Introduction
Breast cancer (BC) is the most common type of cancer
and the leading cause of cancer-associated mortalities (1). An improved understanding of the key
molecules involved in the pathogenesis of BC is essential for
personalized treatment. Ki67 is a proliferation marker that is
expressed in all the phases of the cell cycle, with the exception
of the G0 phase (2–4). A number
of studies have demonstrated the prognostic value of Ki67 in BC
(5,6).
In addition, Ki67 levels are useful in identifying patients that
are most likely to benefit from chemotherapy (7). Furthermore, changes in Ki67 levels have
been used as a primary efficacy endpoint in clinical trials
(8,9).
At present, Ki67 is an important biomarker used in
routine clinical pathological practice, with potential applications
in prognosis, used to predict responses or resistance to
chemotherapy and endocrine therapy, estimate residual risks in
patients receiving standard therapies and as a dynamic biomarker to
measure treatment efficacies in samples obtained prior to, during
and subsequent to neoadjuvant therapy (10). Increasingly, Ki67 levels are used for
clinical research purposes, including as a primary efficacy
endpoint during clinical trials and, in certain circumstances, for
clinical management. The 2011 St Gallen Expert Panel indicated that
a Ki67 level of ≥14% distinguished luminal B from luminal A tumors
in BC molecular subtyping (11,12).
Therefore, understanding the association of Ki67 with pathological
characteristics (including histological grade, tumor size and lymph
node metastasis) and immunohistochemical indexes [including the
estrogen receptor (ER), progesterone receptor (PR) and human
epidermal growth factor receptor 2 (HER2) status] is important for
clinical evaluations and guiding treatment strategies.
Until recently, limited information was available
regarding the expression of Ki67 among Chinese females with BC.
Furthermore, previous studies on the association between Ki67 and
other clinicopathological parameters have only included small
sample sizes (13–15); therefore, this association remains
controversial. The aim of the present study was to investigate the
distribution of Ki67 expression and its association with other
clinicopathological parameters. In addition, Ki67 expression in
molecular subtypes with various tumor sizes or lymph node statuses
were analyzed.
Materials and methods
Patients
The present study was population-based and included
a cohort of females with newly-diagnosed BC who had been treated in
the breast centers of four hospitals in Wuhan, China, between June
2011 and December 2012. The study included patients from the Renmin
Hospital of Wuhan University, Zhongnan Hospital of Wuhan
University, Hubei Cancer Hospital and the Central Hospital of
Wuhan. In total, 1,535 cases were included in the study. Only
invasive BC cases were included; male patients or those with
incomplete medical records were excluded. Overall, 1,259 patients
were eligible to for inclusion in the this study.
Clinical information regarding age at diagnosis,
ethnicity, clinical stage, pre-surgical chemotherapy treatments,
type of surgery and post-surgical therapy treatments, was retrieved
from the electronic medical records subsequent to Institutional
Review Board approval. The tumor size and grade, nodal stage,
status of molecules (ER, PR, HER2 and Ki67) and histological
subtype were acquired from the pathology database. As part of the
clinical work-up, these investigations were performed prospectively
upon excision specimens at the time of diagnosis. According to
specific criteria, all the pathological results of this study were
histopathologically analyzed by two experienced histological
pathologists. The classification was performed according to
definitions provided by the World Health Organization (16). The tumors were graded according to the
modified Nottingham grading system (17). The patients were staged according to
the 2010 7th edition of the American Joint Committee on Cancer
tumor-node-metastasis (TNM) staging system for BC (18). The study was approved by the Ethics
Committee of Wuhan University.
ER, PR, HER2 and Ki67 detection
Tumor samples obtained from the patients were
assessed or reassessed (if the initial results were already
available) by two experienced pathologists to obtain the Ki67
score, as well as the ER, PR and HER2 status. The four hospitals
used the same primary antibodies.
