Introduction
In consideration of the increase in tumor incidence
and mortality rates, numerous studies are focusing on the
identification of practical and effective cancer treatment
strategies (1). Currently, the
predominant treatment strategies for the majority of tumors include
surgery, chemotherapy and radiotherapy; however, an effective
strategy for the treatment of cancer remains to be established
(2).
Tumor radiotherapy has been under development for
>100 years and has become an important method of local treatment
for patients in different clinical stages of cancer (3). Recent studies have demonstrated that 70%
of cancer patients require adjuvant radiotherapy during their
treatment course (3,4). For specific tumor types, including
nasopharyngeal, esophageal and prostate cancer tumors, radiotherapy
may even replace surgical treatment (3). Thus, radiotherapy is important in the
treatment of cancer. However, for specific cases of advanced or
metastasized tumors with low radiosensitivity, the increased dose
of radiation required may damage the surrounding healthy tissues
and organs (3). Therefore, the
identification of novel methods to enhance tumor cell
radiosensitivity has become a recent focus of medical radiation
research (4).
Glutamine S-transferases (GSTs) are an most
important enzyme family involved in the redox reaction and are key
in the protection of cells from toxins and carcinogens. For
instance, GST π 1 (GSTP1) is a member of the GST family that is
involved in detoxification (5), with
recent studies identifying GSTP1 as an important marker of
diagnosis, prognosis and chemotherapy resistance in certain tumors
(6–9).
Therefore, the aims of the present study were to investigate the
effect of GSTP1 gene overexpression on the radiosensitivity
of the HeLa human cervical cancer cell line, to preliminarily
investigate the underlying mechanisms of these effects and to
provide an experimental foundation for improving the effects of
clinical radiotherapy in the future.
Materials and methods
Materials
The HeLa human cervical carcinoma cell line was
purchased from the American Type Culture Collection (Manassas, VA,
USA), Dulbecco's modified Eagle's medium (DMEM) and newborn calf
serum was obtained from Gibco Life Technologies (Carlsbad, CA,
USA), G418 from Inalco Pharmaceuticals (San Luis Obispo, CA, USA),
TRIzol reagent and lipofectamine from Invitrogen Life Technologies
(Carlsbad, CA, USA), the anti-cyclin B1 antibody from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA), and the polymerase chain
reaction (PCR) instrument and Gel Doc 2000 gel imager from Bio-Rad
Laboratories, Inc. (Hercules, CA, USA).
Generating stably transfected cell
lines
HeLa cells were cultured in DMEM medium containing
10% newborn calf serum. Prior to transfection, a total of
1.2×106 cells/well were seeded in a 35-mm petri dish for
48 h. Human full-length GSTP1 cDNA (Genecopoeia, Rockville,
MD, USA) was directionally cloned into the eukaryotic expression
vector, pcDNA3 (Genecopoeia). The construct was subsequently
confirmed by restriction endonuclease (Corning Life Sciences-Axygen
Inc., Union City, CA, USA) and DNA sequence analysis. The
pcDNA3/GSTP1 construct or the empty pcDNA3 vector (negative
control) was then transfected into the HeLa cells using a
liposome-mediated method (10) and
cultured in medium containing 500 µg/ml G418 for four weeks at 37°C
for selection. Following transfection, reverse transcription
(RT)-PCR was performed to detect the GSTP1 mRNA expression
levels. HeLa cells transfected with the empty pcDNA3 plasmid were
termed as HeLa/Neo cells and those transfected with
pcDNA3/GSTP1 were termed as HeLa/GSTP1 cells. Prior
to irradiation, all the cells were transferred to normal culture
medium for two days to avoid interference from G418.
Clone formation assay
HeLa cells were seeded into flasks at a
concentration of 1×105/ml. After a 12-h incubation
period, the cells were synchronized by replacing the medium with
serum-free DMEM and cultured for 24 h before irradiation.
Radiation, at a dose rate of 200 cGy/min, a source-skin distance of
100 cm and a dose gradient of 0, 2, 4, 6 and 8 Gy, was then applied
to the cells. Following irradiation, the cells were cultured in
normal medium for two weeks, fixed with methanol for 30 min and
stained in Giemsa (Sigma-Aldrich, St. Louis, MO, USA) for 30 min.
Subsequent to the removal of excess dye, the number of colonies
containing >25 cells were counted under a microscope to
determine the rate of colony formation and calculate the survival
curves.
