Introduction
Hepatocellular carcinoma (HCC) is one of the most
common and lethal cancers in the human population, ranked the third
most common cause of cancer-associated mortality worldwide,
particularly in Africa and Asia (1).
Although persistent viral infections and persistent exposure to
hepatotoxic agents play a role in HCC neoplastic transformation
(1), the underlying mechanism
controlling the development and progression of HCC is largely
unclear.
Recently, c-Jun N-terminal protein kinase (JNK) has
been reported to be involved in regulating liver tumorigenesis. JNK
belongs to the mitogen-activated protein kinase (MAPK) superfamily,
which also includes extracellular signal-regulated kinase (ERK) and
the p38 family of kinases (2–5). The activation of JNK is mediated by
sequential protein phosphorylation through a MAPK module. MAPK
kinase (MKK) 7 and MKK4 play a non-redundant role in the dual
phosphorylation of JNK at Thr183 and Tyr185, which is required for
JNK activity (2–5). Once activated, JNK phosphorylates and
activates c-Jun, a key component of the transcription factor
activator protein-1 (AP-1) (2–5). Elevated
levels of JNK activity have been frequently observed in HCC and
have been demonstrated to contribute to HCC growth by promoting
cell proliferation and resistance to tumor necrosis factor-related
apoptosis inducing ligand (TRAIL)-mediated apoptosis (2–5).
Receptor for activated C kinase 1 (RACK1), coded for
by the GNB2L1 gene, is a scaffold protein with a
propeller-like structure of seven WD40 repeats (6–10).
Numerous studies have suggested that RACK1 plays a pivotal role in
the coordination of cell growth, migration and differentiation
during tumorigenesis (6–10). It has been demonstrated that RACK1 is
up-regulated in HCC and that overexpressed RACK1 augments JNK
activity, thereby promoting HCC growth by directly binding to MKK7
and enhancing MKK7 activity (11).
It has been reported that there is an AP-1 site in
the promoter region of the GNB2L1 gene (12). In addition, AP-1 has been revealed to
mediate RACK1 overexpression in melanoma cells (13). Since enhanced JNK activity can lead to
elevated AP-1 activity in various cell contexts (14), it is important to investigate the
association between RACK1 and the JNK pathway in HCC SMMC-7721
cells. The aim of the present study was to determine whether JNK
activity regulates RACK1 expression and whether RACK1 regulates JNK
activity in HCC SMMC-7721 cells.
Materials and methods
Cell culture and transduction
HCC SMMC-7721 cells were purchased from the Shanghai
Institutes for Biological Sciences (Shanghai, China) and were
cultured in Dulbecco's modified Eagle's medium supplemented with
10% fetal bovine serum, 100 units/ml penicillin and 100 µg/ml
streptomycin, and were maintained at 37°C in a 5% CO2
atmosphere. Lentivirus-based RACK1 short hairpin (sh)RNA,
5′-GGATGAGACCAACTATGGA-3′, JNK shRNA, 5′-AAAGAAUGUCCUACCUUCU-3′,
and control lentivirus were obtained from Shanghai GeneChem Co.,
Ltd. (Shanghai, China). Transduction was performed using
lentivirus, at a multiplicity of infection of 10.