ER and PR immunohistochemical staining was performed
using a mouse monoclonal anti-human ER antibody (clone, 1D5; Dako,
Glostrup, Denmark) and a mouse monoclonal anti-human PR antibody
(clone, 636; Dako) at a 1:100 dilution. The cut-off value for a
positive result was positive staining for ER and PR in ≥1% of tumor
cells in 10 selected tumor sub-regions (19). The results were recorded as the
percentage of positively-stained nuclei, and the intensity was
graded between 0 and 3+ as follows: i) 0 (negative result),
positive staining in <1% of the tumor cells; ii) 1+, mildly
distinct, positive staining in ≤25% of the tumor cells; iii) 2+,
moderately distinct, positive staining in 25%-50% of the tumor
cells; and iv) 3+, strong, positive staining in >50% of the
tumor cells.
HER2 immunohistochemical staining was performed
using the HercepTest™ assay (Dako). The expression of HER2 was
initially determined by immunohistochemistry and graded between 0
and 3+ as follows: i) 0 (negative result), absence or presence of
HER2 in <10% of the tumor cells; ii) 1+ (negative result),
membranous, weak and discontinuous staining in >10% of the tumor
cells; iii) 2+ (questionable result), membranous, low/moderate and
continuous staining in >10% of the tumor cells, or membranous,
intense and continuous staining in ≤30% of the tumor cells; and iv)
3+ (positive result), membranous, intense and continuous staining
in >30% of the tumor cells. Samples with HER2 scores of 2+ were
confirmed to be HER2-negative or HER2-positive using fluorescence
in situ hybridization analysis (20).
Ki67 immunohistochemical staining was performed
using a mouse monoclonal anti-human Ki67 antibody (clone, MIB-1;
Dako) at a 1:100 dilution. At least three fields in particular
staining ‘hot-spots’ were selected in order to represent the
spectrum of staining observed upon the initial overview of the
entire section. The cancer cells in the three micrographs were
manually counted (500–1,000 cells were counted), and the percentage
of positively-stained cancer cells were considered to be the Ki67
score (10).
The BC cases were divided into five subtypes based
on the expression levels of ER, PR and HER2, and the Ki67
proliferation index as follows: i) Luminal A subtype: ER- and/or
PR-positive, HER2-negative and a low Ki67 proliferation index of
≤14%; ii) luminal B (high Ki67) subtype: ER- and/or PR-positive,
HER2-negative and a high Ki67 index (>14%); iii) luminal B
(HER2-positive) subtype: ER- and/or PR-positive, HER2-positive and
any Ki67 index; iv) HER2-positive (non-luminal) subtype: ER- and
PR-negative, HER2-positive and any Ki67 index; and v)
triple-negative subtype: ER-, PR- and HER2-negative, and any Ki67
index (21).
Statistical analysis
The correlation of Ki67 as a categorical variable
was determined using the χ2-test, two-sample t-test or
one-way analysis of variance for continuous variables. When equal
variances were assumed, post hoc multiple comparisons used the
least significant difference and Student-Newman-Keuls tests. When
equal variances were not assumed, Dunnett's T3 and Dunnett's C
tests were used. Statistical analyses were performed using SPSS
version 21.0 software (SPSS Inc., Chicago, IL, USA). Two-tailed
P<0.05 was considered to indicate a statistically significant
difference.
Results
Clinicopathological features of
patients and distribution of Ki67 expression
The clinicopathological characteristics of the BC
patients are listed in Table I. All
the patients were female, with a median age of 50 years (range,
20–91 years). The majority of the tumor sizes ranged between 2 and
5 cm (pT2) (65%). All the patients underwent axillary dissection.
In total, 673/1,259 cases (53%) were lymph node-negative. Grade II
tumors accounted for 62% (782/1,259) of cases, whilst the ER-, PR-
and HER2-positive rates were 60% (758/1,259), 51% (642/1,259) and
35% (439/1,259), respectively.
 | Table I.Clinicopathological characteristics of
patients and Ki67 distribution. |
Table I.