Flow cytometry
Following synchronization, the HeLa cells were
digested using EDTA-free trypsin (Sigma-Aldrich), washed twice in
phosphate-buffered saline (PBS), fixed in 70% ethanol for 12 h and
filtered through a 300 µm mesh nylon screen (Sangon Biotechnology
Co., Ltd., Shanghai, China). The cells were then stained with 200
µl propidium iodide dye (100 µg/ml; Sigma-Aldrich) for 30 min in
the dark prior to cell cycle analysis using an EPICS XL flow
cytometer (Beckman Coulter, Inc., Brea, CA, USA).
RT-PCR
Total RNA was extracted from the HeLa cells using
TRIzol reagent, according to the manufacturer's instructions, and
RNA purity was determined using an ultraviolet spectrophotometer
(NanoDrop 2000c; Thermo Fisher Scientific, Inc., Wilmington, DE,
USA). The Transcriptor First Strand cDNA Synthesis kit, which was
obtained from Roche Diagnostics GmbH (Mannheim, Germany) was used
for the experiments. Briefly, 1 µl total RNA from each group was
added to 1 µl of oligo (dT) and 10 µl diethylpyrocarbonate-treated
water, and incubated in a water bath at 70°C for 5 min, prior to
cooling on ice for 30 sec. Subsequently, 4 µl 5X reaction solution,
1 µl RNase inhibitor (20 U/µl) and 2 µl deoxynucleoside
triphosphate (10 mmol/l) were added to the reaction, which was
heated in a water bath at 37°C for 5 min. Following the addition of
1 µl Moloney-murine leukemia virus reverse transcriptase (20
µg/µl), reverse transcription was allowed to proceed for 1 h at 37
°C before the reaction was inactivated by heat treatment for 10 min
at 70°C.
Primers for GSTP1 (Genebank accession no.
U21689.1) and GAPDH (Genebank accession no. NH-017008) were
designed against their GenBank sequences using Primer Premier 5.0
software (Premier Biosoft, Palo Alto, CA, USA). The primer
sequences for GSTP1 were as follows: Forward,
5′-ATTAACCCTCACTAAAGGGAGATATGGTGAAGGAATGATGGGGT-3′, and reverse,
5′-TAATACGACTCACTATAGGGGGATCTTGGGCCGGGCACTGA-3′; the amplified
product was 650 bp. The primer sequences for GADPH were as
follows: Forward, 5′-CAA CTA CAT GGT CTA CAT GTTCC-3′, and reverse,
5′-CAA CCT GGT CAG TGTAG-3′; the amplicon was 700 bp. The PCR
reaction conditions were as follows: Reverse transcription at 48°C
for 45 min; inactivation and denaturation for 2 min at 94°C; 35
cycles of annealing at 58°C for 45 sec and elongation at 72°C for
45 sec; and extension at 68°C for 7 min. Finally, the PCR product
was electrophoresed through a 2% agarose gel and images were
captured using the Gel Doc 2000 gel imager (Bio-Rad Laboratories,
Inc.).
Western blot analysis
HeLa cells were collected and lysed using
radioimmunoprecipitation lysis buffer (Beyotime Institute of
Biotechnology, Haimen, China). The total protein concentration was
determined using the Lowry method, with 100 µg protein separated by
10% SDS-PAGE and transferred onto nitrocellulose membranes with 5%
skimmed milk powder.
Following addition of the monoclonal mouse
anti-human anti-β-actin (1:5,000; cat. no. sc-24979; Santa Cruz
Biotechnology, Inc.) and monoclonal mouse anti-human cyclin B1
antibodies (1:1,000; cat. no. sc-166757; Santa Cruz Biotechnology,
Inc.), the blots were incubated overnight at a temperature of 4°C
on an oscillating platform and then washed three times in PBS/Tween
20 for a total of 15 min. Finally, the blots were incubated with a
monoclonal mouse anti-human horseradish peroxidase-labeled IgG
secondary antibody (1:5,000; cat. no. sc-9969; Santa Cruz
Biotechnology, Inc.) and developed using a LightShift
Chemiluminescent EMSA Kit (Sangon Biotechnology Co., Ltd.).
Statistical analysis
All the experiments were repeated three times. The
independent samples t-test and correlation analysis were performed
and SAS statistical software (version 8.0; SAS Institute. Inc.,
Cary, NC, USA) was used to analyze the results. P<0.01 was
considered to indicate a statistically significant difference.
