Introduction
Solitary fibrous tumors (SFTs) were first described
in 1931, as a neoplasm usually originating from the pleura
(1). However, over the past 10 years,
increasing numbers of extrapleural SFTs have been reported,
including those of the prostate. Prostate SFT is relatively
uncommon, with <20 cases reported in the literature worldwide
(2,3).
According to these reports, 13 cases of prostate SFT were
identified by prostate needle biopsy or transurethral resection
(TUR) of the prostate. The majority of cases presented with urinary
tract symptoms (4–6), and were treated by complete tumor
resection [cystoprostatectomy (7),
radical prostatectomy (8,9), pelvic exenteration or pelvic tumor
resection (10)] or enucleation and
TUR (11). According to
immunohistochemical analysis of the tumors, all of the cases except
one were immunoreactive for CD34, and all of the cases were
positive for B cell lymphoma-2 (bcl-2), CD99, β-catenin and c-kit
(12–14). In addition, three SFTs demonstrated
≥10% p53 immunoreactivity, and three cases revealed Ki-67 rates of
≥20% (12).
There have also been several case reports of SFTs in
the prostate. For example, a 37-year-old male presented with
irritative lower urinary tract symptoms, as a result of a mass in
the perineum which displaced and distorted the bulbar urethra.
Following enucleation of the tumor, the patient's condition
gradually returned to normal during the 2-year follow-up period.
The tumor was ‘patternless’ with a combination of alternating
hyper- and hypocellular areas, and the tumor cells were
spindle-shaped with bland nuclei, having dispersed chromatin and
inconspicuous nucleoli. Furthermore, the cells were markedly
immunoreactive for CD34 and vimentin, but negative for cytokeratin
AE1/AE3, smooth muscle actin, S-100 protein and desmin (15). Similarly, a 60-year-old male
presenting with lower urinary tract symptoms was found to have an
enlarged and hard left prostate lobe. Based on the results of
histopathological and immunohistochemical analyses, including the
arrangement of the tumor cells in an irregular pattern, the
identification of short-spindled cells possessing meagre amounts of
eosinophilic cytoplasm, the presence of bland nuclei with uniformly
distributed chromatin and inconspicuous nucleoli, immunoreactivity
to CD34 and bcl-2 but negative immunoreactivity to CD117,
anaplastic lymphoma kinase, smooth muscle actin and progesterone
receptors, a diagnosis of SFT was reached. Following treatment with
nerve-sparing retropubic radical prostatectomy, the mass was well
delineated with no apparent invasion of the bladder neck or pelvic
wall identified (2). Finally, two
males aged 66 and 69 years-old, presenting with urinary tract
symptoms, were diagnosed with SFT, by transrectal needle biopsy and
TUR of the prostate, in 2011. The tumors were excised with a low
anterior resection. The two tumors were well-circumscribed,
although a small quantity of infiltration into the prostate glands
was identified. The tumors consisted of storiform bundles of bland
spindle cells, which stained strongly for CD34 and vimentin, but
were negative for the expression of muscle markers. Following
therapy, no relapses have been reported in either of the cases,
although the follow-up periods were short (16). In the present study, one significant
case of SFT is reported, aimed at promoting understanding of the
diagnosis and treatment of prostate SFT. Written informed consent
was obtained from the patient.
Case report
A 46-year-old male with irritative lower urinary
tract symptoms and increasing dysuria was found to have an
enlarged, smooth and tenacious prostate on digital rectal
examination. Serum prostate-specific antigen levels were within the
normal limits (0.68 ng/ml; normal range, 0–4 ng/ml) (17); however, the patient's maximum urinary
flow rate was reduced to 10 ml/s. The prostate was measured at
64×56×57 mm, combined with multiple cystoliths (the largest of
which was ~6×6 mm), using images from a transabdominal ultrasound.
In addition, the International Prostate Symptom Score was 13
(overall score range, 0–35), indicating a moderate grade of benign
prostatic hyperplasia (moderate score range, 8–19) (18). The present case was initially
diagnosed as benign prostatic hyperplasia, complicated with bladder
calculus. The patient received cystoscopy and lithocystotomy per
urethra, which was combined with doxazosin (oral dose, 4 mg/day)
and finasteride (oral dose, 5 mg/day) treatment following surgery.