Clinicopathological characteristics of
patients and Ki67 distribution.
| Characteristic | Number (%) | Ki67
expressiona, number |
χ2-value | P-value | Ki67 level, mean ±
SD, % | One-way ANOVA or
t-test | P-value |
|---|
|
|---|
| Low | High |
|---|
| Age, years |
|
|
|
|
|
|
|
|
| ≤40 | 190 (15) | 64 | 126 | Px=0.758 | 0.685 |
31.37±24.11 | F=0.009 | 0.991 |
| >40,
<50 | 414 (33) | 149 | 265 |
|
|
31.29±25.46 |
|
|
| ≥50 | 655 (52) | 243 | 412 |
|
|
31.13±25.73 |
|
|
| TNM stage |
|
|
|
|
|
|
|
|
| I | 207 (16b) | 101 | 106 | Px=19.394 | <0.001 |
24.96±24.34 | F=9.292 | <0.001 |
| II | 710 (56b) | 251 | 459 |
|
|
31.47±25.57 |
|
|
| III | 342 (27b) | 104 | 238 |
|
|
34.49±25.01 |
|
|
| Tumor size (cm) |
|
|
|
|
|
|
|
|
| pT1
(T≤2) | 295 (23b) | 129 | 166 | Px=9.405 | 0.002 |
28.36±25.58 | F=2.457 | 0.086 |
| pT2
(2<T≤5) | 824 (65b) | 280 | 544 |
|
|
32.12±25.40 |
|
|
| pT3
(T>5) | 140
(11b) | 47 | 93 |
|
|
31.94±24.58 |
|
|
| Node status |
|
|
|
|
|
|
|
|
|
Negative | 673 (53) | 276 | 397 | Cx=13.926 | <0.001 |
28.58±25.01 | t=-3.985 | <0.001 |
|
Positive | 586 (47) | 180 | 406 |
|
|
34.26±25.49 |
|
|
| Tumor grade |
|
|
|
|
|
|
|
|
| 1 | 229 (18) | 136 | 93 | Px=111.703 | <0.001 |
18.18±19.73 | F=103.963 | <0.001 |
| 2 | 782 (62) | 288 | 494 |
|
|
29.56±23.68 |
|
|
| 3 | 248 (20) | 32 | 216 |
|
|
48.50±26.14 |
|
|
| ER status |
|
|
|
|
|
|
|
|
|