Immunoblotting analysis
The SMMC-7721 cells were washed twice with ice-cold
phosphate-buffered saline (PBS) and were then lysed using 20 mM
Tris/HCl (pH 7.6), 250 mM NaCl, 3 mM EDTA, 3 mM EGTA, 0.5% NP40, 1
mM dithiothreitol, 5 mM NaF, 2 mM Na3VO4 and
0.2 µM aprotinin. The whole cell extract was clarified at 10,000 ×
g for 15 min at 4°C. The recovered protein was quantified using a
Bradford protein assay. Equal quantities of proteins were resolved
by sodium dodecy1 sulfate-polyacrylamide gel electrophoresis
(SDS-PAGE) and the proteins were then transferred to Hybond-P
polyvinylidene difluoride (PVDF) membranes (GE Healthcare Life
Sciences, Chalfont, UK). The membranes were initially incubated
with primary antibody over night at 4°C, and then with horseradish
peroxidase-conjugated polyclonal goat anti-rabbit or anti-mouse
secondary antibodies (cat. no. ZB2301 and ZB2305, respectively;
1:5,000; Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd.,
Beijing, China) for 1 h at room temperature. Bound antibody was
detected using an electrochemiluminescence kit (Amersham, Chalfont,
UK) and Kodak X-ray film (Rochester, NY, USA). Rabbit anti-human
polyclonal antibodies against MKK7 (cat. no. 4172; 1:1,000),
phosphorylated MKK7 (P-MKK7; cat. no. 4171; 1:1,000), and
phosphorylated JNK (P-JNK; cat. no. 9251; 1:1,000) were purchased
from Cell Signaling Technology, Inc. (Danvers, MA, USA). Monoclonal
mouse anti-human antibodies against RACK1 (cat. no 610171; 1:5,000)
and JNK (cat. no. 612541; 1:1,000) were obtained from BD
Biosciences (Franklin Lakes, NJ, USA). Monoclonal mouse anti-human
antibody against β-actin (cat. no. sc-8432; 1:5,000) was obtained
from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). All the
chemical inhibitors were purchased from Calbiochem (Billerica, MA,
USA).
Soft-agar assays
Agar (1.2%) was mixed with 2X Dulbecco's modified
Eagle's medium at a ratio of 1:1 to produce a 0.6% agar growth
medium solution. Next, 1.5 ml of the 0.6% growth medium mixture was
pipetted into each well of a six-well cell culture cluster (Corning
Life Sciences, Corning, New York, NY, USA), while avoiding bubble
formation. The mixture was then evenly spread by slowly rotating
the plate. The 0.6% agar growth medium layer was left to harden for
20 min at 4°C and the cells were then seeded at a density of
1×103 cells/ml in 0.3% agar diluted with 2X Dulbecco's
modified Eagle's medium, at a ratio of 1:1. Cell suspension (1 ml)
was plated onto the 0.6% agar growth medium plate and cultured at
37°C in a 5% CO2 atmosphere for 14 days. The colony
numbers were counted using a microscope (Nikon Eclipse TS100; Nikon
Corporation, Tokyo, Japan), based on colonies >400 µm in
diameter.
Apoptosis analysis
The cells were adjusted to a density of
2×105 cells/ml and were added to 24-well plates, with
0.5 ml in each well. TRAIL was purchased from Sigma-Aldrich (St.
Louis, MO, USA) and was used to treat the SMMC-7721 cells according
to the manufacturer's instructions. The cells were washed with PBS
twice and stained with Annexin V-phycoerythrin and
7-aminoactinomycin D (Nanjing KeyGen Biotech, Nanjing, Jiangsu,
China) for 15 min at room temperature in the dark. The level of
apoptosis was determined by measuring the fluorescence of the cells
using a flow cytometer (BD Biosciences).
Statistical analysis
Statistically significant differences between groups
were identified using a two-tailed Student's t-test. P<0.05 was
considered to indicate a statistically significant result.