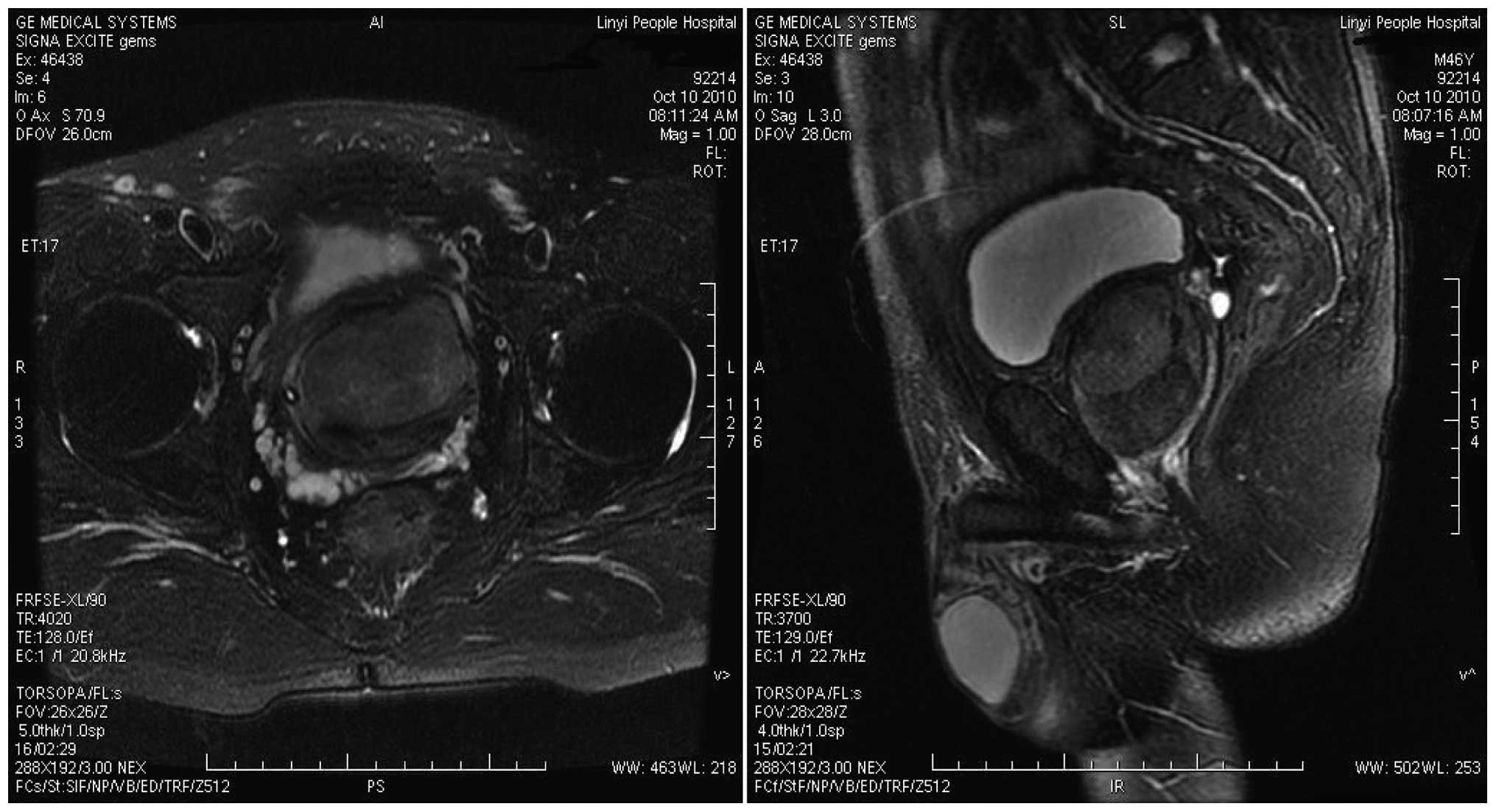
However, the symptoms were not improved. Subsequently, a large
circumscribed tumor within the prostate, which appeared to be
focally intimate with the bladder neck and partially invading the
urinary bladder was identified on a magnetic resonance imaging
(MRI) scan (Fig. 1). Approximately
two weeks later, TUR of the prostate (TURP) was performed and ~80 g
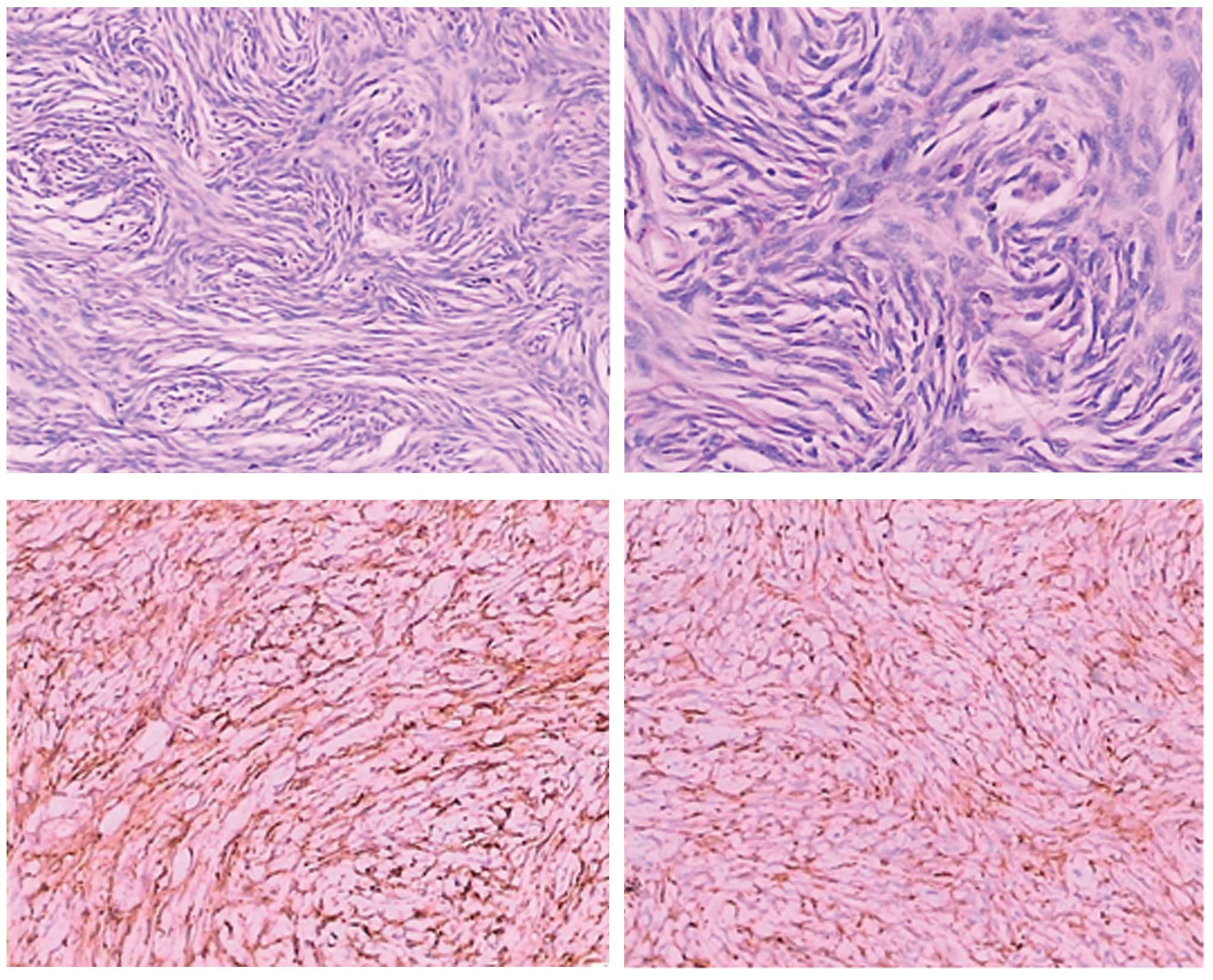
tissue was removed. Histopathological and immunohistochemical
analyses were performed and it was identified that the neoplastic
cells were spindle cells arranged in storiform. In addition, CD34
and bcl-2 were highly expressed in the tumor cells (Fig. 2). However, the cells were negative for
CD117 (c-kit), S-100, discovered on GIST-1 (Dog-1), Ki-67 and CD68.
Approximately 20 days subsequent to TURP, the patient exhibited a
recurrence of aggravating dysuria, and a locoregional recurrence of
tumor was identified by ultrasound and MRI. Subsequently, a
nerve-sparing retropubic radical prostatectomy was performed, and
the mass appeared to be well ablated, with no invasion of the
bladder neck or pelvic wall identified. Postoperatively, the
patient exhibited normal erectile and voiding function, with no
locoregional recurrence identified in follow-ups over the
subsequent 18 months. The final diagnosis of this lesion was
borderline prostatic SFT.
Discussion
As a result of the lack of typical clinical
presentations, ultrasound, MRI or computerised tomography (CT) are
always required for the diagnosis of SFT lesions. However, SFTs
cannot be definitively differentiated from other tumors by imaging
alone. Specimens of prostatic SFT are frequently isolated from fine
needle aspiration biopsies, TUR or open surgery. The tumors are
characterized histologically by uniform spindle-shaped cells, which
are arranged in storiform, herringbone or with a ‘patternless’
growth pattern of alternating hyper- and hypocellular areas, or a
combination of these patterns (15).
Furthermore, tumor cells are invariably positive for CD34, CD99 and
bcl-2, but negative for S-100 protein, actin, desmin and epithelial
markers, which therefore represent valuable diagnostic supports
(2,15). As previously reported, the diagnosis
of SFTs is conclusively based on the histopathological and
immunohistochemical characteristics of the tumor (7,8,19). The present case did not significantly
differ from those previously reported. The neoplastic cells were
identified to be spindle cells, which were arranged in storiform.