Negative | 501 (40) | 103 | 398 | Cx=87.220 | <0.001 |
43.65±26.97 | t=14.580 | <0.001 |
|
Positive | 758 (60) | 353 | 405 |
|
|
23.00±20.51 |
|
|
| PR status |
|
|
|
|
|
|
|
|
|
Negative | 617 (49) | 156 | 461 | Cx=61.712 | <0.001 |
39.69±27.08 | t=12.218 | <0.001 |
|
Positive | 642 (51) | 301 | 341 |
|
|
23.08±20.60 |
|
|
| HER2 status |
|
|
|
|
|
|
|
|
|
Negative | 820 (65) | 360 | 460 | Cx=59.114 | <0.001 |
28.81±26.23 | t=-4.816 | <0.001 |
|
Positive | 439 (35) | 96 | 343 |
|
|
35.72±23.08 |
|
|
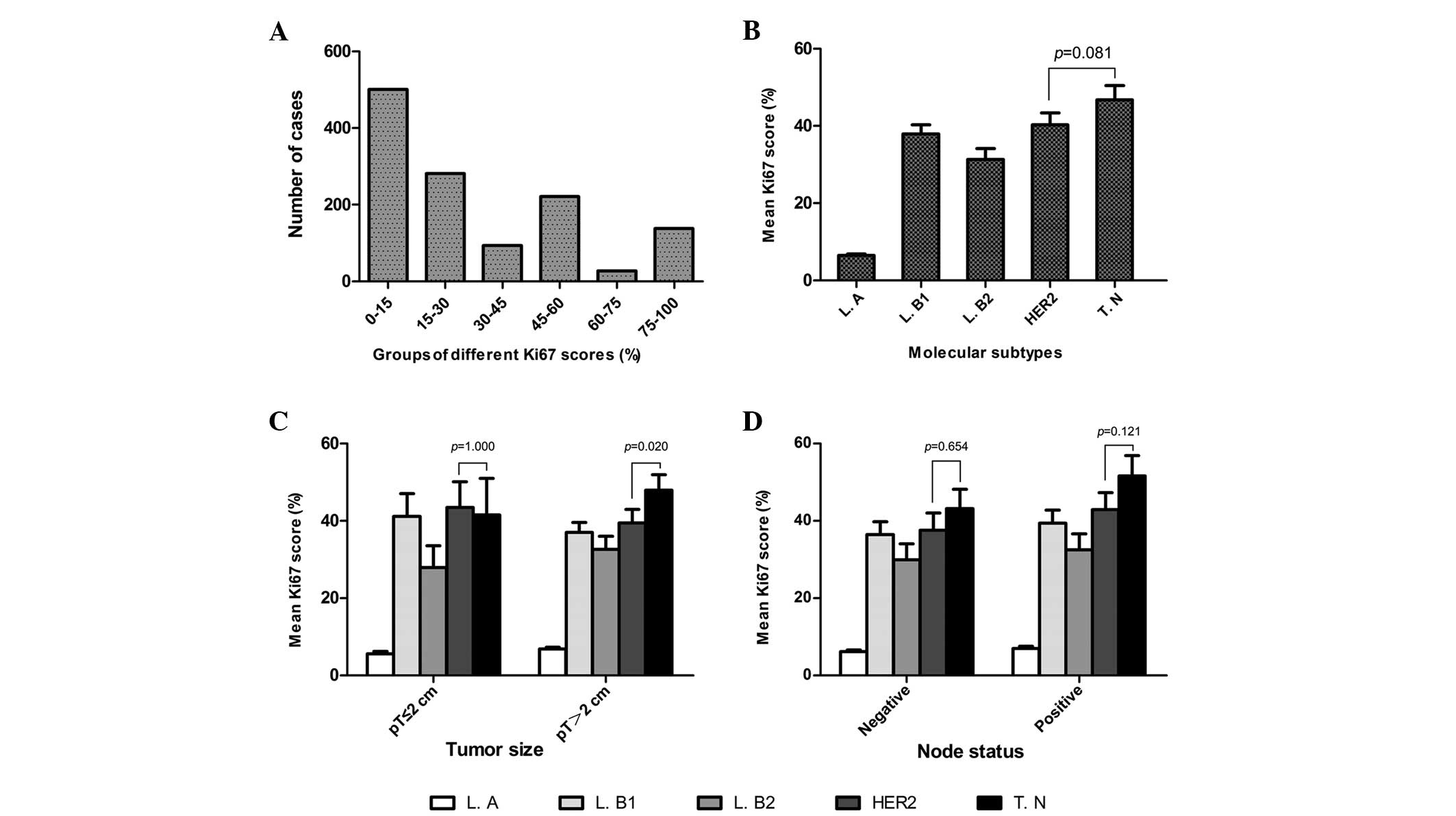
The mean Ki67 score was 31.22% (median, 25%; range,
0–91%). Overall, the Ki67 scores were as follows: ≤15% in 500
cases; >15% but ≤30% for 281 cases; >30% but ≤45% in 93
cases; >45% but ≤60% for 221 cases; >60% but ≤75% in 27
cases; and >75% in 137 cases (Fig.
1A). The clinicopathological characteristics, mean Ki67 scores
and Ki67 expression levels are listed in Table I.
Of the 1,259 eligible patients, 308 (24%) were
classified with a luminal A subtype, 274 (22%) with luminal B (high
Ki67), 211 (17%) with luminal B (HER2-positive), 230 (18%) with
HER2-positive (non-luminal) and 236 (19%) with a triple-negative
subtype. The mean Ki67 scores of these subtypes were 6.48, 37.94,
31.31, 40.29 and 46.79%, respectively. Significant differences were
identified between the Ki67 scores of different BC subtypes
(Fig. 1B).