Results
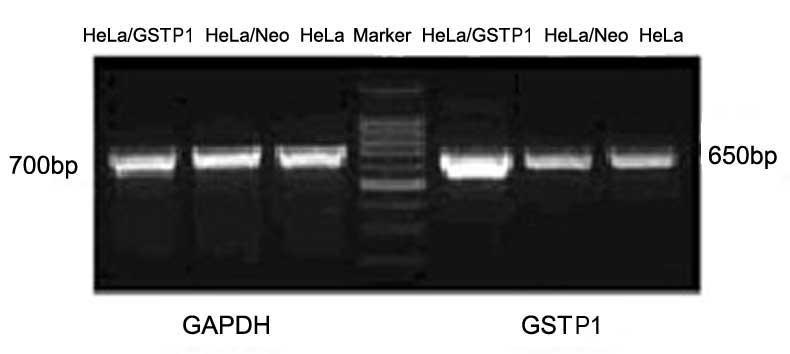
High GSTP1 gene expression in stably
transfected HeLa cell lines
HeLa cells stably transfected with GSTP1 and
empty plasmid were successfully established, and their GSTP1
mRNA expression levels were determined using RT-PCR. GSTP1
mRNA expression was markedly higher in the HeLa/GSTP1 cells
compared with the non-transfected HeLa cells or the cells
transfected with the empty vector (HeLa/Neo cells), as indicated in
Fig. 1. By contrast, GSTP1
mRNA expression in the HeLa/Neo cells was almost equivalent to the
expression in the normal HeLa cells. The results indicated that the
GSTP1 recombinant plasmid was successfully constructed and
highly expressed in HeLa cells, obtaining the HeLa/GSTP1
cells.
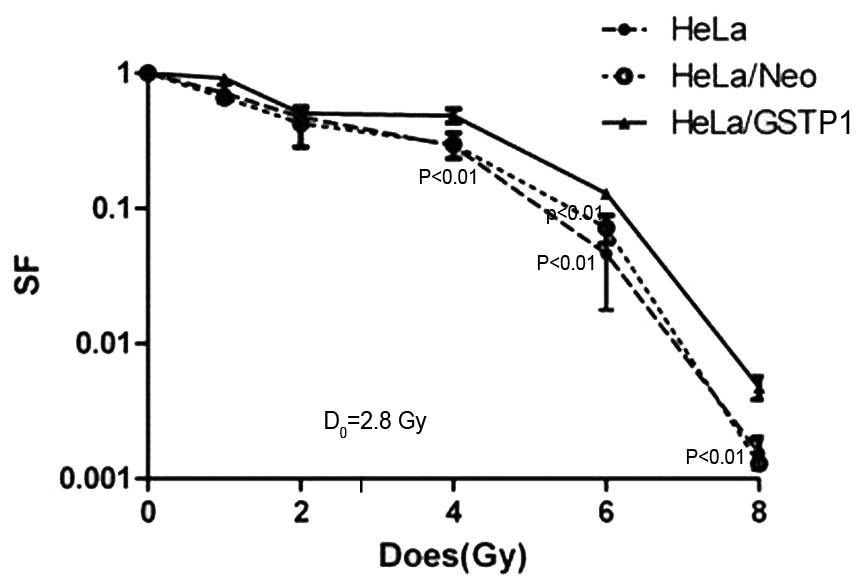
GSTP1 gene overexpression reduces
radiosensitivity in HeLa cells
As indicated in Fig.
2, the administration of increasing doses of radiation resulted
in a dose-dependent downward trend in the cell survival rate of the
non-transfected HeLa, HeLa/Neo and the HeLa/GSTP1 cells. In
particular, the survival rate was significantly higher in the
HeLa/GSTP1 cells irradiated with 2.8 Gy compared with the
control group (P<0.01), with the HeLa/GSTP1 cells
demonstrating radiation resistance. Furthermore, the D0
value (incremental dose required for reducing the fraction of
colonies to 37%, indicative of single event killing) of the HeLa
and HeLa/Neo cells was ~2.4 Gy; however, the D0 value
was 2.8 Gy in the HeLa/GSTP1 cells, demonstrating a
statistically significant difference (P<0.01). These
experimental data indicated that the radiosensitivity in HeLa cells
may be reduced by increasing the expression of GSTP1.
