Introduction
Breast cancer is a major health concern worldwide.
In the United States, the breast cancer incidence is 120.7 per
100,000 women and breast cancer mortality is 24 per 100,000 women;
the lifetime risk of breast cancer is 12.2% (1). The breast cancer incidence in China is
3.5 times lower than in the United States (2), but models strongly suggest that breast
cancer will soon reach epidemic proportions in China (3). Invasive ductal carcinomas represent
about ~75% of all breast cancers (4).
A number of lifestyle habits are associated with the risk of
developing breast cancer (5), and
recent changes in Chinese lifestyle, such as a shift toward a
Western diet and increased stress, may explain a part of this
increase (6). Our understanding of
the pathogenesis of breast cancer has gaps (7,8), and there
is an urgent requirement to improve this knowledge.
The paired box (PAX) family member, PAX6, is located
on chromosome 11p13 in humans, and encodes the PAX6 protein that
plays an important role in the development of human neuroectodermal
epithelial tissues (9). A previous
study reported that PAX6 participates in the regulation of
neuroectodermal cell differentiation and apoptosis (10). PAX6 has also been suggested as a tumor
suppressor gene for glioblastoma and prostate cancer, and as an
early differentiation marker for neuroendocrine cells (11).
PAX genes are often expressed in solid cancers and
are necessary for cancer cell survival (12). In prostate cancer tissues, the
expression level of PAX6 in cancer cells is significantly lower
than in normal epithelial cells (11). An epigenetic study revealed that the
promoter of PAX6 was hypermethylated in breast cancer (13). Our previous study revealed that PAX6
is involved in breast cancer cell proliferation and tumor
progression in vitro (14),
while a screening of cancer cell lines revealed that PAX6 was
highly expressed in breast cancer cell lines (12).
However, to the best of our knowledge, no study has
yet detected the expression of PAX6 in invasive ductal breast
cancer tissues and evaluated its prognostic significance. The
present study aimed to investigate PAX6 protein expression in an
invasive ductal breast cancer tissue microarray (TMA) using
immunohistochemistry (IHC), and to evaluate the association between
PAX6 expression and the prognosis of invasive ductal breast cancer,
in order to show the potential of PAX6 for improving the assessment
of invasive ductal breast cancer prognosis and as an eventual
treatment target.
Subjects and methods
Human subjects and tissue
specimens
The cohort included a total of 119 patients with
invasive ductal breast cancer, diagnosed and surgically treated
between January 2001 and December 2003 at the Department of Breast
Cancer, Zhejiang Cancer Hospital (Hangzhou, Zhejiang, China). All
patients had received no radiotherapy or neoadjuvant therapy prior
to surgery. Of these 119 cases, 8 patients were excluded due to
incomplete medical records. The remaining 111 patients with primary
breast cancer received conventional post-operative treatments,
depending on the extent of the disease. Patients without axillary
lymph node involvement were treated with a modified radical
mastectomy and 6 cycles of cyclophosphamide (100 mg/m2,
orally, days 1–14), methotrexate (40 mg/m2, days 1 and
8) and 5-fluorouracil (600 mg/m2, days 1 and 8)
chemotherapy, cycled every 28 days (if the tumor size was ≥1 cm).
Patients with axillary lymph node involvement received
5-fluorouracil (500 mg/m2, day 1), epirubicin (100
mg/m2, day 1) and cyclophosphamide (500
mg/m2, day 1) chemotherapy, cycled every 21 days for 3
cycles, followed by docetaxel chemotherapy (100 mg/m2,
day 1), cycled every 21 days for 3 cycles. Alternatively, certain
patients received epirubicin (100 mg/m2, day 1) and
cyclophosphamide (500 mg/m2, day 1) chemotherapy, cycled
every 21 days for 4 cycles, followed by docetaxel chemotherapy (100
mg/m2, day 1), cycled every 21 days for 4 cycles.
Patients with positive nodes or a tumor size of ≥5 cm received
postoperative radiotherapy to the breast (50 Gy in 25 fractions). A
boost to the tumor bed was applied in patients at higher risk
(those aged <50 years and those with high-grade disease).