Association between Ki67 and other
clinicopathological parameters
As a continuous variable, statistically significant
differences were observed between the mean Ki67 scores and the
lymph node status, tumor grade, ER, PR and HER2 status, clinical
stage and molecular subtypes (P<0.001; Fig. 2). When Ki67 was categorized into high
(>14%) and low (≤14%) level groups, the χ2 test was
used to verify these results. No statistically significant
associations were identified between the mean Ki67 scores and age
(P=0.991) or tumor size (P=0.086). When Ki67 was a categorized
variable, a statistically significant association was revealed
between Ki67 expression and tumor size (P=0.002). The data are
presented in Table II. Fig. 2 demonstrates the correlation tendency
between the mean Ki67 scores and the clinicopathological parameters
of tumor size, lymph node status, tumor grade, ER, PR and HER2
status, age, TNM stage and molecular subtype.
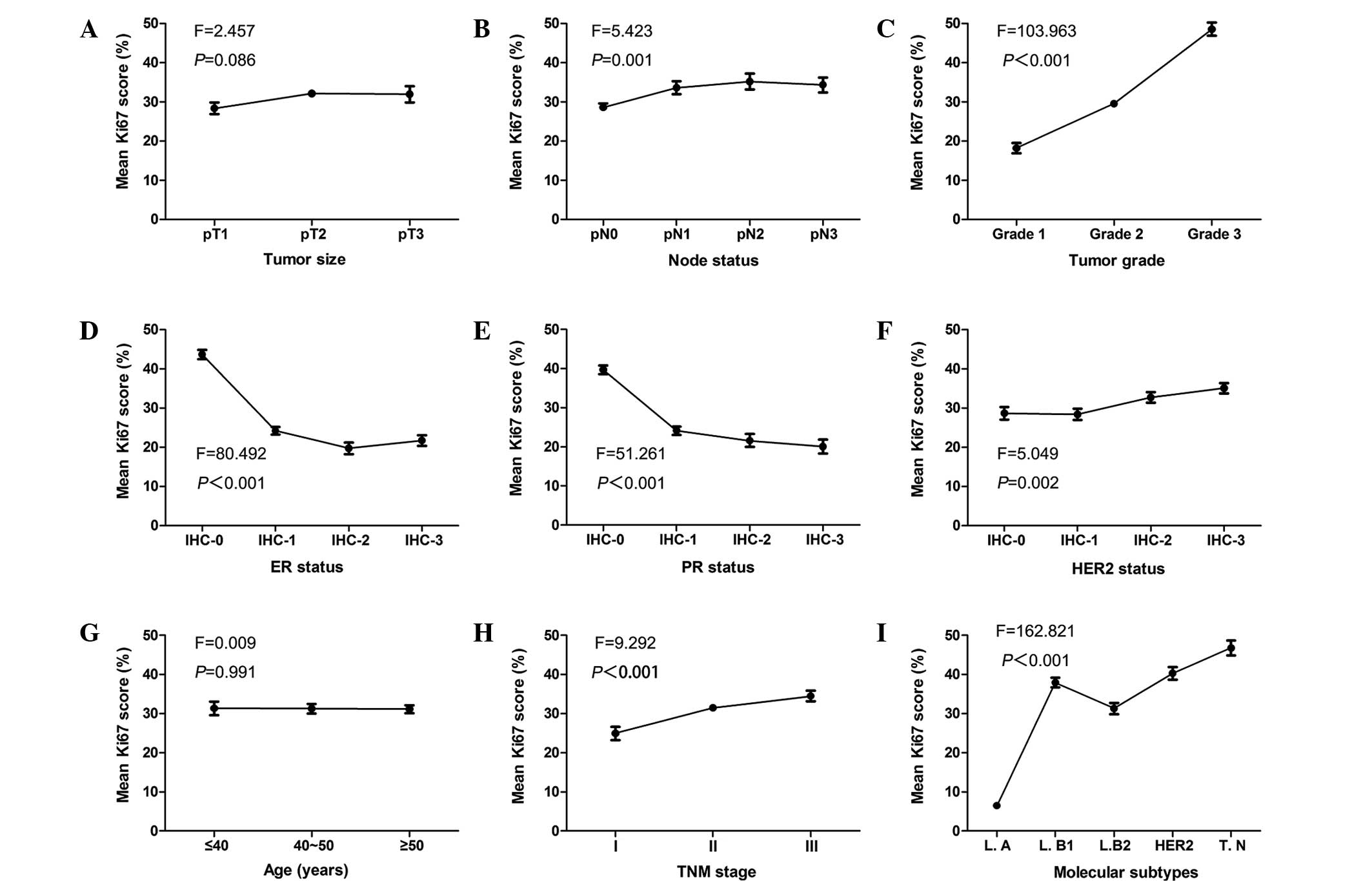 | Figure 2.Correlation tendencies between mean
Ki67 scores and other clinicopathological parameters. Mean Ki67
scores and correlation with (A) tumor size, (B) lymph node status,
(C) tumor grade, (D) ER status, (E) PR status, (F) HER2 status, (G)
age, (H) TNM stage and (I) molecular subtypes. ER, estrogen
receptor; PR, progesterone receptor; HER2, human epidermal growth
factor receptor 2; IHC, immunohistochemical stage; TNM,
tumor-node-metastasis; L.A, luminal A; L.B1, luminal B (high Ki67);
L.B2, luminal B (HER2-positive); HER2, HER2-positive (non-luminal);
T.N, triple-negative. |
 | Table II.Ki67 distribution according to node,