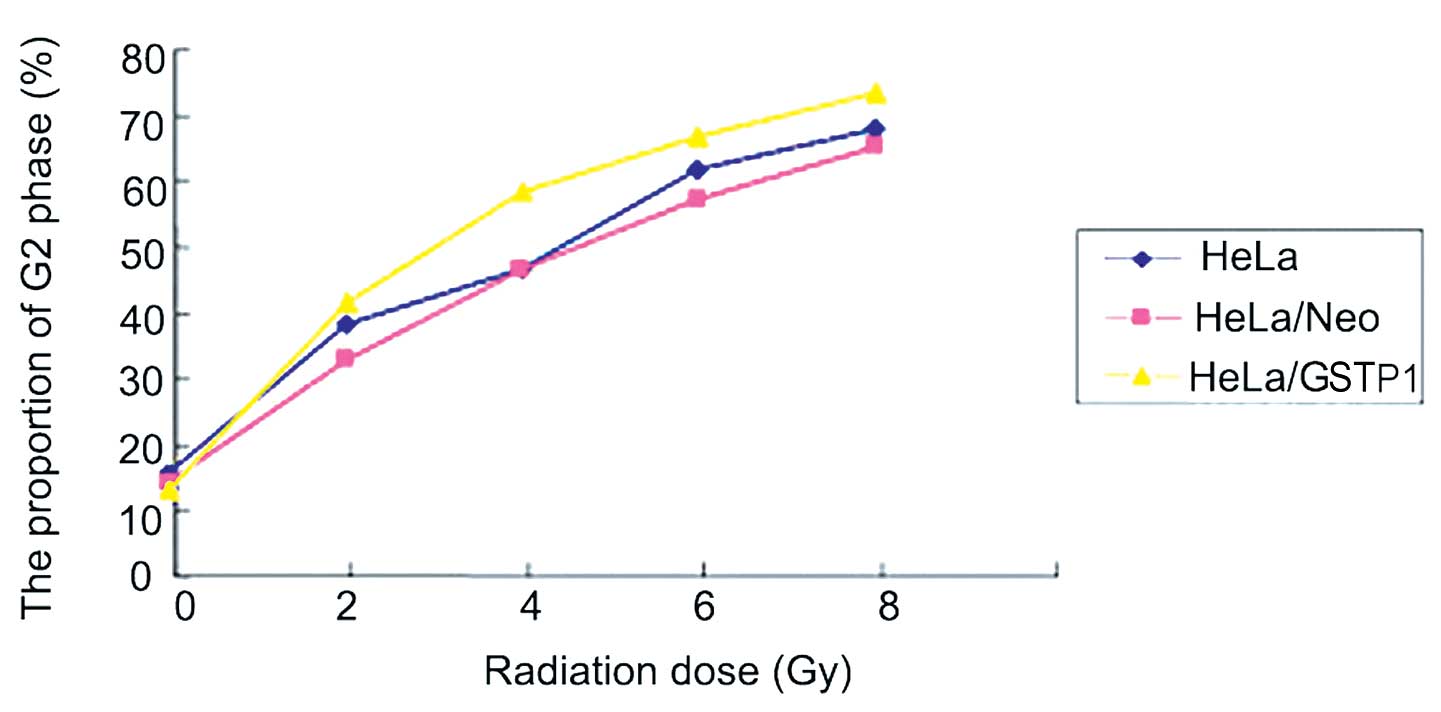
GSTP1 overexpression alters cell cycle
progression in HeLa cells
As indicated in Fig.
3, the three groups of HeLa cells were treated with an X-ray
radiation dose gradient of 0, 2, 4, 6 and 8 Gy. The results
indicated that G2 phase arrest occurred in a
dose-dependent manner in the three groups of cells; however,
G2 arrest was more evident in the HeLa/GSTP1
cells compared with the control group. The proportion of cells in
the G2 phase was significantly higher in the HeLa/GSTP1 cells when
compared with the negative control group (HeLa/Neo)
(P<0.01).