Patients with estrogen receptor (ER)+/progesterone
receptor (PR)+ tumors were treated for 5 years with
tamoxifen or aromatase inhibitors. Patient characteristics,
including age, menopausal status and clinical stage
(tumor-node-metastasis classification defined by the International
Union against Cancer, 2003) (15),
were assessed.
All patients were followed up for at least 4 months,
and up to 131 months or until mortality. Patients who were lost to
follow-up due to mortality or any other reasons were censored at
last contact. Overall survival (OS) time was calculated as the time
between surgery and mortality from any cause. This study was
approved by the Clinical Research Ethics Board of the Zhejiang
Cancer Hospital. Written informed consent was obtained from each
patient.
TMAs and IHC
TMAs were constructed using a Beecher Instruments
Tissue Array (Beecher Instruments, Silver Spring, MD, USA)
(16). Briefly, archival paraffin
blocks containing invasive ductal carcinoma tissues were selected.
Two cores of 1.5 mm in diameter were sampled from the tumor area of
each specimen (donor block), and transferred into the TMA block
(recipient block). Consecutive 4-µm thick sections were cut from
the TMA blocks and placed on a poly-L-lysine-coated slide for IHC
analysis. Antigens were retrieved for 5 min in a microwave at high
power followed by 10 min at low power in citrate-buffered saline
(pH 6.0). ER, PR, human epidermal growth factor receptor 2 (HER2)
and PAX6 were detected by IHC. The following antibodies were used:
mouse monoclonal anti-PAX6 (clone D2.38; cat. no. ab78545; 1:150
dilution; Abcam, Cambridge, MA, USA), rabbit monoclonal anti-ER
(clone SP1; cat. no. ab16660; 1:100 dilution; Abcam), mouse
monoclonal anti-PR (clone IA6; cat. no. M3569; 1:200 dilution;
Dako, Carpinteria, CA, USA), and rabbit polyclonal anti-HER2/neu
(clone SP3; cat. no. ab2428; 1:100 dilution; Abcam). Developing
human brain tissues (paraffin-embedded frontal cortex tissues from
children with glioblastoma who had undergone biopsy at the
hospital) known to express high levels of PAX6 were used as the
positive control. Samples from patients with cancers known to be
HER2-amplified or with ER/PR overexpression were used as positive
controls for HER2, ER and PR. Negative controls were performed by
omission of the primary antibody.
ER, PR and HER2 scoring
TMA was scored by two pathologists for the
percentage of tumor cell nuclear positivity. ER and PR were scored
as follows: -, <1%; 1+, 1–25%; 2+, 25–75%; or 3+, >75%. IHC
ER and PR scores were dichotomized as follows: 0, negative; and
≥1+, positive. HER2 was scored as: 0, no staining or faint membrane
staining; 1+, faint membrane staining in <10% of tumor cells,
incomplete membrane staining; 2+, weak to moderate membrane
staining in >10% of tumor cells; and 3+, strong complete
membrane staining in >10% of tumor cells. IHC HER2 scores were
considered negative at 0/1+. All samples with a HER2 score of 2+
were tested for gene amplification by fluorescence in situ
hybridization (FISH; Vysis PathVysion; Abbott Laboratories,
Chicago, IL, USA). Slides were hybridized with probes to HER-2/neu
and CEP17 using the PathVysion HER-2 DNA Probe Kit, according to
the manufacturer's instructions. Sections were counterstained with
DAPI and visualized on a fluorescent microscope (DM4000B, Leica
Biosystems Nussloch GmbH, Nussloch, Germany). Scoring was performed
by one pathologist according to the manufacturer's guidelines,
yielding a HER-2/CEP17 ratio. A HER-2/CEP17 ratio of ≥2 was
considered amplified. IHC HER2 scores of 3+ and FISH-amplified
patients were considered positive. Pathologists were blinded to the
clinical outcomes.
PAX6 scoring
The IHC expression of PAX6 was scored independently
using a semi-quantitative scoring system by two pathologists who
were blinded to the characteristics and outcomes of the patients.
