Introduction
Infantile hemangioma (IH) is the most common
soft-tissue tumor of infancy. It is benign tumor with an incidence
of up to 10%. IH is typically not present at birth, however, it may
arise following rapid proliferation within the first weeks of life.
This is followed by a plateau stage, and a subsequent slow
involution of the lesion, such that 60% of 4-year-olds and 76% of
7-year-olds with the condition will experience complete regression
of the hemangioma. Characterized by a proliferation of endothelial
cells, the pathogenesis of hemangioma is currently unclear
(1,2).
In recent years, the development of advanced molecular and cellular
biology technologies has led to more in depth research on the cell
cycle and its regulation mechanisms. Tumor suppressor genes, with
their unique structures, biological activity and essential roles in
the cell cycle, have accordingly become an important area of
investigation.
Tumor suppressor genes, also known as
anti-oncogenes, are present in normal cells and inhibit cell
transformation and tumor occurrence (3,4). p16 is a
tumor suppressor gene that is able to block the G1 to S
phase transition of the cell cycle, thereby inhibiting cell
proliferation; in certain tumors, abnormal expression of this gene
and protein may also promote the apoptosis of tumor cells. Aberrant
p16 underexpression may cause cell proliferation and lead to
cancer, the mechanism of which has been confirmed through research
conducted in numerous tumor types; p16 inactivation most commonly
occurs as a result of gene mutation or 5′CpG island methylation
(5). The p16 gene and its protein
have been found to be altered in the majority of human primary
tumors, including melanoma, head and neck cancers, glioma and
gastric cancer (6,7). However, the expression of p16 protein in
IH has not been reported.
The purpose of the present study was to detect p16
protein expression by immunohistochemical methods in hemangioma and
normal tissues, and to investigate the mechanism and significance
of the protein in the occurrence, development and regression of
hemangioma, in order to provide novel insights into the clinical
treatment of hemangioma.
Materials and methods
Patients
Between January 2001 and June 2006, a total of 80
patients with IH, who had undergone surgery and were pathologically
diagnosed with hemangioma at Stomatological Hospital of Jiamusi
University (Jiamusi, China), were enrolled in this study.
Paraffin-embedded hemangioma tissue blocks and clinical data were
obtained for 38 males and 42 females, who were of a minimum age of
2 months and a maximum age of 4 years. The hemangiomas were located
on the scalp, forehead, eyelids, neck, back, arms, legs, hands or
feet. The patients underwent no auxiliary treatment. Paraffin
blocks were cut into 4-µm thick sections, which were each cut into
two pieces, for the detection of p16 protein and hematoxylin and
eosin (HE) staining. After HE staining, the paraffin blocks were
grouped, according to the Mulliken classification standard
(8), into 40 cases of proliferating
hemangioma and 40 cases of involuting hemangioma, with
tumor-adjacent normal tissues in 26 cases used as controls. The
study was approved by the Ethics Committee of the Stomatological
Hospital of Jiamusi University. Written informed consent was
obtained from the patient's families.
Immunohistochemistry
The 4-µm sections were deparaffinized in xylene
followed by hydration in a graded series of alcohols. Antigen
retrieval was performed by immersing the section in 0.5 M citrate
buffer (pH 6) at room temperature, and placing it into a microwave
oven for 20 min. Endogenous peroxidase activity was blocked by
incubation in 3% H2O2 for 20 min at room
temperature. Subsequent to being rinsed in Tris-buffered saline
(TBS; pH 7.4), the sections were incubated with primary rabbit
anti-p16INK4a monoclonal antibody (Wuhan Boster Biological
Technology Ltd., Wuhan, China; dilution, 1:2 in TBS) at 4°C
overnight. In the negative control, the primary antibody was
replaced with phosphate-buffered saline. Subsequent to being washed
with TBS three times for 5 min each, the sections were incubated
with a biotin-labeled goat anti-rabbit IgG secondary antibody
(Wuhan Boster Biological Technology Ltd.). The sections were
counterstained with 3–3′-diaminobenzidine (Wuhan Boster Biological
Technology Ltd.) followed by hematoxylin. The slides were washed
under running water and mounted with DPX. The protein expression
was then scored as follows: negative (−), <25% positive cells;
weak (+), 25–50% positive cells; strong (++), >50–75% positive
cells; and very strong (+++), >75% positive cells.
Statistical analysis
The data are expressed as number of cases and
percentage. Categorical data was analyzed using the χ2
test. SPSS 15.0 software (SPSS Inc., Chicago, IL, USA) was used for
all data analyses. P<0.05 was considered to indicate a
statistically significant difference.
