Introduction
Gastric cancer (GC) ranks the fifth most prevalent
type of cancer and the third leading cause of cancer-associated
mortality worldwide, accounting for 952,000 novel diagnoses (6.8%
incidence) and 723,000 mortalities in 2012 (1). GC in developing countries accounts for
>70% of worldwide total cases; currently China, Japan and Korea
account for 60% of total cases (2,3). The USA
has the lowest incidence of GC, comprising 1.3% (~22,000) and 1.9%
(~11,000) of all novel cancer cases and cancer-associated
mortalities, respectively, in 2014 (4).
Small non-coding RNAs, known as microRNAs (miRNA),
regulate gene expression at the post-transcriptional level by
binding to complementary messenger (m)RNA sequences, primarily in
the 3′-untranslated region (UTR) of the target. Subsequently,
target mRNA is degraded or its translation into protein is
inhibited (5,6). miRNAs have been implicated in numerous
biological processes, including cell proliferation, metabolism,
apoptosis, morphogenesis, developmental timing and stress-response
(7–9).
In relation to cancer, miRNAs have been reported to be
differentially expressed, acting as oncogenes as well as tumor
suppressors (10,11); in addition, numerous miRNA may have
the potential for use as diagnostic and prognostic markers in
cancer as well as possible therapeutic targets (12–17).
A limited number of previous studies have
investigated the role of miRNA (miR)-577 in cancer. One such study
reported that miR-577 regulated testis-specific gene 10 protein
expression, inactivated the p53 and retinoblastoma protein pathways
as well as modulated the G1/S phase transition, leading
to an increased rate of malignancy and progression of esophageal
squamous cell carcinomas (18). In GC
patients, miR-577 expression was reported to correlate with
chemosensitivity (19), possibly
through modulating cytochrome P450 protein expression (20). E2F3 is a member of the E2F family of
transcription factors and is important in the control of cell cycle
during tumor development including GC (21). Several miRNA have been demonstrated to
repress tumor growth by regulating E2F3 (22).
The present study aimed to investigate the
expression of miR-577 in GC tissues and cell lines in order to
provide evidence for miR-577 as a diagnostic and prognostic marker
of GC as well as a potential therapeutic target.
Materials and methods
Samples
The present study was approved by the Medical Ethics
Committee of Qiqihar Medical University (Qiqihar, China). None of
the patients in the study received chemotherapy or radiation
treatment prior to surgery. Written informed consent was obtained
from all patients. GC and adjacent normal tissues (≥5 cm from the
edge of the tumor) were collected by debulking surgery from 30
patients who were recruited into a clinical trial at the Third
Affiliated Hospital of Qiqihar Medical University between 2012 and
2013. Clinicopathologic information was obtained and two
pathologists independently determined diagnoses according to the
International Union Against Cancer (7th edition).
Cell lines
Human GC cell lines HGC-27, SGC-7901, MKN-28,
NCI-N87 and MKN-74 as well as the immortalized human gastric
epithelial mucosa cell line GES1 were purchased from the Cell Bank
of Type Culture Collection of Chinese Academy of Sciences
(Shanghai, China). All cell lines were cultured in Dulbecco's
modified Eagle's medium supplemented with 10% fetal bovine serum,
100 U/ml penicillin and 100 µg/ml streptomycin (all purchased from
Gibco Life Technologies, Grand Island, NY, USA). All cells were
cultured at 37°C in a 5% CO2 atmosphere.
RNA extraction and reverse
transcription quantitative polymerase chain reaction (RT-qPCR)
Total miRNA from cultured cells and surgical GC
tissues was extracted using TRIzol® reagent (Invitrogen Life
Technologies, Carlsbad, CA, USA) according to the manufacturer's
instructions. Complementary DNA was synthesized from 5 ng total RNA
using the TaqMan miRNA reverse transcription kit (Invitrogen Life
Technologies). Expression levels of miR-577 were quantified by qPCR
using the reverse transcription products, TaqMan 2 Universal PCR
Master Mix without UNG Amperase (Applied Biosystems Life
Technologies, Foster City, CA, USA), miRNA-specific TaqMan probes,
and primers (Applied Biosystems) on a 7500 Fast Real Time PCR
system (Applied Biosystems) with an initial denaturation at 95°C,
followed by 40 cycles at 95°C for 15 s and 60°C for 1 min. The
threshold cycle (CT) was then determined and defined as the
fractional cycle number at which the fluorescence detected passes a
fixed threshold. The Applied Biosystems 7500 Fast software (Applied
Biosystems) was used to analyze the CT values of different miRNAs
normalized to an endogenous control U6. The normalized values (dCT)
from tumorous tissue were then compared with its paired nontumorous
tissue, yielding miRNA differential expression profiles. Primers
were used as follows: miR-577 F 5′-ACA CTC CAG CTG GGT AGA TAA AAT
ATT GG-3′ and R 5′-CTC AAC TGG TGT CGT GGA GTC GGC AAT TCA GTT GAG
CAG GTA CC-3′; U6, 5′-CTC GCT TCG GCA GCA CA-3′, R 5-AAC GCT TCA
CGA ATT TGC GT-3′. miRNA mimics, small interfering (si)RNA and
plasmids. miR-577, anti-miR-577 and control miRNA encoding plasmids
were purchased from Shanghai GenePharma Co., Ltd (Shanghai, China).