Discordant scores were re-evaluated by the investigators and
consensus scores were used for further analyses. The intensity and
the extent of IHC staining were assessed. Staining intensity in the
nucleus was defined as follows: No staining, 0 points; light brown
particles, 1 point; moderate brown particles, 2 points; and dark
brown particles, 3 points. The percentage of positive cells was
scored as: 1, <25% positive cells; 2, 25–50% positive cells; 3,
51–75% positive cells; and 4, >75% positive cells. The staining
index (SI) was calculated as the product of the intensity and the
percentage of positive staining, and PAX6 expression was defined as
high (SI≥6) or low (SI<6).
Statistical analysis
Statistical analyses were performed using SPSS 19.0
(IBM, Armonk, NY, USA). Correlations between PAX6 expression and
the clinicopathological variables were analyzed using the Pearson
χ2 analysis. Survival was analyzed using the
Kaplan-Meier method, and differences were evaluated using the
log-rank test. The Cox proportional hazards model was used for
univariate and multivariate analyses to examine the potential
prognostic value of different variables on OS. All tests were
two-sided. P<0.05 was considered to indicate a statistically
significant difference.
Results
Patient characteristics
Table I presents the
clinical characteristics of the patients with invasive ductal
carcinoma of the breast. At the time of surgery, the median age was
50 years (range, 29–82 years). The histological grade of the tumor
was grade I in 37 (33.3%) patients, grade II in 71 (64.0) patients
and grade III in 3 (2.7%) patients. Lymph node status was N0 in 42
(37.8%) patients, N1 in 36 (32.5%), N2 in 25 (22.5%) and N3 in 8
(7.2%). Tumor size was <2 cm in 21 (18.9%) patients, 2–5 cm in
78 (70.3%) and >5 cm in 12 (10.8%). ER was positive in 63.1% of
tumors, PR was positive in 55.9%, HER2 was positive in 10.8%, and
ER, PR and HER2 were all negative in 25.2%.