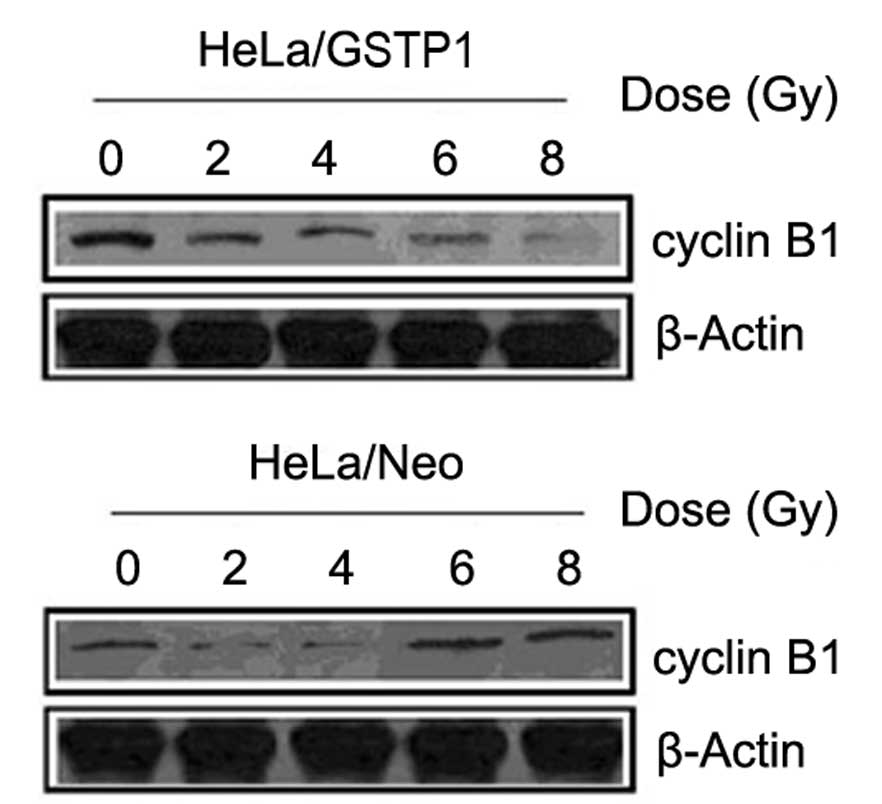
Impact of GSTP1 overexpression on
cyclin B1 protein expression levels
Since cell cycle progression is affected by
radiation, the expression of the cell cycle regulation protein
cyclin B1 was detected by western blot analysis. As indicated in
Fig. 4, the protein expression of
cyclin B1 in the HeLa/GSTP1 cells markedly decreased with an
increasing radiation dose, while in the control group, cyclin B1
expression was unchanged.
Discussion
The underlying mechanisms that cause certain tumors
to exhibit low radiosensitivity remain unclear. A previous study
identified that various growth factors, including the insulin-like
growth factor-1 receptor and epidermal growth factor, are involved
in radiosensitivity (11). In
addition, nuclear expression of anti-tumor genes or oncogenes, such
as p53 and B-cell lymphoma-2, may alter radiosensitivity in
cells (12). Therefore, genes that
affect the cell cycle, apoptosis and genomic stability may also
affect the radiosensitivity of tumor cells (13–15).
The majority of studies have reported that
G2/M phase arrest is negatively correlated with cell
radiosensitivity, with significant radiation resistance occurring
in the S phase or in G2/M phase arrest (16,17).
Particularly in the G2 phase, longer duration of the
cell cycle arrest results in more frequent occurrence of
potentially lethal DNA damage repair. Therefore, cell survival
eventually increases as a function of radiation resistance
(15). The results of the present
study demonstrated that GSTP1 may induce G2 phase
arrest by regulating the expression of the cyclin B1 cell cycle
protein and, thus, reducing the radiosensitivity of HeLa cervical
cancer cells.
Members of the GST super-gene family function in
cell protection, storage, binding, transport and detoxification. In
addition, overexpression of the isoenzyme, GSTP1, is closely
associated with tumor occurrence; therefore, increased GSTP1
expression may be used in the diagnosis, prognosis and
determination of chemotherapy resistance in a variety of tumor
tissues (18).
In conclusion, only a limited number of studies have
thus far been conducted regarding the effect of GSTP1
expression on tumor radiosensitivity (15,19,20).
However, combined with the existing data on GSTP1, the
present study hypothesized that GSTP1, as well as acting as
a marker for diagnosis, prognosis and chemotherapy resistance, may
serve as an important therapeutic agent in the treatment of various
tumors.
Acknowledgements
The present study was supported by grants from the
National Science Foundation of China (nos. 81072175, 81372854 and
81102010) and the Shanghai Committee of Science and Technology,
China (no. 13NM1401504).
References
|
1
|
Aneja P, Rahman M, Beg S, Aneja S, Dhingra
V and Chugh R: Cancer targeted magic bullets for effective
treatment of cancer. Recent Pat Antiinfect Drug Discov. Apr
15–2015.(Epub ahead of print). View Article : Google Scholar
|
|
2
|
Souza VB, Silva EN, Ribeiro ML and Martins
Wde A: Hypertension in patients with cancer. Arq Bras Cardiol.