E2F transcription factor 3 (E2F3)-small interfering (si)RNA and
scrambled control siRNA were purchased from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA). siRNA and miRNA transfection
were performed using Lipofectamine 2000 (Invitrogen Life
Technologies) according to the manufacturer's instructions. The
Renilla and firefly luciferase activities in cell lysates were
measured 48 h after transfection using the dual-luciferase reporter
assay system (Promega Corporation, Madison, WI, USA), and the
results were presented as the ratio of Renilla activity/firefly
activity in the lysate. Renilla activity was used to normalize the
relative luciferase values. Each transfection was performed in
triplicate.
Cell proliferation assay
Cell viability was measured using an MTS assay kit
(Promega Corp.), as described previously (23). Briefly, at 2 days post transfection,
2,000 cells/well were seeded into 96-well plates and following 24
h, the medium was replaced with fresh medium and the cells were
further cultured for 1, 2 or 3 days. Following culture, MTS (20 µl)
solution was added to each well and plates were incubated for 1 h
at 37°C. Absorbance (A) was recorded at 490 nm using a 3500
microplate reader (Bio-Rad Laboratories, Inc., Richmond, CA, USA).
Each individual experiment was performed in six replicates for
three independent times.
Cell cycle analysis
Cells (2×106/ml) were transfected with
miR-577, control miRNA or E2F3 siRNA and cultured for 2 days. Cells
were then collected, washed twice with phosphate-buffered saline
and fixed with ice-cold 70% ethanol, then incubated with RNase I (1
µg/ml; Sigma-Aldrich, St. Louis, MO, USA) at 37°C for 1 h. Samples
were analyzed using a flow cytometer (LSRII; BD Biosciences, San
Jose, CA, USA) following the addition of propidium iodide (20
µg/ml).
Protein isolation and western blot
analysis
Total proteins were extracted using
radioimmunopreciptiation assay lysis buffer with
proteinase/phosphatase inhibitors (Thermo Fisher Scientific,
Cambridge, MA, USA). Lysate was separated using 10% sodium dodecyl
sulfate polyacrylamide gel electrophoresis (Bio-Rad Laboratories,
Inc., Hercules, CA, USA), which were then blotted onto
polyvinylidene difluoride membranes (Millipore, Bedford, MA, USA).
The membrane was blocked in 5% non fat milk (Fisher Scientific Co.,
Fair Lawn, NJ, USA) for 1 h and subsequently incubated with either
rabbit polyclonal antibody against human E2F3 (sc-878; 1:500
dilution) or goat polyclonal antibody against human GAPDH
(sc-20357; 1:1,000 dilution) at 4°C overnight. The membrane was
washed 3 times and then treated with horseradish
peroxidase-conjugated mouse anti-rabbit or anti-goat second
antibodies (1:10,000; Santa Cruz Biotechnology, Inc.) for 1 h at
room temperature. The bands were detected using an enhanced
chemiluminescence kit (Thermo Fisher Scientific).
Statistical analysis
Values are expressed as the mean ± standard error of
the mean. Differences between groups were analyzed using a one way
analysis of variance followed by Bonferroni post-hoc analyses, as
appropriate. All data were analyzed using SPSS software, version
19.0 (IBM SPSS, Armonk, NY, USA). P<0.05 was considered to
indicate a statistically significant difference between values.