 | Table I.Association between PAX6 expression
and the clinicopathological features of invasive ductal breast
cancer in 111 patients. |
Table I.
Association between PAX6 expression
and the clinicopathological features of invasive ductal breast
cancer in 111 patients.
|
|
| PAX6 expression, n
(%) |
|
|---|
|
|
|
|
|
|---|
| Variables | Total, n (%) | Low (n=75) | High (n=36) | P-values |
|---|
| Age, years |
|
|
|
|
|
<50 | 58 (52.3) | 40 (69.0) | 18 (31.0) | 0.938 |
|
50–69 | 39 (35.1) | 25 (64.1) | 14 (35.9) |
|
|
>70 | 14 (12.6) | 10 (71.4) | 4 (28.6) |
|
| Histological
grade |
|
|
|
|
| G1 | 37 (33.3) | 28 (75.7) | 9 (24.3) | 0.431 |
| G2 | 71 (34.0) | 44 (62.0) | 27 (38.0) |
|
| G3 | 3 (2.7) | 3 (100.0) | 0 (0.0) |
|
| Lymph node
status |
|
|
|
|
| N0 | 42 (37.9) | 25 (59.5) | 17 (40.5) | 0.063 |
| N1 | 36 (32.4) | 24 (66.7) | 12 (33.3) |
|
| N2 | 25 (22.5) | 19 (76.0) | 6 (24.0) |
|
| N3 | 8 (7.2) | 7 (87.5) | 1 (12.5) |
|
| Tumor size, cm |
|
|
|
|
| 2 | 21 (18.9) | 15 (71.4) | 6 (28.6) | 0.976 |
| 2–5 | 78 (70.3) | 51 (65.4) | 27 (34.6) |
|
|
>5 | 12 (10.8) | 9 (75.0) | 3 (25.0) |
|
| ER |
|
|
|
|
|
Negative | 41 (36.9) | 23 (56.1) | 18 (43.9) | 0.049 |
|
Positive | 70 (63.1) | 52 (74.3) | 18 (25.7) |
|
| PR |
|
|
|
|
|
Negative | 49 (44.1) | 32 (65.3) | 17 (34.7) | 0.654 |
|
Positive | 62 (55.9) | 43 (69.4) | 19 (30.6) |
|
| HER2 |
|
|
|
|
|
Negative | 99 (89.2) | 68 (68.7) | 31 (31.3) | 0.930 |
|
Positive | 12 (10.8) | 7 (58.3) | 5 (41.7) |
|
| TNBC |
|
|
|
|
| No | 83 (74.8) | 60 (72.3) | 23 (27.7) | 0.101 |
|
Yes | 28 (25.2) | 15 (53.6) | 13 (46.4) |
|
| Menopausal
status |
|
|
|
|
|
Premenopausal | 53 (47.7) | 38 (71.7) | 15 (28.3) | 0.084 |
|
Postmenopausal | 58 (52.3) | 37 (67.6) | 21 (32.4) |
|
PAX6 staining
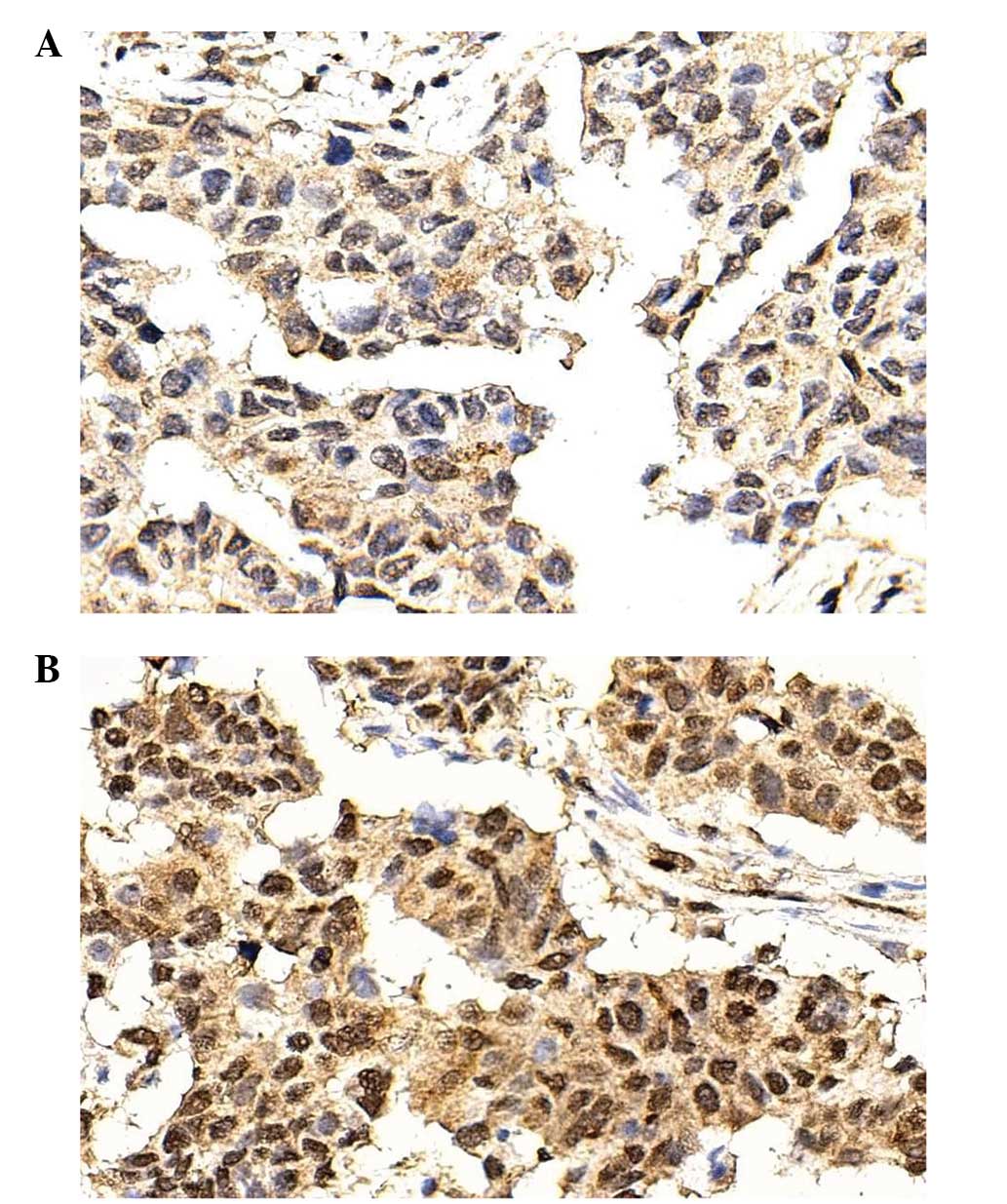
PAX6 was mainly expressed in the nucleus. PAX6 was
expressed at a low (SI<6; Fig. 1A)
or high (SI>6, Fig. 1B) level.