Results
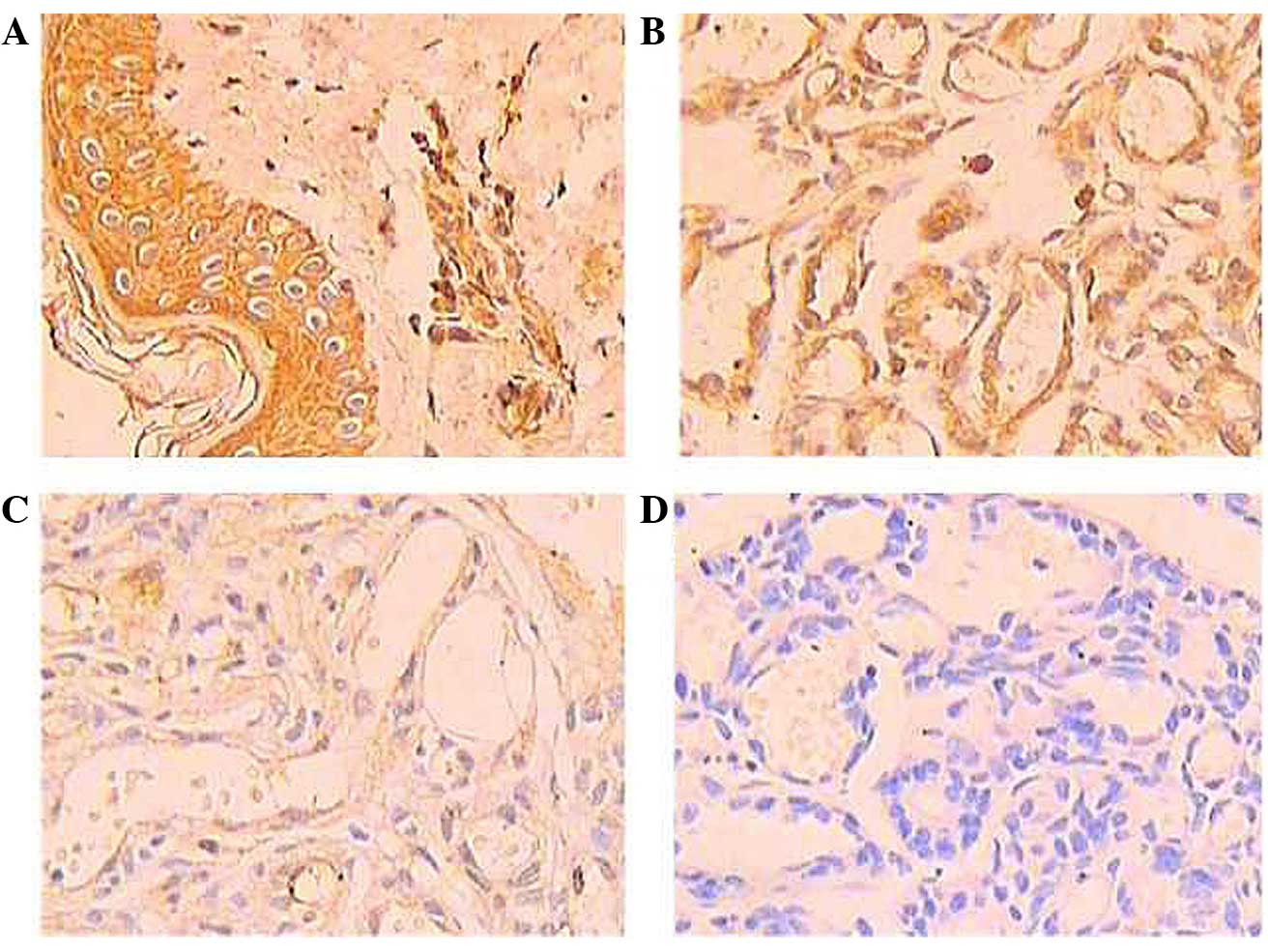
Upon immunohistochemical staining, the positive
expression of p16 protein was shown as brown-yellow granules,
mainly in the cytoplasm and partially in the nucleus. The normal
tissue cells exhibited a large number of brown-yellow granules,
indicating that the expression of p16 protein was very strong
(Fig. 1A), with a positive rate of
84.62%. The expression level of p16 protein was different in the
different types of hemangioma. In the involuting hemangioma,
numerous brown granules were deposited in the endothelial cell,
indicating that the expression of p16 protein was strong (Fig. 1B), with a positive rate of 52.5%. In
the proliferating hemangioma, less brown granules were deposited in
the endothelial cells, indicating that the expression of p16
protein was weak (Fig. 1C), with a
positive rate of 22.5%. The expression level of p16 protein in the
proliferating hemangioma was lower than that in the involuting
hemangioma. The expression level of p16 protein in the involuting
hemangioma was lower than that in normal tissue. These differences
were statistically significant (P<0.05) (Table I).
 | Table I.p16 expression in normal tissue,
involuting hemangioma tissue and proliferating hemangioma
tissue. |
Table I.
