur RJ, Scarborough MT, et
al: Stage IE primary non-Hodgkin's lymphoma of bone. Clin Orthop
Relat Marshall Res. 405:216–222. 2002. View Article : Google Scholar
|
|
59
|
Christie D, Dear K, Le T, et al: Limited
chemotherapy and shrinking field radiotherapy for osteolymphoma
(primary bone lymphoma): results from the trans-Tasman Radiation
Oncology Group 99.04 and Australasian Leukaemia and Lymphoma Group
LY02 prospective trial. Int J Radiat Oncol Biol Phys. 80:1164–1170.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Lowry L, Smith P, Qian W, et al: Reduced
dose radiotherapy for local control in non-Hodgkin lymphoma: a
randomised phase III trial. Radiother Oncol. 100:86–92. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Scoccianti G, Rigacci L, Puccini B, et al:
Primary lymphoma of bone: outcome and role of surgery. Int Orthop.
37:2437–2442. 2013. View Article : Google Scholar : PubMed/NCBI
|