According to the SI, 75 (67.6%) patients exhibited low PAX6
expression, and 36 (32.4%) exhibited high PAX6 expression.
Association between PAX6 and
clinicopathological characteristics
High PAX6 expression was associated with a lower
proportion of ER positivity (25.7 vs. 74.3%; P=0.049) (Table I). PAX6 expression was not associated
with age, histological grade, lymph node status, tumor size, PR,
HER2 and triple-negative breast cancer (TNBC) (all P>0.05).
Survival
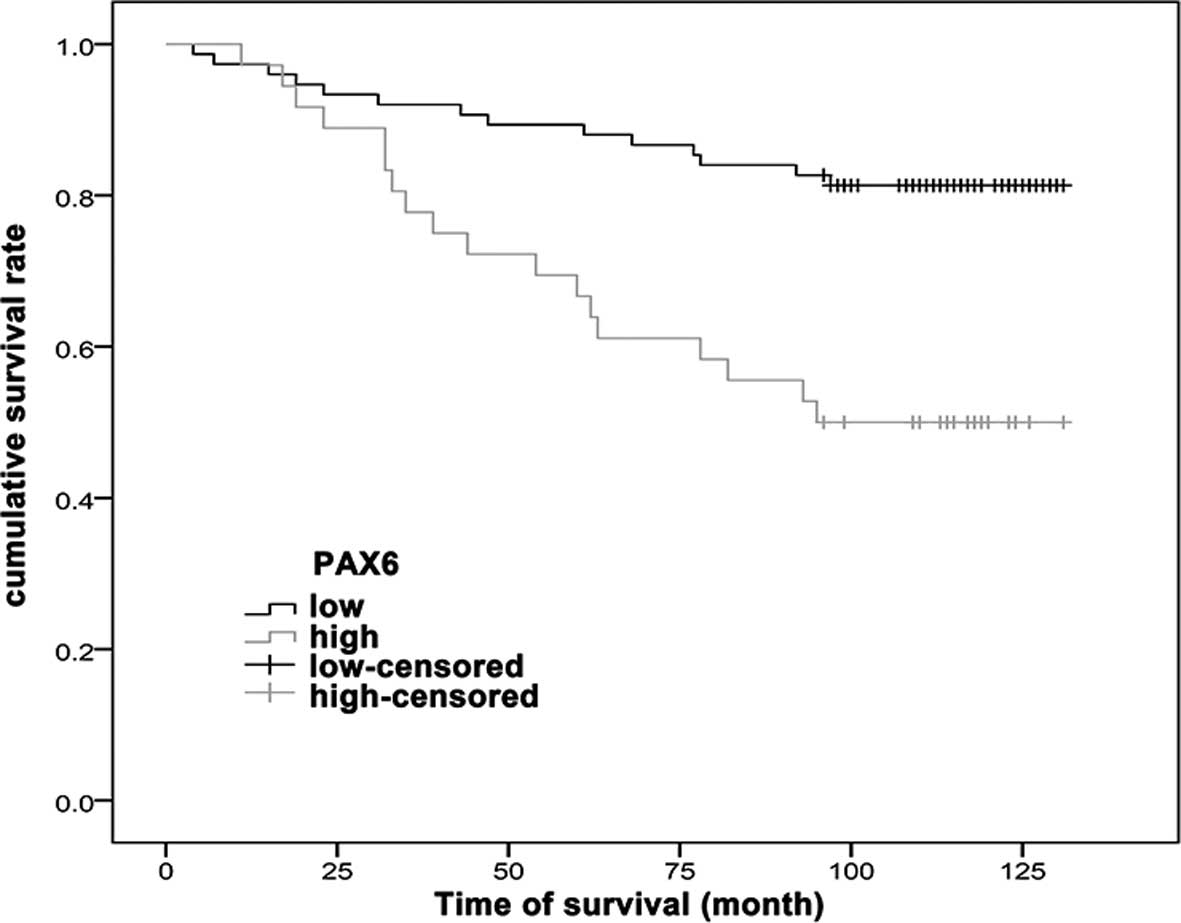
After a median follow-up time of 110 months, the
patients with low PAX6 expression exhibited an improved survival
rate compared with the patients with high PAX6 expression
(P<0.001) (Fig. 2).