Results
miR-577 is downregulated in human GC
tissues and cell lines
In order to investigate the role of miR-577 in human
GC, miR-577 expression was analyzed in GC patient samples and
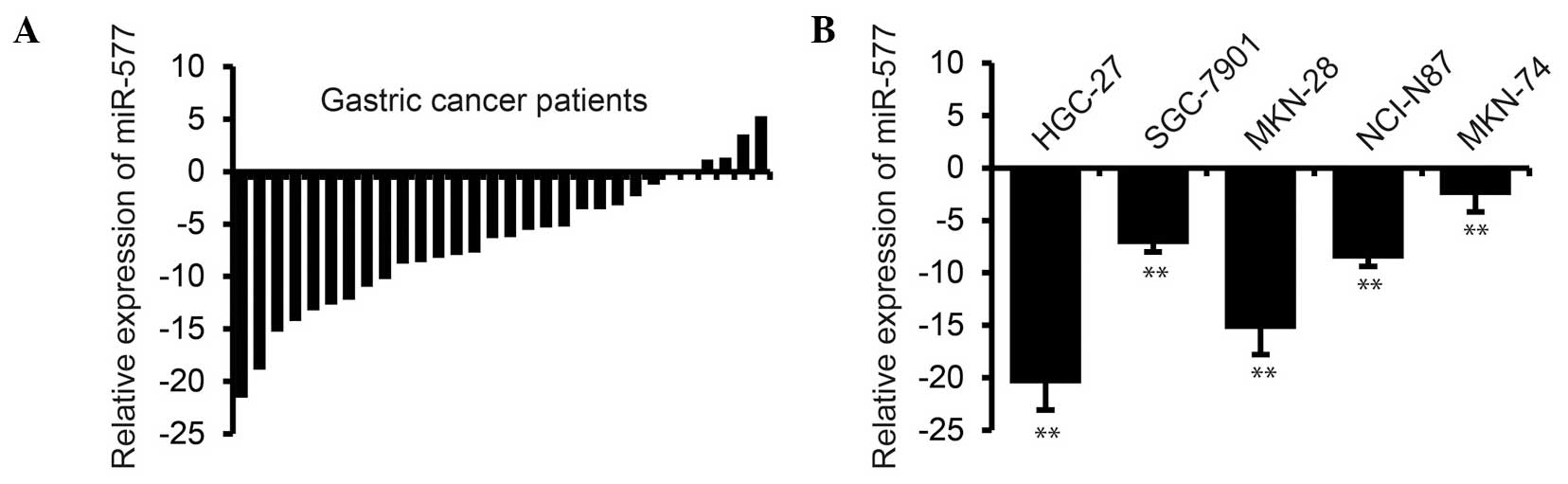
adjacent healthy tissue controls. The results demonstrated that
miR-577 was significantly downregulated in 24 out of 30 GC patient
samples compared with paired control samples (P<0.01) (Fig. 1A). To further evaluate the association
of miR-577 and GC, miR-577 expression was examined in GC cancer
cell lines using RT-qPCR. The results revealed that miR-577
expression was significantly decreased in all five cell lines
(HGC-27, SGC-7901, MKN-28, NCI-N87 and MKN-74) derived from GC,
compared with the normal GES1 gastric mucosa cell line (Fig. 1B). These data therefore suggested that
miR-577 expression was downregulated in GC.
miR-577 inhibits GC cell
proliferation