ER, PR and HER2 status. |
Table II.
Ki67 distribution according to node,
ER, PR and HER2 status.
| Characteristic | Number (%) | Ki67
expressiona, number |
χ2-value | P-value | Ki67 level, mean ±
SD, % | One-way ANOVA or
t-test | P-value |
|---|
|
|---|
| Low | High |
|---|
| pN status |
|
|
|
|
|
|
|
|
| pN0
(0) | 673 (53) | 276 | 397 | Px=16.632 | 0.001 |
28.58±25.01 | F=5.423 | 0.001 |
| pN1
(1–3) | 258 (20) | 82 | 176 |
|
|
33.59±26.03 |
|
|
| pN2
(4–9) | 170 (14) | 57 | 113 |
|
|
35.19±26.20 |
|
|
| pN3
(>9) | 158 (13) | 41 | 117 |
|
|
34.35±23.89 |
|
|
| ER status |
|
|
|
|
|
|
|
|
|
IHC-0 | 501 (40) | 103 | 398 | Px=90.973 | <0.001 |
43.65±26.97 | F=80.492 | <0.001 |
|
IHC-1+ | 488 (39) | 237 | 251 |
|
|
24.18±22.33 |
|
|
|
IHC-2+ | 114 (9) | 51 | 63 |
|
|
19.73±15.61 |
|
|
|
IHC-3+ | 156 (12) | 65 | 91 |
|
|
21.72±17.20 |
|
|
| PR status |
|
|
|
|
|
|
|
|
|
IHC-0 | 617 (49) | 156 | 461 | Px=63.707 | <0.001 |
39.69±27.08 | F=51.261 | <0.001 |
|
IHC-1+ | 437 (35) | 210 | 227 |
|
|
24.13±21.94 |
|
|
|
IHC-2+ | 106 (8) | 45 | 61 |
|
|
21.58±16.95 |
|
|
|
IHC-3+ | 99 (8) | 46 | 53 |
|
|
20.06±17.58 |
|
|
| HER2 status |
|
|
|
|
|
|
|
|
|
IHC-0 | 246 (20) | 102 | 144 | Px=48.552 | <0.001 |
28.65±25.72 | F=5.049 | 0.002 |
|
IHC-1+ | 364 (29) | 168 | 196 |
|
|
28.40±26.94 |
|
|
|
IHC-2+ | 353 (28) | 124 | 229 |
|
|
32.69±25.46 |
|
|
|
IHC-3+ | 296 (24) | 62 | 234 |
|
|
35.07±22.34 |
|
|
Ki67 expression in molecular subtypes
with various lymph node statuses or tumor sizes
Ki67 expression in the five molecular subtypes and
its association with the various lymph node statuses or tumor sizes
are listed in Table III.