Cox regression analysis between
clinicopathological factors and survival
Univariate Cox regression analysis revealed that
high PAX6 expression [hazard ratio (HR), 3.246; 95% confidence
interval (CI), 1.610–6.539; P=0.001], ER positivity (HR, 0.434; 95%
CI, 0.219–0.869; P=0.018), PR positivity (HR, 0.355; 95% CI,
0.171–0.737; P=0.005) and TNBC (HR, 2.773; 95% CI, 1.383–5.583;
P=0.004) were associated with survival. HER2, histological grade,
tumor size, lymph node status and menopausal status were not
associated with survival (all P>0.05) (Table II).
 | Table II.Cox proportional hazards model was
used for univariate and multivariate analysis to examine the
potential prognostic value of different variables on cumulative
survival in the invasive ductal breast cancer patients. |
Table II.
Cox proportional hazards model was
used for univariate and multivariate analysis to examine the
potential prognostic value of different variables on cumulative
survival in the invasive ductal breast cancer patients.
|
| Univariable
analysis | Multivariable
analysis |
|---|
|
|
|
|
|---|
| Variable | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| PAX6 (high vs.
low) | 3.246
(1.610–6.539) | 0.001 | 3.458
(1.575–7.593) | 0.002 |
| ER (positive vs.
negative) | 0.434
(0.219–0.869) | 0.018 | 5.140
(0.343–7.014) | 0.236 |
| PR (positive vs.
negative) | 0.355
(0.171–0.737) | 0.005 | 0.298
(0.089–1.004) | 0.051 |
| TNBC (yes vs.
no) | 2.773
(1.383–5.583) | 0.004 | 1.665
(0.637–4.301) | 0.301 |
| HER2 (positive vs.
negative) | 1.257
(0.484–3.265) | 0.638 |
|
|
| Histological grade
(G2+G3 vs. G1) | 1.096
(0.579–2.315) | 0.810 |
|
|
| Tumor size (>5
vs. ≤5 cm) | 1.841
(0.654–5.252) | 0.254 |
|
|
| Lymph node status
(N1+N2+N3 vs. N0) | 1.204
(0.838–1.729) | 0.316 |
|
|
| Menopausal status
(pre- vs. post-) | 1.310
(0.646–2.654) | 0.454 |
|
|
Multivariate Cox regression analysis showed that
high PAX6 expression (HR, 3.458; 95% CI, 1.575–7.593; P=0.002) was
independently associated with survival. Additionally, there was a
tendency toward an association between PR and survival, but this
was not statistically significant (P=0.051) (Table II).
Discussion
Previous studies have suggested a possible
association between PAX6 and breast cancer. However, no previous
study has investigated PAX6 expression in breast cancer according
to IHC and its association with prognosis. Therefore, the present
study aimed to investigate PAX6 protein expression in a breast
cancer TMA using IHC, and to evaluate the association between PAX6
expression and the breast cancer prognosis.
The results showed that PAX6 staining intensity was
not associated with histological grade, lymph node status, tumor
size, PR or HER2. High PAX6 staining was more frequent in
ER-negative cases compared with ER-positive cases. After a median
follow-up time of 110 months, patients with low PAX6 expression
exhibited an improved survival rate compared with patients with
high PAX6 expression. Cox analysis showed a worst survival rate in
patients with high PAX6 staining (HR, 3.458; 95% CI, 1.575–7.593).
Results suggest that high PAX6 expression in breast cancer is
associated with a worse prognosis. PAX6 may be used to improve the
assessment of breast cancer prognosis, and may eventually be a
treatment target.