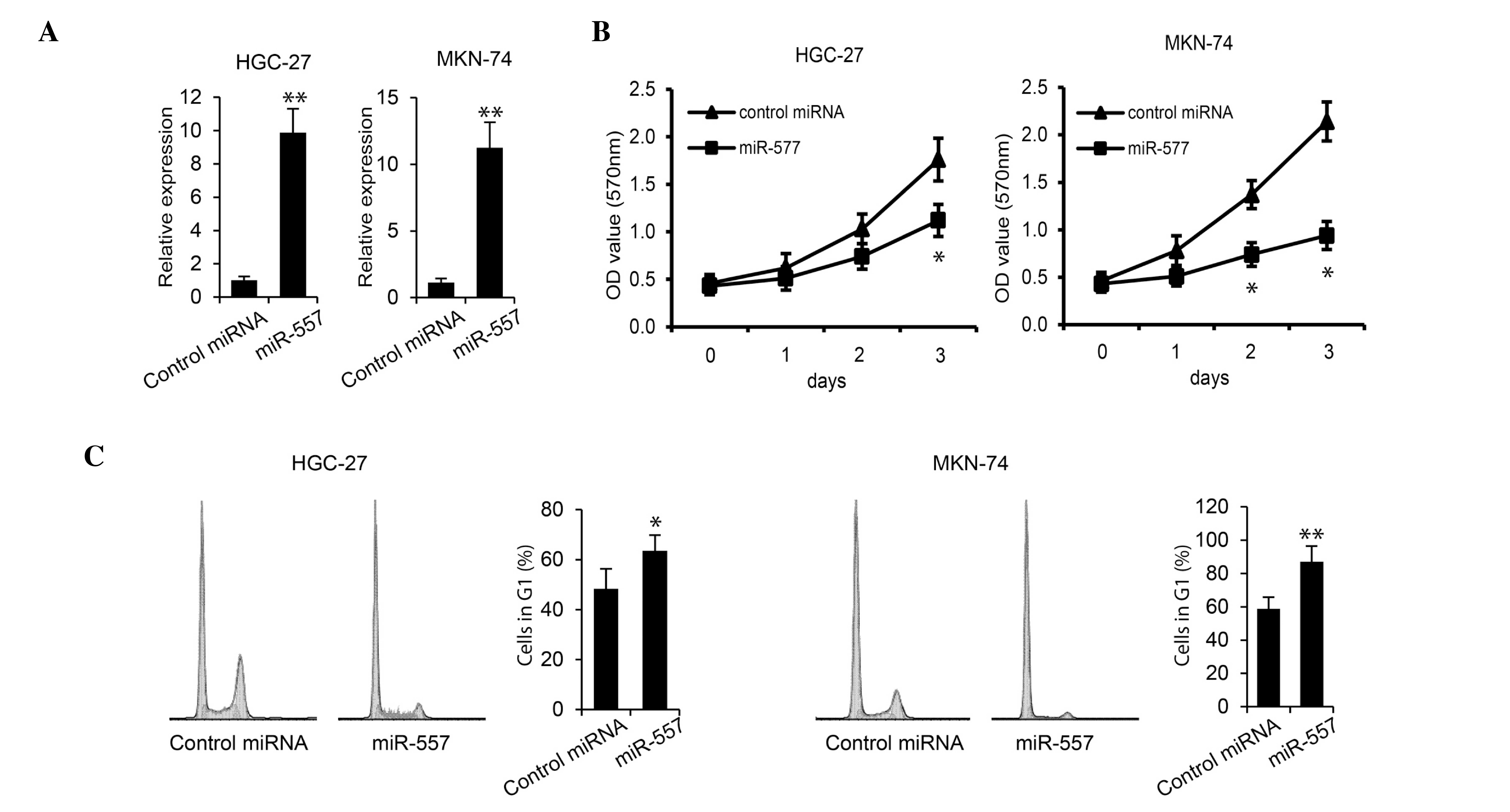
In order to determine the role of miR-577 in GC
tumorigenesis, the effect of miR-577 overexpression on HGC-27 and
MKN-74 GC cell proliferation was examined. Cells were transfected
with miR-577 or control miRNA. RT-qPCR revealed that miR-577
expression levels were significantly increased in cells transfected
with miR-577 in each GC cell line (Fig.
2A). In addition, an MTS assay demonstrated that the
overexpression of miR-577 inhibited proliferation of HGC-27 and
MKN-74 cells (Fig. 2B). Furthermore,
flow cytometric analysis was performed on transfected cells in
order to determine cell cycle progression. As shown in Fig. 2C, transfection with miR-577
significantly increased the percentage of HGC-27 and MKN-74 cells
in G1 phase (P<0.05 and P<0.01); in addition, S
phase peaks were decreased in miR-577-transfected cells. These
results indicated that miR-577 inhibited GC cell growth.