Results
Enhancement of MKK7/JNK activity by
RACK1 in SMMC-7721 cells
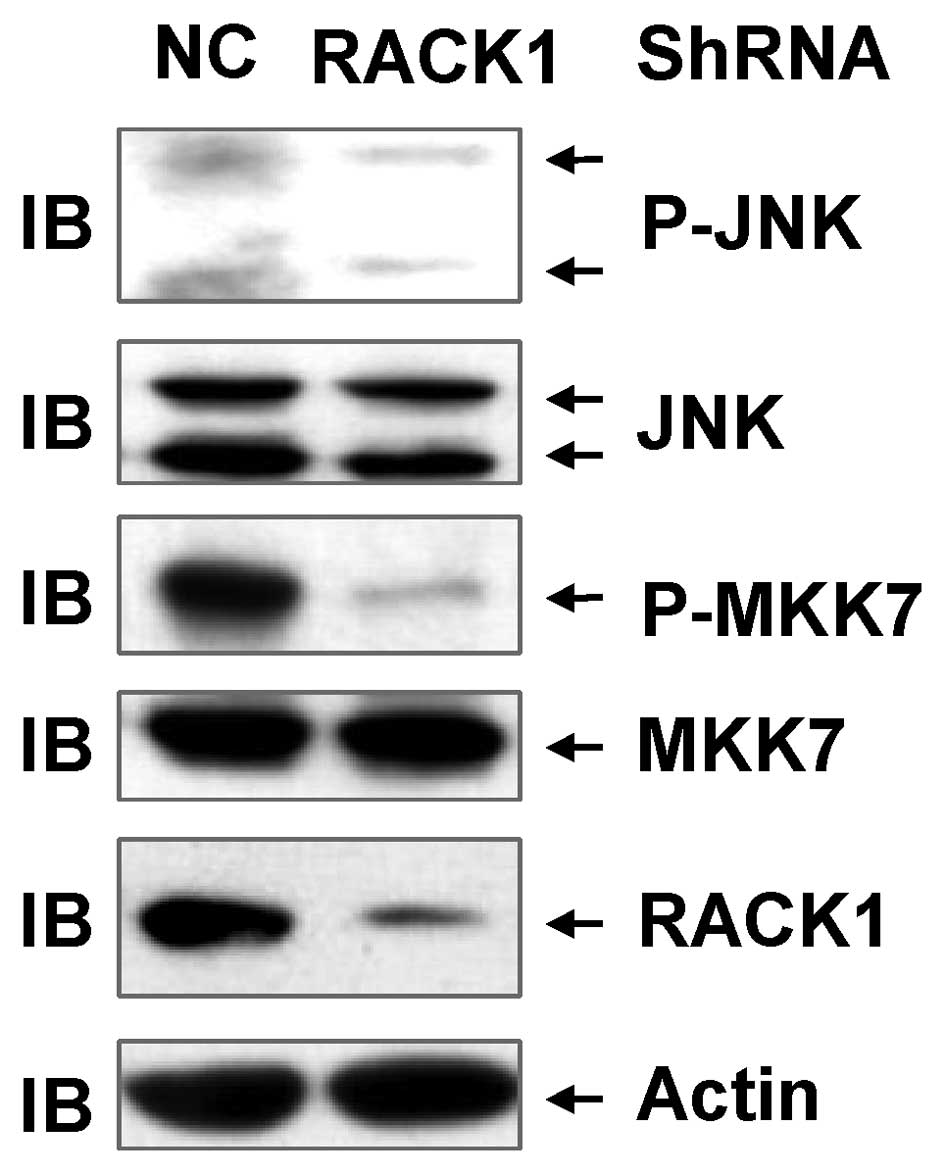
To investigate the correlation between RACK1 and the
JNK pathway in SMMC-7721 cells, endogenous RACK1 expression was
transiently silenced using lentivirus-based RACK1 shRNA. The
expression of RACK1 protein was detected by immunoblotting analysis
with an antibody against RACK1, whereas JNK activity and MKK7
activity was measured by immunoblotting analysis of P-JNK and
P-MKK7, respectively. The present results demonstrate that
transduction of SMMC-7721 cells with the lentivirus carrying RACK1
shRNA compared with the control lentivirus, significantly decreased
RACK1 expression (Fig. 1). Silencing
of endogenous RACK1 expression by RACK1 shRNA in SMMC-7721 cells
significantly suppressed the basal level of P-JNK (Fig. 1). In addition, the present data also
revealed that a decreased level of P-JNK in RACK1-knockdown cells
was associated with a reduced P-MKK7 level (Fig. 1). These data collectively indicate
that RACK1 contributes to the enhancement of MKK7/JNK activity in
SMMC-7721 cells.