Shyr et al (11) used IHC to show that the PAX6
expression level in normal prostate epithelial cells was higher
than that in prostate cancer cells. The results of the present
study showed that PAX6 was expressed in the normal tissues
bordering the tumor and in the breast cancer cells, and that the
staining intensity varied between patients within the range from 2+
to 3+. A previous study (9) suggested
that PAX6 was mainly expressed in stem cells and progenitor cells,
and that PAX6 activation could lead to mitotic arrest, premature
neurogenesis and apoptosis. This may explain the finding of the
present study that patients with high PAX6 expression only had a
survival rate of 50%, suggesting that high PAX6 expression may be
an indicator of the poor prognosis of breast cancer. Lang et
al (17) suggested that PAX6
could be used as a molecular target in cancer therapies.
The present results suggested that PAX6 was an
independent prognostic factor. To the best of our knowledge, this
is the first study suggesting this association in breast cancer,
and no other study is available for direct comparison. A previous
study in gastric cancer showed that methylation of the PAX6
promoter was associated with reduced survival (18). Another study in gastric cancer showed
that PAX6 was associated with markers of a poor prognosis, such as
lymph node metastasis (19). In
addition, PAX6 was upregulated in alveolar soft part sarcoma, a
type of cancer with an extremely poor prognosis (20). In pancreatic cancer, PAX6 actively
participates in tumor growth via the MET tyrosine kinase (21). However, certain studies have suggested
that PAX6 may have a tumor suppressor effect, such as in
glioblastoma (22,23). Therefore, further studies are
necessary to assess the role of PAX6 in tumorigenesis.
Nevertheless, our previous in vitro study showed that PAX
suppression significantly decreased cell viability, DNA synthesis
and the colony formation of MCF-7 and MDA-MB-231 cells, and
inhibited tumorigenesis in xenograft nude mice (14). However, another study showed that the
promoter of PAX6 was frequently hypermethylated in breast cancer
(13), adding to the controversy.
The present results showed that PR-negative staining
may be a factor for a poor prognosis in breast cancer. There are
few studies reporting PR-negative staining as an independent factor
for breast cancer poor prognosis. Only Chen et al (24) suggested that no PR expression was an
independent factor for the early recurrence of breast cancer, which
is consistent with the present results. However, PR-negative
patients include patients with certain triple-negative cancers,
which may cause this difference. Further studies are required to
assess this point.
The present study is not without limitation. First,
the sample size was small. Second, even though the follow-up was
long (median, 110 months), the survival time of breast cancer
patients is generally good and a longer period may be necessary to
more precisely observe the effect of PAX6 expression on
prognosis.
In conclusion, a high tumor PAX6 staining intensity,
as observed by IHC, was independently associated with a poor
prognosis in the breast cancer patients of the present study. PAX6
may be a novel prognosis marker, and may eventually be tested as a
target for therapy.
References
|
1
|
Kohler BA, Ward E, McCarthy BJ, et al:
Annual report to the nation on the status of cancer, 1975–2007,
featuring tumors of the brain and other nervous system. J Natl
Cancer Inst. 103:714–736. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang YC, Wei LJ, Liu JT, Li SX and Wang
QS: Comparison of cancer incidence between China and the USA.
Cancer Biol Med. 9:128–132. 2012.PubMed/NCBI
|
|
3
|
Linos E, Spanos D, Rosner BA, Linos K,
Hesketh T, Qu JD, Gao YT, Zheng W and Colditz GA: Effects of
reproductive and demographic changes on breast cancer incidence in
China: A modeling analysis. J Natl Cancer Inst. 100:1352–1360.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li CI, Anderson BO, Daling JR and Moe RE:
Trends in incidence rates of invasive lobular and ductal breast
carcinoma. JAMA. 289:1421–1424. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
McPherson K, Steel CM and Dixon JM: ABC of
breast diseases. Breast cancer-epidemiology, risk factors, and
genetics. BMJ. 321:624–628. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chia KS, Reilly M, Tan CS, Lee J, Pawitan
Y, Adami HO, Hall P and Mow B: Profound changes in breast cancer
incidence may reflect changes into a Westernized lifestyle: A
comparative population-based study in Singapore and Sweden. Int J
Cancer. 113:302–306. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yager JD and Davidson NE: Estrogen
carcinogenesis in breast cancer. N Engl J Med. 354:270–282. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
McDonnell DP, Park S, Goulet MT, Jasper J,
Wardell SE, Chang CY, Norris JD, Guyton JR and Nelson ER: Obesity,
cholesterol metabolism and breast cancer pathogenesis. Cancer Res.