Statistically significant differences were identified between BC
subtypes with different Ki67 scores and the various lymph node
statuses and tumor sizes. In addition, the mean Ki67 scores were
found to be higher in the triple-negative subtype compared with the
HER2-positive (non-luminal) subtype. However, no statistically
significant differences were evident between the mean Ki67 scores
in the HER2-positive (non-luminal) and triple-negative subtypes,
with the exception of patients with tumors with a size of >2 cm
(P=0.02; Fig. 1B–D).
 | Table III.Ki67 expression in different
molecular breast cancer subtypes. |
Table III.
Ki67 expression in different
molecular breast cancer subtypes.
| Variables | T≤2 cm | T>2 cm | Node-negative | Node-positive | Total |
|---|
| Luminal A |
|
|
|
|
|
| Low
Ki67 expressiona | 88 | 220 | 188 | 120 | 308 |
| High
Ki67 expressiona | 0 | 0 | 0 | 0 | 0 |
| Ki67
level (%)b | 5.55±3.21 | 6.85±3.23 | 6.16±3.35 | 6.97±3.09 | 6.48±3.27 |
| Luminal B (high
ki67) |
|
|
|
|
|
| Low
Ki67 expressiona | 0 | 0 | 0 | 0 | 0 |
| High
Ki67 expressiona | 60 | 214 | 138 | 136 | 274 |
| Ki67
level (%)b | 41.17±22.59 | 37.03±18.92 | 36.49±19.64 | 39.41±19.94 | 37.94±19.81 |
| Luminal B
(HER2-positive) |
|
|
|
|
|
| Low
Ki67 expressiona | 20 | 34 | 27 | 27 | 54 |
| High
Ki67 expressiona | 38 | 119 | 72 | 85 | 157 |
| Ki67
level (%)b | 27.91±21.51 | 32.60±21.22 | 29.92±20.84 | 32.54±21.81 | 31.31±21.35 |
| HER2-positive
(non-luminal) |
|
|
|
|
|
| Low
Ki67 expressiona | 8 | 32 | 21 | 19 | 40 |
| High
Ki67 expressiona | 39 | 151 | 92 | 98 | 190 |
| Ki67
level (%)b | 43.49±22.63 | 39.47±24.34 | 37.59±23.70 | 42.90±24.11 | 40.29±24.00 |
| Triple
negative |
|
|
|
|
|
| Low
Ki67 expressiona | 13 | 41 | 40 | 14 | 54 |
| High
Ki67 expressiona | 29 | 153 | 95 | 87 | 182 |
| Ki67
level (%)b | 41.55±30.32 | 47.93±28.46 | 43.17±29.79 | 51.63±26.90 | 46.79±28.83 |
| Statistical
analysis (P-value) |
|
|
|
|
|
|
χ2 test | <0.001 | 0.001 | 0.001 | 0.001 | 0.001 |
| One-way
ANOVA | <0.001 | 0.001 | 0.001 | 0.001 | 0.001 |
|
Dunnett's T3 testc | 1 | 0.020 | 0.654 | 0.121 | 0.081 |
Discussion
To the best of our knowledge, this is the first
study to describe the distribution of Ki67 expression in BC.
However, Ki67 expression levels were previously reported in several
small-sample BC studies (13,21,22).
Haroon et al (13) identified
that ~39.8% (78/194) of the BC patients included in the study
exhibited a low Ki67 expression (cut-off, 15%), while Fasching
et al observed the same in ~29.3% (162/552; cut-off, 13%) of
the included patients (21).
Furthermore, ~36.2% (471/1,302) of invasive ductal BC patients and
59.7% (237/397) of invasive lobular BC patients demonstrated a low
Ki67 expression (cut-off, 14%) in a study by Heusinger et al
(22). Although the analysis of Ki67
expression may differ from previous studies, the present study
demonstrated similar findings, with 36.2% (456/1,259) of patients
with a low Ki67 expression (cut-off, 15%). The findings of the
present study also indicated that the distribution of Ki67
expression in a Chinese cohort of BC cases may be the same as that
in other countries.