miR-577 directly targets E2F3
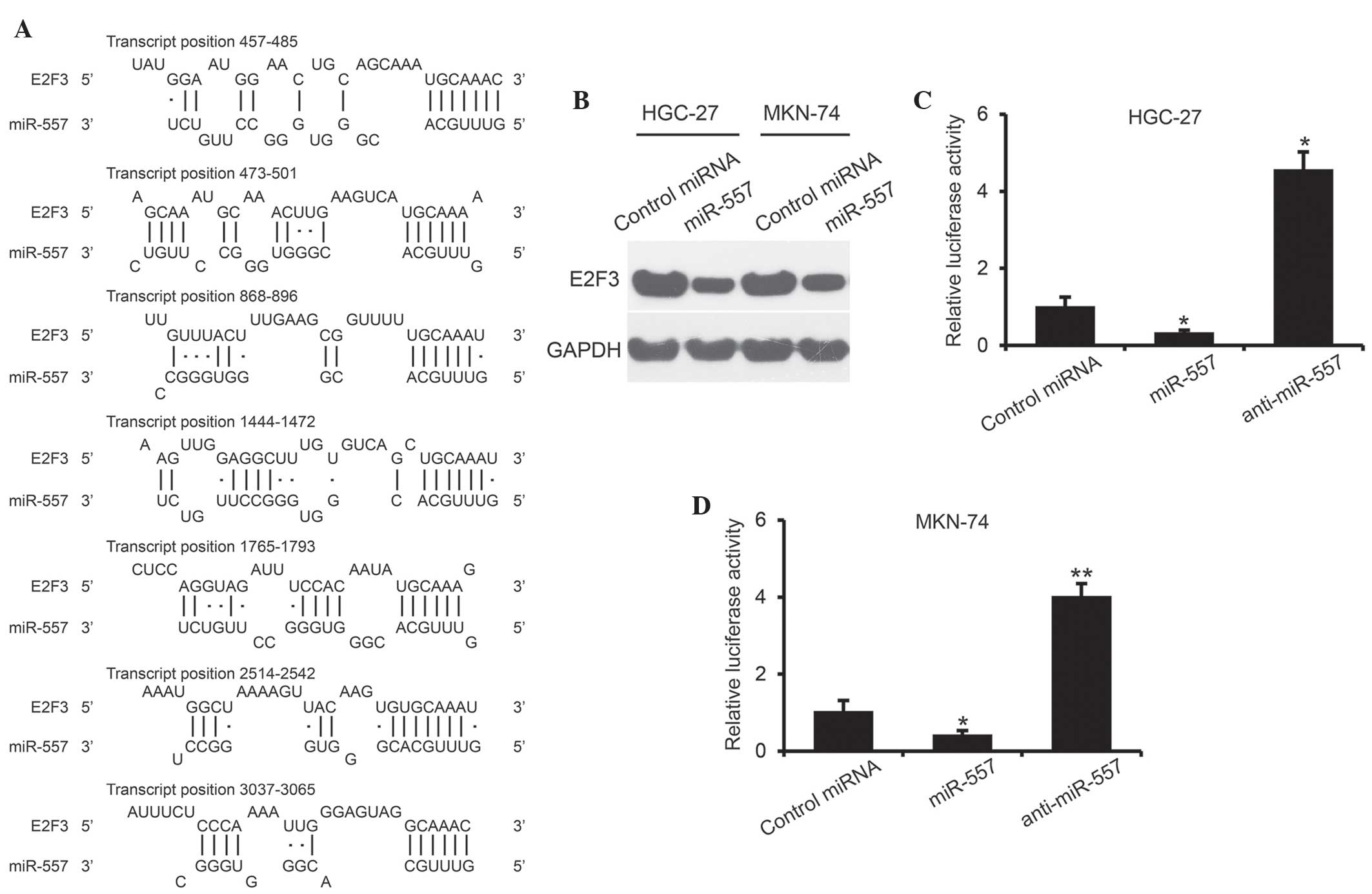
In order to elucidate the biological implications of
miR-577 on GC tumorigenesis, TargetScan human 6.2 (www.targetscan.org), DNA Intelligent Analysis
(diana.cslab.ece.ntua.gr) and MiRanda software -aug2010 (www.microrna.org) were used for putative human
protein-coding gene targets of miR-577. The results revealed seven
putative binding sites of miR-577, which are broadly conserved in
vertebrates E2F3 3′-UTR (Fig.
3A). In order to confirm that miR-577 targets the E2F3
3′-UTR, HGC-27 and MKN-74 cells were transiently transfected with
miR-577 and control miRNA. Western blot analysis revealed that
expression of E2F3 was substantially decreased at 48 h following
miR-577 transfection in each GC cell line (Fig. 3B). Furthermore, a luciferase reporter
assay was performed, in which wild-type full length 3′-UTR of
E2F3 was cloned downstream of the luciferase gene in a pGL3
vector. The results demonstrated that miR-577, rather than control
miRNA, significantly suppressed the luciferase activity of reporter
genes containing E2F3 3′-UTR in HGC-27 and MKN-74 cells
(P<0.05) (Fig. 3C and D).