In addition, CD34 and bcl-2 were highly expressed, but the tumor
was negative for CD117 (c-kit), S-100, Dog-1, Ki-67 and CD68.
Therefore, the case was ultimately diagnosed as SFT.
Therapeutic strategies for the treatment of
prostatic SFTs, include TUR, enucleation and complete tumor
resection (cystoprostatectomy), radical prostatectomy, pelvic
exenteration and pelvic tumor resection. Due to the fact that it is
difficult to predict the clinical behaviour of SFTs, undergoing
complete tumor resection currently has the greatest influence on
prognosis, emphasizing the importance of resection margins
(12).
In the present case, in view of the large size and
rapid growth of the tumor following TURP, retropubic prostatectomy
was performed, which was concerned with the preservation of sexual
function. Following surgery, the patient exhibited normal erectile
and voiding function, with no locoregional recurrence within the 18
month follow-up period.
References
|
1
|
Klemperer P and Rabin CB: Primary
neoplasms of the pleura. A report of the five cases. Arch Pathol.
11:385–412. 1931.
|
|
2
|
Galosi AB, Mazzucchelli R, Scarpelli M, et
al: Solitary fibrous tumour of the prostate identified on needle
biopsy. Eur Urol. 56:564–567. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Parada Domínguez D and Peña González K:
Morente Laguna V and Riu Ferrando F: Solitary fibrous tumor of the
prostate. Actas Urol Esp. 34:119–121. 2010.(In Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Herawi M and Epstein JI: Solitary fibrous
tumor on needle biopsy and transurethral resection of the prostate:
A clinicopathologic study of 13 cases. Am J Surg Pathol.
31:870–876. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen KT: Hemangiopericytoma of the
prostate. J Surg Oncol. 35:42–43. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Grasso M, Blanco S, Franzoso F, Lania C,
Di Bella C and Crippa S: Solitary fibrous tumor of the prostate. J
Urology. 168:11002002. View Article : Google Scholar
|
|
7
|
Kelly PM and Baxter GM: Solitary fibrous
tumour of the prostate. Brit J Radiol. 71:1086–1088. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mentzel T, Bainbridge TC and Katenkamp D:
Solitary fibrous tumour: Clinicopathological, immunohistochemical,
and ultrastructural analysis of 12 cases arising in soft tissues,
nasal cavity and nasopharynx, urinary bladder and prostate.
Virchows Arch. 430:445–453. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Noguchi M, Hirabayashi Y, Kato S and Noda
S: Solitary fibrous tumor arising from the prostatic capsule. J
Urol. 168:1490–1491. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pins MR, Campbell SC, Laskin WB,
Steinbronn K and Dalton DP: Solitary fibrous tumor of the prostate:
A report of 2 cases and review of the literature. Arch Pathol Lab
Med. 125:274–277. 2001.PubMed/NCBI
|
|
11
|
Reyes JW, Shinozuka H, Garry P and Putong
PB: A light and electron microscopic study of a hemangiopericytoma
of the prostate with local extension. Cancer. 40:1122–1126. 1977.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sekine H, Ohya K, Kojima S and Mizuguchi
K: Solitary fibrous tumor of the prostate. Int J Urol. 8:137–138.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Takeshima Y, Yoneda K, Sands N and Inai K:
Solitary fibrous tumor of the prostate. Pathol Int. 47:713–717.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Westra WH, Gerald WL and Rosai J: Solitary
fibrous tumor. Consistent CD34 immunoreactivity and occurrence in
the orbit. Am J Surg Pathol. 18:992–998. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nair B, Nambiar A, Hattangadi SB, Sukumar
S and Saifuddin MS: Solitary fibrous tumour of prostate: Evaluation
and management of a rare tumour. Scand J Urol Nephrol. 41:442–444.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Talvitie H, Aström K, Larsson O, Ahlén J,
Bergh A and Egevad L: Solitary fibrous tumor of the prostate: A
report of two cases. Pathol Int. 61:536–538. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Park KK, Lee SH, Choi YD and Chung BH:
Optimal baseline prostate-specific antigen level to distinguish
risk of prostate cancer in healthy men between 40 and 69 years of
age. J Korean Med Sci. 27:40–45. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Roehrborn CG: Focus on lower urinary tract
symptoms: Nomenclature, diagnosis, and treatment options:
Highlights from the 5th international consultation on benign
prostatic hyperplasia June 25–27, 2000, Paris, France. Rev Urol.
3:139–145. 2001.PubMed/NCBI
|
|
19
|
Vodovnik A, Rogawski K and Bolton JF: A
case of malignant solitary fibrous tumor of the prostate. Pathol
Int. 55:807–808. 2005. View Article : Google Scholar : PubMed/NCBI
|