JNK activity plays no role in RACK1
overexpression in SMMC-7721 cells
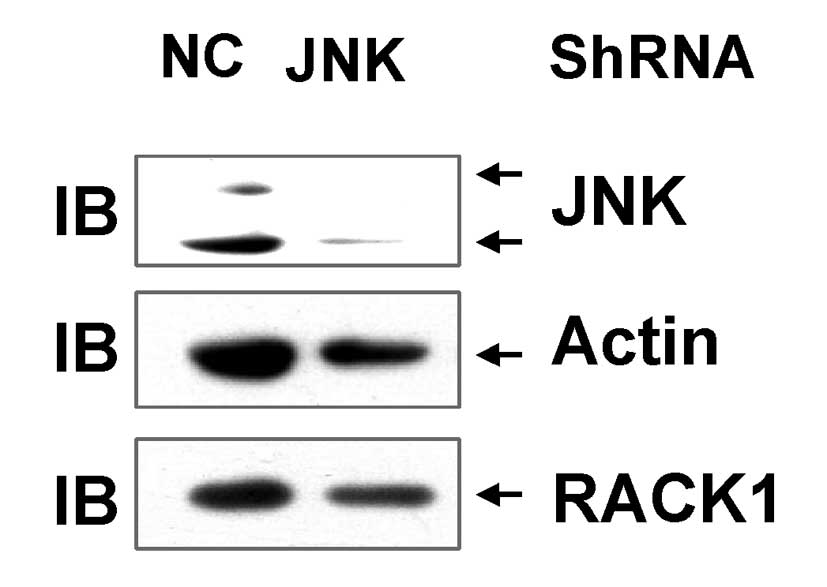
Endogenous JNK expression was transiently silenced
using lentivirus-based JNK shRNA. The present results demonstrate
that transduction of SMMC-7721 cells with the JNK shRNA-carrying
lentivirus significantly decreased the expression of the p54 JNK
and p46 JNK proteins compared to transfection with the control
lentivirus (Fig. 2). However, the
protein level of RACK1 was not reduced when JNK was knocked down
(Fig. 2). These data suggest that JNK
activity does not contribute to RACK1 overexpression in SMMC-7721
cells.
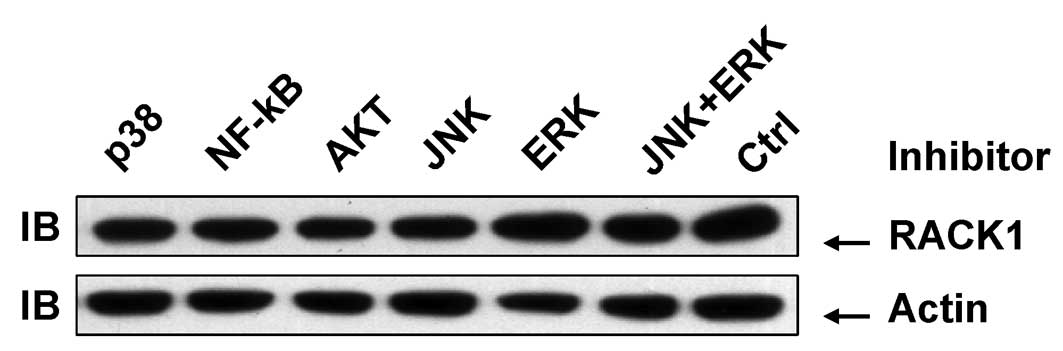
As AP-1 activity is affected by other MAPK
superfamily members in addition to JNK (14), chemically synthesized inhibitors were
used to block the activity of ERK, p38 and JNK. Akt and NF-κB
inhibitors were also included. Treatment of SMMC-7721 cells with
these inhibitors for 48 h exhibited no significant effects on the
RACK protein level (Fig. 3). These
data further confirm that JNK activity plays no role in RACK1
overexpression in SMMC-7721 cells. However, these data also suggest
that other factors, but not AP-1, mediate RACK1 overexpression in
SMMC-7721 cells.