p16 expression in normal tissue,
involuting hemangioma tissue and proliferating hemangioma
tissue.
|
|
| p16 expression,
n |
|
|
|---|
|
|
|
|
|
|
|---|
| Groups | n | – | + | ++ | +++ | Positive rate, % | P-value |
|---|
| Normal tissue | 26 | 4 | 2 | 4 | 16 | 84.62 | 0.001a |
| Involuting
hemangioma | 40 | 19 | 4 | 7 | 10 | 52.50 | 0.018b |
| Proliferating
hemangioma | 40 | 31 | 5 | 2 | 2 | 22.50 | 0.016c |
Discussion
Molecular studies have shown that tumor development
is closely associated with the cell cycle. Normal cells possess a
dynamic balance between proliferation and the inhibition of
proliferation, so as to ensure the normal growth of the organism.
When this balance is broken, the components for promoting
proliferation cause overexpression or the components for inhibiting
proliferation cause underexpression; with this, cell proliferation
becomes uncontrolled and tumors form. p16 is a tumor suppressor
gene, located in chromosomal region 9p21, with a total length of
8.5 kb and containing 3 exons and 2 introns. The p16 protein is
encoded by a single polypeptide chain of 15.84 kDa, containing 148
amino acid residues, and is involved in the regulation of cell
growth. The p16 protein is a cyclin dependent kinase (CDK)4/CDK6
inhibitor, and CDK4/CDK6 is the key factor for the G1/S
phase transition in the cell cycle. p16 protein is specifically
combined with CDK4/CDK6, and its inactivation prevents the
phosphorylation of Rb protein, which means that the Rb gene cannot
relieve the inhibition of transcription factors, thereby preventing
the cells from passing from the G1 phase to the S phase.
This therefore inhibits cell proliferation, making p16 a key
negative regulatory factor in the process of cell proliferation
(9–11). The p16 gene and its protein are
altered in the majority of human primary tumors, including gliomas,
melanoma, head and neck cancer and gastric cancer (12–14).
Inactivation (deletion or mutation) and 5′ CpG
island methylation are the main forms of change for the p16
protein. Kamb et al (15)
analyzed 290 tumor cell lines and found that 133 of these cell
lines, including lung cancer and leukemia cell lines, showed
deletion of the p16 gene; the gene deletion rate was 25% in glioma
and the mutation rate was 85%. In breast cancer, and cancer of the
head and neck, the mutation rates were 60 and 30%, respectively.
p16 gene deletion, mutation or hypermethylation cause cells to
undergo uncontrolled proliferation, and are thus some of the major
factors in the occurrence and development of tumors (7,15).
Hemangioma is a benign tumor with endothelial cell
hyperplasia; the condition occurs in infants, with a pathogenesis
that is unknown. The current view is that endothelial cell
hyperplasia, the aggregation process and microvessel lumen
formation are the core of vascular tumor growth. A previous study
revealed that endothelial cells in the proliferative phase were in
an immature state and had strong proliferative capability (16).
The expression of p16 protein in IH was detected by
immunohistochemistry in the present study. The experimental results
showed that the expression level of p16 protein in the
proliferating hemangioma was lower than that in the involuting
hemangioma, with a significant difference (P<0.05), and that the
expression level of p16 protein in the involuting hemangioma was
lower than that in the normal tissue, with a significant difference
(P<0.05). Based on the results of the present study, it is
hypothesized that the p16 protein is present at a low level of
expression in proliferating hemangioma and is only partially
expressed by endothelial cells; it therefore cannot inhibit the
hemangioma endothelial cell CDK4/CDK6 activity, and is unable to
prevent the cell from entering the G1 phase to S phase
transition. Thus, cell proliferation becomes uncontrolled, leading
to the excessive proliferation of the endothelial cells,
accelerating hemangioma proliferation. In the period of hemangioma
regression, the expression of p16 protein is increased, thus
inhibiting the hemangioma endothelial cell CDK4/CDK6 activity,
leading to cell cycle arrest, the inhibition of the proliferation
of the hemangioma endothelial cells, and promoting the involution
of IH.
Hemangioma is a type of angiogenic disease (17). The results of the present study
indicate that endothelial cell proliferation and angiogenesis are
not the result of a single factor; each process may be a result of
the participation of multiple genes and factors. The p16 protein
was the first protein molecule identified to have tumor suppressor
functions (9). Due to the high
frequency of deletion and mutation in numerous primary tumors,
restoring normal function of the p16 gene in tumor cells is a
potential method of inhibiting tumor cell proliferation. The p16
gene has the following features: cDNA molecules are small and easy
to manipulate; the p16 protein is also able to specifically inhibit
CDK4/CDK6 so, as an antitumor drug, it has a specific target; in
comparison to other genes the mutation rate is high, and mutations
are observed in numerous primary tumors (18). Therefore, the p16 gene may become an
important candidate gene for gene therapy. By increasing the p16
protein level of a patient, it may be possible to prevent the
proliferation of hemangioma endothelial cells.