104:246–252. 2015.(In English, Portuguese). PubMed/NCBI
|
|
3
|
Gómez-Millán J, Lara MF, Correa Generoso
R, Perez-Rozos A, Lupiáñez-Pérez Y and Medina Carmona JA: Advances
in the treatment of prostate cancer with radiotherapy. Crit Rev
Oncol Hematol. Feb 25–2015.(Epub ahead of print). View Article : Google Scholar
|
|
4
|
Belfatto A, Riboldi M, Ciardo D, et al:
Kinetic models for predicting cervical cancer response to radiation
therapy on individual basis using tumor regression measured in vivo
with volumetric imaging. Technol Cancer Res Treat.
Mar;10:2015.(Epub ahead of print).
|
|
5
|
Jardim BV, Moschetta MG, Gelaleti GB, et
al: Glutathione transferase pi (GSTpi) expression in breast cancer:
an immunohistochemical and molecular study. Acta Histochem.
114:510–517. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
von Knebel Doeberitz M: New markers for
cervical dysplasia to visualise the genomic chaos created by
aberrant oncogenic papillomavirus infections. Eur J Cancer.
38:2229–2242. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nagle CM, Chenevix-Trench G, Spurdle AB
and Webb PM: The role of glutathione-S-transferase polymorphisms in
ovarian cancer survival. Eur J Cancer. 43:283–290. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang W, Liu G and Zheng J: Human renal
UOK130 tumor cells: a drug resistant cell line with highly
selective over-expression of glutathione S-transferase-pi isozyme.
Eur J Pharmacol. 568:61–67. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zon G, Barker MA, Kaur P, et al: Formamide
as a denaturant for bisulfite conversion of genomic DNA: Bisulfite
sequencing of the GSTPi and RARbeta2 genes of 43 formalin-fixed
paraffin-embedded prostate cancer specimens. Anal Biochem.
392:117–125. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Saxena V, Gacchina Johnson C, Negussie AH,
Sharma KV, Dreher MR and Wood BJ: Temperature-sensitive
liposome-mediated delivery of thrombolytic agents. Int J
Hyperthermia. 31:67–73. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shen Z, Wu X, Wang Z, Li B and Zhu X:
Effect of miR-18a overexpression on the radiosensitivity of
non-small cell lung cancer. Int J Clin Exp Pathol. 8:643–648.
2015.PubMed/NCBI
|
|
12
|
Liu S, Song L, Zhang L, Zeng S and Gao F:
miR-21 modulates resistance of HR-HPV positive cervical cancer
cells to radiation through targeting LATS1. Biochem Biophys Res
Commun. Mar 10–2015.(Epub ahead of print).
|
|
13
|
Hopkins TG, Burns PA and Routledge MN: DNA
methylation of GSTP1 as biomarker in diagnosis of prostate cancer.
Urology. 69:11–16. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yan WL and Huang G: Factors implicated to
radioresistance of breast cancer and their possible roles. Int J
Radiat Med Nucl Med. 30:193–195. 2006.
|
|
15
|
Sartor CI: Epidermal growth factor family
receptors and inhibitors: radiation response modulators. Semin
Radiat Oncol. 13:22–30. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Alshatwi AA, Athinarayanan J and Vaiyapuri
Subbarayan P: Green synthesis of platinum nanoparticles that induce
cell death and G2/M-phase cell cycle arrest in human cervical
cancer cells. J Mater Sci Mater Med. 26:53302015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Duangmano S, Sae-Lim P, Suksamrarn A,
Patmasiriwat P and Domann FE: Cucurbitacin B causes increased
radiation sensitivity of human breast cancer cells via G2/M cell
cycle arrest. J Oncol. 2012:6016822012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rotili D, De Luca A, Tarantino D, Pezzola
S, Forgione M, Morozzo Della Rocca B, Falconi M, Mai A and Caccuri
AM: Synthesis and structure - activity relationship of new
cytotoxic agents targeting human glutathione-S-transferases. Eur J
Med Chem. 89:156–171. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Su F, Hu X, Jia W, Gong C, Song E and
Hamar P: Glutathion S transferase pi indicates chemotherapy
resistance in breast cancer. J Surg Res. 113:102–108. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhu J, Tan Y, He L, Si MJ and Yin ZM: A
novel function for glutathione S-transferase π in MAPK pathway. Xi
Bao Sheng Wu Xue Za Zhi. 26:252–256. 2004.(In Chinese).
|