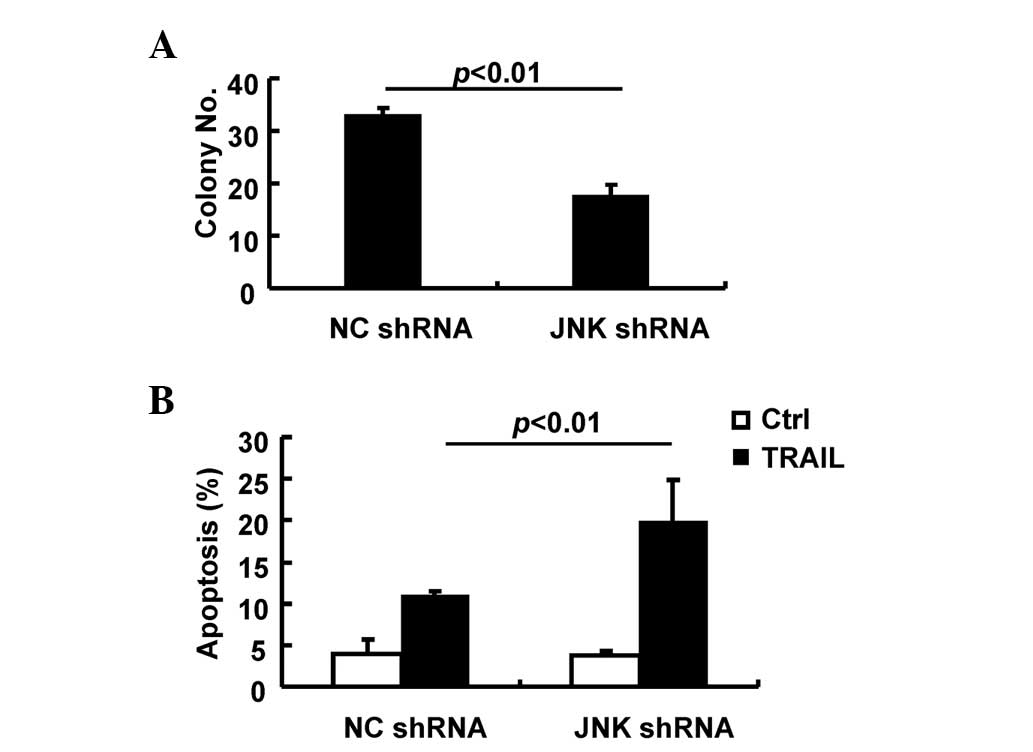
JNK activity contributes to the
oncogenic growth of SMMC-7721 cells
It is of importance to clarify the roles of JNK
activity in the tumorigenic growth of SMMC-7721 cells. In this
scenario, anchorage-independent growth and apoptosis in response to
TRAIL were analyzed in SMMC-7721 cells transduced with the control
lentivirus or the lentivirus carrying JNK shRNA. The present data
reveal that transduction of SMMC-7721 cells with the lentivirus
carrying JNK shRNA significantly inhibited the
anchorage-independent growth compared with the control lentivirus
(Fig. 4A) and led to an increased
proportion of apoptotic cells in response to TRAIL (Fig. 4B). Thus, JNK activity makes an
essential contribution to the oncogenic growth of SMMC-7721
cells.
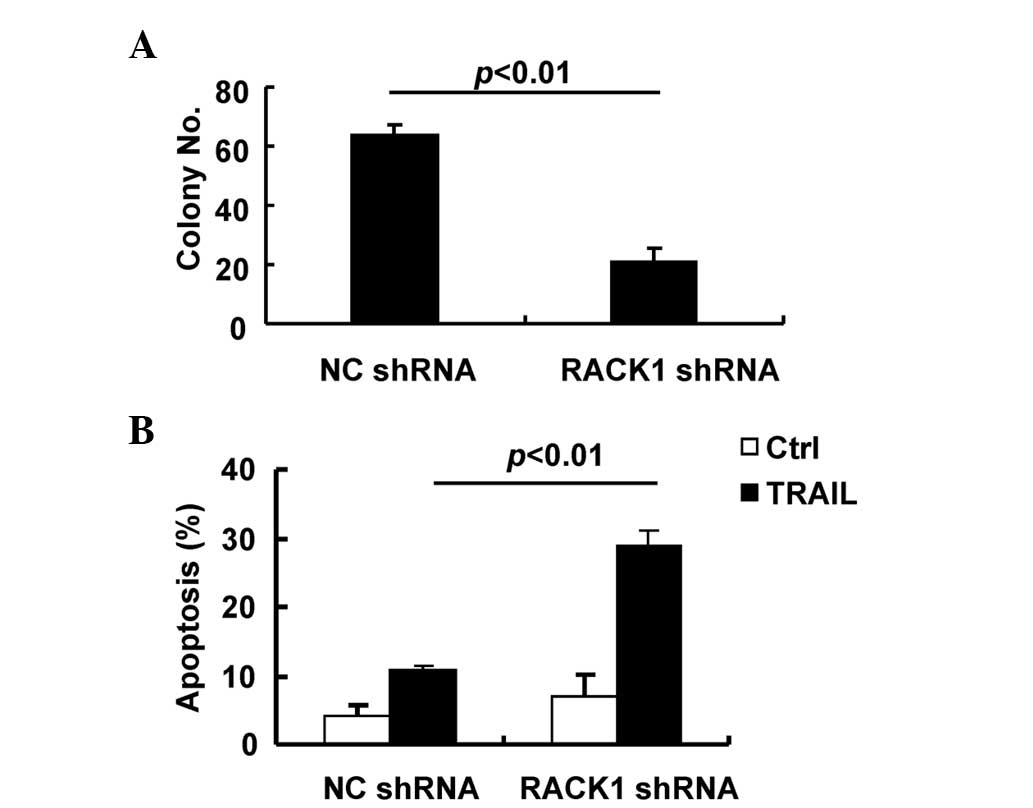
RACK1 facilitates the oncogenic growth
of SMMC-7721 cells
The previous data suggested that JNK activity
contributes to the oncogenic growth of SMMC-7721 cells by promoting
cell proliferation and resistance to TRAIL-mediated apoptosis.
Since RACK1 enhances MKK7/JNK activity in this cell line, it is
important to investigate how RACK1 may affect tumorigenic growth.
Therefore, anchorage-independent growth and apoptosis in response
to TRAIL were analyzed in SMMC-7721 cells transduced with the
control lentivirus or the lentivirus carrying RACK1 shRNA. As
expected, transduction of SMMC-7721 cells with the lentivirus
carrying RACK1 shRNA significantly inhibited anchorage-independent
growth compared with the control lentivirus (Fig. 5A) and led to increased apoptosis in
response to TRAIL (Fig. 5B). Thus,
RACK1 facilitates the oncogenic growth of SMMC-7721 cells,
partially due to the enhancement of JNK activity.
Discussion
It has been reported that RACK1 promotes HCC growth
by enhancing MKK7 activity (11). The
correlation between the levels of RACK1 protein and the activity of
the JNK pathway was observed in clinical HCC tissues and various
HCC cell lines (11). However, it
should be noted that SMMC-7721, BEL-7402 and BEL-7404 cells exhibit
significantly elevated RACK1 expression, but the levels of P-JNK in
these cells are only weakly up-regulated (11). This issue promoted an examination of