Furthermore, luciferase activity was increased ~4-fold compared
with control miRNA in HGC-27 and MKN-74 cells transfected with
anti-miR-577 (P<0.05 and 0.01, respectively). These results
suggested that E2F3 is a direct target of miR-577.
miR-577 inhibits cell growth by
suppressing E2F3 expression
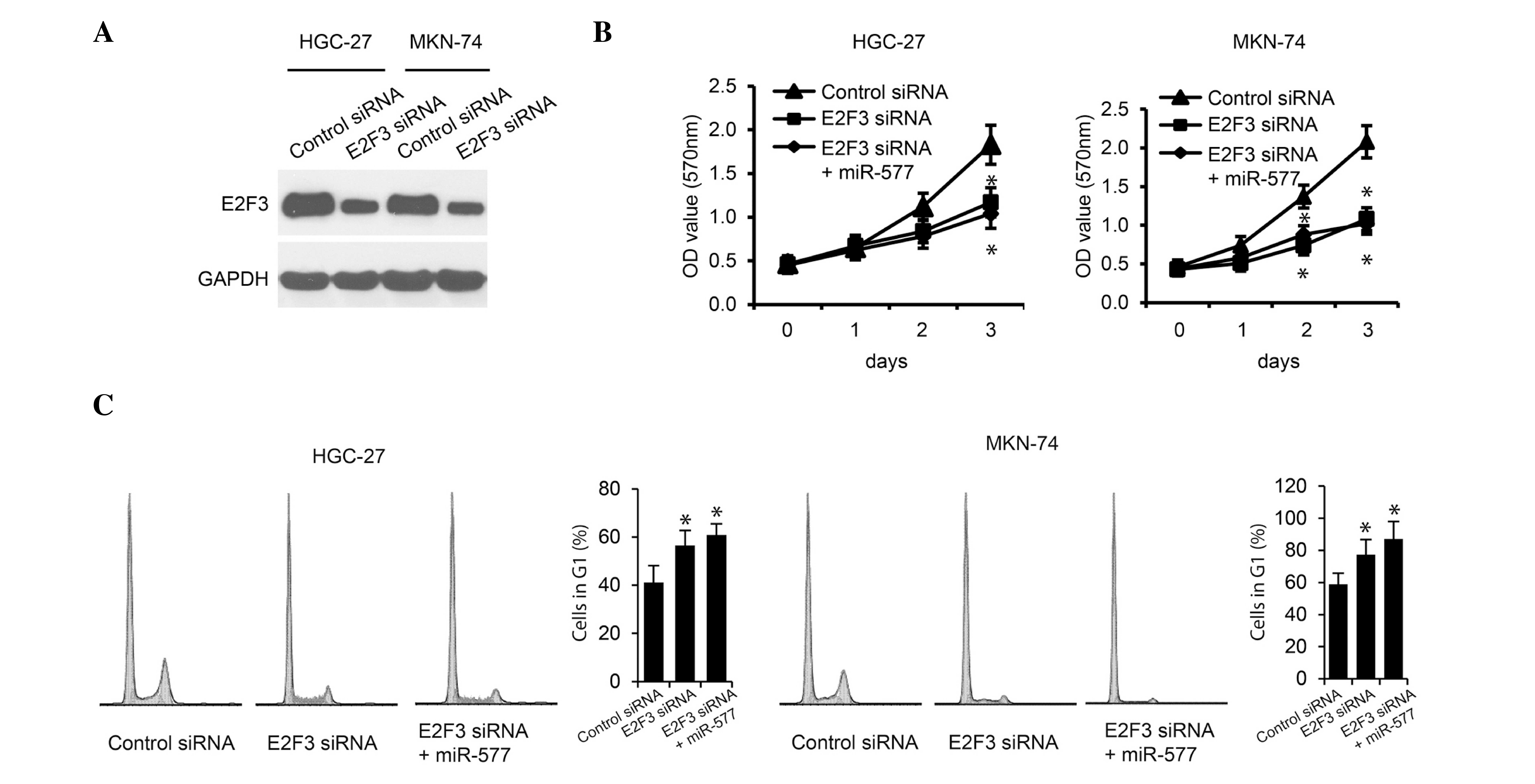
In order to further investigate the role of E2F3
repression in miR-577-inhibited GC proliferation, the effects of
E2F3 downregulation on GC cell proliferation were investigated. As
shown in Fig. 4A, E2F3 silencing by
specific E2F3 siRNAs significantly decreased E2F3 protein levels in
HGC-27 and MKN-74 cells. In addition, downregulation of E2F3
markedly suppressed HGC-27 and MKN-74 cell proliferation. However,
further overexpression of miR-577 in the E2F3-silenced cells
exhibited no additive effect on cell proliferation, as determined
using MTS assays and cell cycle analysis (Fig. 4B and C). These results demonstrated
that E2F3 expression correlated with GC cell proliferation and
indicated that E2F3 may be required for GC cells to proliferate; in
addition, the expression of E2F3 was suggested to be regulated by
miR-577.