From the results of the present study, we speculated
that the p16 protein plays a role in inhibiting the proliferation
of hemangioma endothelial cells and in anti-angiogenesis. There is
a certain association between p16 and the regression of hemangioma.
This provides a theoretical basis for the further study of the
pathological mechanisms of p16 in hemangioma and potential gene
therapies that may treat this disease.
References
|
1
|
Sans V, Dumas de la Roque ED, Berge J,
Grenier N, Boralevi F, Mazereeuw-Hautier J, Lipsker D, Dupuis E,
Ezzedine K, Vergnes P, et al: Propanolol for severe infantile
hemangiomas: Follow-up report. Pediatrics. 124:e423–e431. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen TS, Eichenfield LF and Friedlander
SF: Infantile hemangiomas: An update on pathogenesis and therapy.
Pediatrics. 131:99–108. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang B, House MG, Guo M, Herman JG and
Clark DP: Promoter methylation profiles of tumor suppressor genes
in intrahepatic and extrahepatic cholangiocarcinoma. Mod Pathol.
18:412–420. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ekholm SV and Reed SI: Regulation of G(1)
cyclin-dependent kinases in the mammalian cell cycle. Curr Opin
Cell Biol. 12:676–684. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li J, Poi MJ and Tsai MD: Regulatory
mechanisms of tumor suppressor P16(INK4A) and their relevance to
cancer. Biochemistry. 50:5566–5582. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bachmann IM, Straume O and Akslen LA:
Altered expression of cell cycle regulators Cyclin D1, p14, p16,
CDK4 and Rb in nodular melanomas. Int J Oncol. 25:1559–1565.
2004.PubMed/NCBI
|
|
7
|
Witkiewicz AK, Knudsen KE, Dicker AP and
Knudsen ES: The meaning of p16(ink4a) expression in tumors:
Functional significance, clinical associations and future
developments. Cell Cycle. 10:2497–2503. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mulliken JB and Glowacki J: Hemangiomas
and vascular malformations in infants and children: A
classification based on endothelial characteristics. Plast Reconstr
Surg. 69:412–422. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Serrano M, Hannon GJ and Beach D: A new
regulatory motif in cell-cycle control causing specific inhibition
of cyclin D/CDK4. Nature. 366:704–707. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li W, Sanki A, Karim RZ, Thompson JF, Soon
Lee C, Zhuang L, McCarthy SW and Scolyer RA: The role of cell cycle
regulatory proteins in the pathogenesis of melanoma. Pathology.
38:287–301. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Khoo CM, Carrasco DR, Bosenberg MW, Paik
JH and Depinho RA: Ink4a/Arf tumor suppressor does not modulate the
degenerative conditions or tumor spectrum of the
telomerase-deficient mouse. Proc Natl Acad Sci U S A.
104:3931–3936. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Horbinski C, Nikiforova MN, Hagenkord JM,
Hamilton RL and Pollack IF: Interplay among BRAF, p16, p53, and
MIB1 in pediatric low-grade gliomas. Neuro Oncol. 14:777–789. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
de Andrade BA, León JE, Carlos R,
Delgado-Azañero W, Mosqueda-Taylor A and de Almeida OP:
Immunohistochemical expression of p16, p21, p27 and cyclin D1 in
oral nevi and melanoma. Head Neck Pathol. 6:297–304. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Goto T, Mizukami H, Shirahata A, Yokomizo
K, Kitamura YH, Sakuraba K, Saito M, Ishibashi K, Kigawa G, Nemoto
H, et al: Methylation of the p16 gene is frequently detected in
lymphatic-invasive gastric cancer. Anticancer Res. 30:2701–2703.
2010.PubMed/NCBI
|
|
15
|
Kamb A, Gruis NA, Weaver-Feldhaus J, Liu
Q, Harshman K, Tavtigian SV, Stockert E, Day RS III, Johnson BE and
Skolnick MH: A cell cycle regulator potentially involved in genesis
of many tumor types. Science. 264:436–440. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lo K, Mihm M and Fay A: Current theories
on the pathogenesis of infantile hemangioma. Semin Ophthalmol.
24:172–177. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Phung TL, Hochman M and Mihm MC: Current
knowledge of the pathogenesis of infantile hemangiomas. Arch Facial
Plast Surg. 7:319–321. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Volgareva G, Zavalishina L, Andreeva Y,
Frank G, Krutikova E, Golovina D, Bliev A, Spitkovsky D, Ermilova V
and Kisseljov F: Protein p16 as a marker of dysplastic and
neoplastic alterations in cervical epithelial cells. BMC Cancer.
4:582004. View Article : Google Scholar : PubMed/NCBI
|