the association between RACK1 and JNK in SMMC-7721 cells. The
present data provided additional support for a pivotal role of
RACK1 in mediating enhanced JNK activity and in HCC growth.
The present data revealed that the JNK activity,
although weakly detected, is essential to the oncogenic growth of
SMMC-7721 cells. Consistent with the present observations, a recent
study indicates that eupolyphaga sinensis walker extract (ESWE)
demonstrated significant inhibition on the growth of SMMC-7721
cells, which was associated with decreased JNK1 protein expression
(15). Silencing of endogenous RACK1
expression leads to reduced JNK activity and impaired oncogenic
growth of SMMC-7721 cells. Thus, RACK1 promotes the tumorigenic
growth of SMMC-7721 cells through an at least partial enhancement
of JNK activity, although the role of RACK1 on the activity of the
JNK pathway may be compromised by other genetic mutations in
SMMC-7721 cells. Further explorations are required to clarify the
genetic mutations that compromise JNK activity and the consequent
effects in HCC development and progression.
The molecular mechanisms underlying the elevated
RACK1 expression are undefined. It has been reported that there is
an AP-1 site in the promoter region of the GNB2L1 gene
(12). Additionally, AP-1 has been
revealed to mediate RACK1 overexpression in melanoma cells
(13). Since enhanced JNK activity
can lead to elevated AP-1 activity in various cell contexts
(14), the present study detected
RACK1 protein levels subsequent to knockdown of JNK or inhibition
of JNK activity by a chemical inhibitor. The data revealed that
expression of RACK1 is independent of the activation of JNK
signaling. As p38 and ERK also contribute to AP-1 activity
(14), and it has been reported that
a NF-κB site in the promoter region of the GNB2L1 gene
mediates up-regulation of RACK1 (16,17),
chemical inhibitors of p38, ERK, NF-κB and Akt were also
investigated in the present study. Furthermore, neither the
blockade of JNK signaling nor a blockade of p38, ERK, AKT or NF-κB
signaling for 48 h demonstrated any effect on the RACK1 protein
level. Therefore, there may be alternative mechanisms underlying
RACK1 upregulation.