Discussion
In the present study, miR-577 expression was
analyzed in human GC tissue samples and cell lines, the results of
which demonstrated a significant reduction in miR-577 expression in
the majority of tissue samples and in all tested cell lines
compared with adjacent health tissue and GES1 control cells,
respectively. In addition, the role of miR-577 in cell
proliferation was investigated; overexpression of miR-577
significantly inhibited proliferation as well as increased the
percentage of cells in G1 phase and decrease the
percentage of cells in S phase. During the G1 phase,
cells grow and express mRNA and proteins involved in mitosis; S
phase follows, which involves DNA replication in preparation for
cell division. The transition from G1 to S phase is a
major checkpoint in cell cycle regulation, which is commonly
disrupted in cancers (24,25). The results of the present study
demonstrated that miR-577 expression significantly affected the
G1 to S phase transition in GC cells.
Based on the computational prediction of likely
targets for miR-577, an expression study and luciferase reporter
assay were conducted in order to investigate the regulation of E2F3
transcription by miR-577. The results revealed that miR-577
directly targeted and inhibited E2F3 expression. In addition,
silencing E2F3 with siRNA demonstrated that downregulation of this
transcription factor significantly suppressed the proliferation of
GC cells.
The transcription factor E2F3 modulates expression
of proteins involved in cell/centrosome cycles and mitosis and is
critical for cellular proliferation (25). In addition, the E2F-family of
transcription factors were reported to control gene expression
vital to angiogenesis, extracellular matrix remodeling, tumor cell
survival and interactions with vascular endothelial cells, which
facilitates metastasis to the lungs (26). Expression of several genes that
control the timing of G1 to S phase transition, the rate
of DNA synthesis and act as rate limiting factors of cellular
proliferation, have been reported to be dependent on the presence
of E2F3 (27–30). Increased expression of this
transcription factor has been reported in other types of cancer and
has been associated with altered miRNA expression (26,29,31,32).
These previous studies are consistent with the findings of the
present study, which indicated that the downregulation of miR-577
allows for increased E2F3 expression, leading to aberrant cell
proliferation.
In conclusion, the results of the present study
demonstrated that downregulated expression of miR-577 in GC
affected core mechanisms in the regulation of cell cycle
progression, resulting in aberrant proliferative ability. In
addition, the differential expression of miR-577 in GC allowed for
the increased expression of the transcription factor E2F3, which
was proposed to result in the increased transcription of factors
required for G1 to S phase transition. These results
enhance the current understanding of oncogenesis in GC and may
potentially be used to develop novel diagnostic and therapeutic
applications for GC treatment.
Acknowledgements
The present study was supported by the Department of
Education Science and Technology Research Project in Heilongjiang
Province (12531830).
References
|
1
|
Globocan 2012: Estimated Cancer Incidence,
Mortality and Prevalence Worldwide in 2012. http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspxMarch
31–2014
|
|
2
|
Fock KM: Review article: The epidemiology
and prevention of gastric cancer. Aliment Pharmacol Ther.
40:250–260. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ferlay J, Soerjomataram I, Ervik M, et al;
International Agency for Research on Cancer. GLOBOCAN 2012 Cancer
incidence, mortality and prevalence worldwide in 2012. http://globocan.iarc.frNovember 20–2014
|
|
4
|
Howlader N, Noone AM, Krapcho M, et al;
National Cancer Institute. SEER Cancer statistics review,
1975–2011. http://seer.cancer.gov/csr/1975_2011/June
21–2014
|
|
5
|
Makeyev EV and Maniatis T: Multilevel
regulation of gene expression by microRNAs. Science. 319:1789–1790.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bushati N and Cohen SM: microRNA
functions. Annu Rev Cell Dev Biol. 23:175–205. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Alvarez-Garcia I and Miska EA: MicroRNA
functions in animal development and human disease. Development.
132:4653–4662. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Leung AK and Sharp PA: MicroRNA functions
in stress responses. Mol Cell. 40:205–215. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yan B, Zhao LH, Guo JT and Zhao JL:
miR-429 regulation of osmotic stress transcription factor 1 (OSTF1)
in tilapia during osmotic stress. Biochem Biophys Res Commun.
426:294–298. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
DeSano JT and Xu L: MicroRNA regulation of
cancer stem cells and therapeutic implications. AAPS J. 11:682–692.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Esquela-Kerscher A and Slack FJ:
Oncomirs-microRNAs with a role in cancer. Nat Rev Cancer.