74:4976–4982. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang J, Lu JP, Suter DM, Krause KH, Fini
ME, Chen B and Lu Q: Isoform- and dose-sensitive feedback
interactions between paired box 6 gene and delta-catenin in cell
differentiation and death. Exp Cell Res. 316:1070–1081. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang X, Huang CT, Chen J, et al: Pax6 is
a human neuroectoderm cell fate determinant. Cell Stem Cell.
7:90–100. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shyr CR, Tsai MY, Yeh S, Kang HY, Chang
YC, Wong PL, Huang CC, Huang KE and Chang C: Tumor suppressor PAX6
functions as androgen receptor co-repressor to inhibit prostate
cancer growth. Prostate. 70:190–199. 2010.PubMed/NCBI
|
|
12
|
Muratovska A, Zhou C, He S, Goodyer P and
Eccles MR: Paired-Box genes are frequently expressed in cancer and
often required for cancer cell survival. Oncogene. 22:7989–7997.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Moelans CB, Verschuur-Maes AH and van
Diest PJ: Frequent promoter hypermethylation of BRCA2, CDH13, MSH6,
PAX5, PAX6 and WT1 in ductal carcinoma in situ and invasive breast
cancer. J Pathol. 225:222–231. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zong X, Yang H, Yu Y, Zou D, Ling Z, He X
and Meng X: Possible role of Pax-6 in promoting breast cancer cell
proliferation and tumorigenesis. BMB Rep. 44:595–600. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sobin LH and Wittekind CH: TNM
Classification of Malignant tumours6th. John Wiley & Sons;
Hoboken, NJ, USA: 2002
|
|
16
|
Zhang B, Cao X, Liu Y, et al:
Tumor-derived matrix metalloproteinase-13 (MMP-13) correlates with
poor prognoses of invasive breast cancer. BMC Cancer. 8:832008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lang D, Powell SK, Plummer RS, Young KP
and Ruggeri BA: PAX genes: Roles in development, pathophysiology,
and cancer. Biochem Pharmacol. 73:1–14. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang Q, Shao Y, Shi J, Qu Y, Wu K, Dang S,
Shi B and Hou P: Concomitant PIK3CA amplification and RASSF1A or
PAX6 hypermethylation predict worse survival in gastric cancer.
Clin Biochem. 47:111–116. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yao D, Shi J, Shi B, et al: Quantitative
assessment of gene methylation and their impact on clinical outcome
in gastric cancer. Clin Chim Acta. 413:787–794. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Selvarajah S, Pyne S, Chen E, et al:
High-resolution array CGH and gene expression profiling of alveolar
soft part sarcoma. Clin Cancer Res. 20:1521–1530. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mascarenhas JB, Young KP, Littlejohn EL,
Yoo BK, Salgia R and Lang D: PAX6 is expressed in pancreatic cancer
and actively participates in cancer progression through activation
of the MET tyrosine kinase receptor gene. J Biol Chem.
284:27524–27532. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhou YH, Wu X, Tan F, et al: PAX6
suppresses growth of human glioblastoma cells. J Neurooncol.
71:223–229. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhou YH, Tan F, Hess KR and Yung WK: The
expression of PAX6, PTEN, vascular endothelial growth factor, and
epidermal growth factor receptor in gliomas: relationship to tumor
grade and survival. Clin Cancer Res. 9:3369–3375. 2003.PubMed/NCBI
|
|
24
|
Chen L, Romond E, Chokshi S, Saeed H,
Hodskins J, Stevens M, Pasley G, Weiss H and Massarweh S: A
prognostic model of early breast cancer relapse after standard
adjuvant therapy and comparison with metastatic disease on initial
presentation. Breast Cancer Res Treat. 136:565–572. 2012.
View Article : Google Scholar : PubMed/NCBI
|