References
|
1
|
Llovet JM, Burroughs A and Bruix J:
Hepatocellular carcinoma. Lancet. 362:1907–1917. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hui L, Zatloukal K, Scheuch H, et al:
Proliferation of human HCC cells and chemically induced mouse liver
cancers requires JNK1-dependent p21 downregulation. J Clin Invest.
118:3943–3953. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sakurai T, Maeda S, Chang L and Karin M:
Loss of hepatic NF-kappa B activity enhances chemical
hepatocarcinogenesis through sustained c-Jun N-terminal kinase 1
activation. Proc Natl Acad Sci USA. 103:10544–10551. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mucha SR, Rizzani A, Gerbes AL, et al: JNK
inhibition sensitises hepatocellular carcinoma cells but not normal
hepatocytes to the TNF-related apoptosis-inducing ligand. Gut.
58:688–698. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kuntzen C, Sonuc N, De Toni EN, et al:
Inhibition of c-Jun-N-terminal-kinase sensitizes tumor cells to
CD95-induced apoptosis and induces G2/M cell cycle arrest. Cancer
Res. 65:6780–6788. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hermanto U, Zong CS, Li W and Wang LH:
RACK1, an insulin-like growth factor I (IGF-I) receptor-interacting
protein, modulates IGF-I-dependent integrin signaling and promotes
cell spreading and contact with extracellular matrix. Mol Cell
Biol. 22:2345–2365. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kadrmas JL, Smith MA, Pronovost SM and
Beckerle MC: Characterization of RACK1 function in Drosophila
development. Dev Dyn. 236:2207–2215. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hoffmann B, Wanke C, Lapaglia SK and Braus
GH: c-Jun and RACK1 homologues regulate a control point for sexual
development in Aspergillus nidulans. Mol Microbiol. 37:28–41. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
McLeod M, Shor B, Caporaso A, et al: Cpc2,
a fission yeast homologue of mammalian RACK1 protein, interacts
with Ran1 (Pat1) kinase to regulate cell cycle progression and
meiotic development. Mol Cell Biol. 20:4016–4027. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rothberg KG, Burdette DL, Pfannstiel J, et
al: The RACK1 homologue from Trypanosoma brucei is required for the
onset and progression of cytokinesis. J Biol Chem. 281:9781–9790.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Guo Y, Wang W, Wang J, et al: Receptor for
activated C kinase 1 promotes hepatocellular carcinoma growth by
enhancing mitogen-activated protein kinase kinase 7 activity.
Hepatology. 57:140–151. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chou YC, Chou CC, Chen YK, et al:
Structure and genomic organization of porcine RACK1 gene. Biochim
Biophys Acta. 1489:315–322. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lopez-Bergami P, Huang C, Goydos JS, et
al: Rewired ERK-JNK signaling pathways in melanoma. Cancer Cell.
11:447–460. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Karin M: The regulation of AP-1 activity
by mitogen-activated protein kinases. J Biol Chem. 270:16483–16486.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang Y, Zhan Y, Zhang D, et al:
Eupolyphaga sinensis walker displays inhibition on hepatocellular
carcinoma through regulating cell growth and metastasis signaling.
Sci Rep. 4:55182014.PubMed/NCBI
|
|
16
|
Choi DS, Young H, McMahon T, et al: The
mouse RACK1 gene is regulated by nuclear factor-kappa B and
contributes to cell survival. Mol Pharmacol. 64:1541–1548. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jia D, Duan F, Peng P, et al:
Up-regulation of RACK1 by TGF-β1 promotes hepatic fibrosis in mice.
PLoS One. 8:e601152013. View Article : Google Scholar : PubMed/NCBI
|