6:259–269. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhu W, He J, Chen D, et al: Expression of
miR-29c, miR-93 and miR-429 as potential biomarkers for detection
of early stage non-small lung cancer. PLoS One. 9:e877802014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schultz NA, Dehlendorff C, Jensen BV, et
al: MicroRNA biomarkers in whole blood for detection of pancreatic
cancer. JAMA. 311:392–404. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ahmed FE, Ahmed NC, Vos PW, et al:
Diagnostic microRNA markers to screen for sporadic human colon
cancer in stool: I. Proof of principle. Cancer Genomics Proteomics.
10:93–113. 2013.
|
|
15
|
Li J, Du L, Yang Y, et al: MiR-429 is an
independent prognostic factor in colorectal cancer and exerts its
anti-apoptotic function by targeting SOX2. Cancer Lett. 329:84–90.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang Y, Li M, Zang W, et al: MiR-429
up-regulation induces apoptosis and suppresses invasion by
targeting Bcl-2 and SP-1 in esophageal carcinoma. Cell Oncol
(Dordr). 36:385–394. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Krützfeldt J, Rajewsky N, Braich R, et al:
Silencing of microRNAs in vivo with ‘antagomirs’. Nature.
438:685–689. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yuan X, He J, Sun F and Gu J: Effects and
interactions of MiR-577 and TSGA10 in regulating esophageal
squamous cell carcinoma. Int J Clin Exp Pathol. 6:2651–2667.
2013.PubMed/NCBI
|
|
19
|
Kim CH, Kim HK, Rettig RL, et al: miRNA
signature associated with outcome of gastric cancer patients
following chemotherapy. BMC Med Genomics. 4:792011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wei Z, Jiang S, Zhang Y, et al: The effect
of microRNAs in the regulation of human CYP3A4: A systematic study
using a mathematical model. Sci Rep. 4:42832014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Suzuki T, Yasui W, Yokozaki H, Naka K,
Ishikawa T and Tahara E: Expression of the E2F family in human
gastrointestinal carcinomas. Int J Cancer. 81:535–538. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ciafre SA and Galardi S: microRNAs and
RNA-binding proteins: A complex network of interactions and
reciprocal regulations in cancer. RNA biol. 10:935–942. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lai JP, Chien JR, Moser DR, et al: hSulf1
Sulfatase promotes apoptosis of hepatocellular cancer cells by
decreasing heparin-binding growth factor signaling.
Gastroenterology. 126:231–248. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nojima H: G1 and S-phase checkpoints,
chromosome instability, and cancer. Methods Mol Biol. 280:3–49.
2004.PubMed/NCBI
|
|
25
|
Humbert PO, Verona R, Trimarchi JM, Rogers
C, Dandapani S and Lees JA: E2f3 is critical for normal cellular
proliferation. Genes Dev. 14:690–703. 2000.
Bartek J and Lukas J: Mammalian G1- and
S-phase checkpoints in response to DNA damage. Curr Opin Cell Biol.
13:738–747. 2001.PubMed/NCBI
|
|
26
|
Lee MY, Moreno CS and Saavedra HI: E2F
activators signal and maintain centrosome amplification in breast
cancer cells. Mol Cell Biol. 34:2581–2599. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nevins JR: The Rb/E2F pathway and cancer.
Hum Mol Genet. 10:699–703. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xiao F, Zhang W, Chen L, et al:
MicroRNA-503 inhibits the G1/S transition by downregulating cyclin
D3 and E2F3 in hepatocellular carcinoma. J Transl Med. 11:1952013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Leone G, DeGregori J, Yan Z, et al: E2F3
activity is regulated during the cell cycle and is required for the
induction of S phase. Genes Dev. 12:2120–2130. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Olsson AY, Feber A, Edwards S, et al: Role
of E2F3 expression in modulating cellular proliferation rate in
human bladder and prostate cancer cells. Oncogene. 26:1028–1037.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ren XS, Yin MH, Zhang X, et al:
Tumor-suppressive microRNA-449a induces growth arrest and
senescence by targeting E2F3 in human lung cancer cells. Cancer
Lett. 344:195–203. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hollern DP, Honeysett J, Cardiff RD and
Andrechek ER: The E2F transcription factors regulate tumor
development and metastasis in a mouse model of metastatic breast
cancer. Mol Cell Biol. 34:3229–3243. 2014. View Article : Google Scholar : PubMed/NCBI
|