A number of previous studies have investigated the
correlation between Ki67 and other clinicopathological parameters
(13–15); however, the findings were
controversial. A study that included a cohort of Pakistani patients
revealed a significant association between Ki67 expression and
tumor grade, PR, HER2 and lymph node status. However, no
correlation was identified between the ER status and tumor size
(13). The earliest study conducted
in the United Kingdom, demonstrated a significant association
between the Ki67 index and the histological grade, size and type of
the tumors (14). However, these
studies included only a small number of samples. The present
population-based study revealed that Ki67 was significantly
associated with all the clinicopathological parameters, with the
exception of age. In accordance with previous studies, the present
study confirmed the importance of the Ki67 level in predicting the
prognosis of BC (23–26).
The results of the present study also identified
marked differences between the Ki67 scores and the levels of the
ER, PR and HER2. Higher levels of ER and PR were correlated with
declining Ki67 scores, while higher levels of HER2 were associated
with increasing Ki67 scores. These results indicated that there was
an increased proliferative activity in the BC cells with lower
levels of ER and PR, or higher levels of HER2, and that Ki67 is an
accurate biomarker that reflects tumor cellular proliferate
activity. Furthermore, the results demonstrated that the Ki67 score
increased with increasing tumor size in the early stages of BC.
However, when the BC progressed to a certain stage, the Ki67 score
did not increase accordingly. This indicated that the proliferative
activity increases with the progression of a tumor to a certain
stage, at which it no longer significantly changes. This may be due
to insufficient blood supply and nutrition, which is unable to
support tumor growth after a certain point.
A previous study also identified higher Ki67
expression levels in triple-negative and HER2-positive subtypes
compared with the luminal subtypes (27). However, whether the levels of Ki67 are
highest in the triple-negative or the HER2-positive subtype
required further investigation. In the present study, the
expression of molecular subtypes with various tumor sizes and lymph
node statuses were also identified. The results revealed that the
mean Ki67 scores were not significantly different between the
HER2-positive (non-luminal) and triple-negative subtypes, with the
exception of patients with a tumor size of >2 cm. This indicated
the presence of stronger proliferative activity in the
triple-negative subtype compared with the HER2-positive
(non-luminal) subtype, with regard to patients with a tumor size of
>2cm BC patients.
The present study also has certain limitations. The
immunohistochemical approaches that were used had limited technical
reproducibility, subjective interpretation and qualitative
readouts. In addition the samples and data obtained for the study
were from different hospitals and pathology laboratories, which may
have lead to specific biases. Nevertheless, this population-based
study from central China demonstrated the correlation between Ki67
and other clinicopathological parameters in invasive BC cases.
Furthermore, to the best of our knowledge, this study contained the
greatest number of samples used in China and in other counties to
investigate the distribution of Ki67 in BC patients.
In conclusion, in the present study a significant
correlation was identified between Ki67 and ER, PR, and HER2
status, tumor size, lymph node status, tumor grade and molecular
subtypes in invasive BC, which indicates that Ki67 presents an
important biomarker. Thus, analysis of Ki67 expression may be
useful in clinical practice and may present an option for the
personalized treatment of BC patients in the future.
Acknowledgements
This study was supported by grants from the National
Science Foundation of China (nos. 81201196, 81302314 and 81230031),
the Fundamental Research Funds for the Central Universities (no.
121004), the Natural Science Foundation of Hubei Province, China
(nos. 301130851, 2011CBD489 and 2013CFB374), the Research
Foundation of Public Health Bureau of Hubei Province (nos.
JS-2011018, JX4B19 and JX3A14) and the National Science and
Technology Major Project of the Ministry of Science and Technology
of China (no. 2012YQ16020306).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gerdes J, Schwab U, Lemke H and Stein H:
Production of a mouse monoclonal antibody reactive with a human
nuclear antigen associated with cell proliferation. Int J Cancer.
31:13–20. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gerdes J, Lemke H, Baisch H, Wacker HH,
Schwab U and Stein H: Cell cycle analysis of a cell
proliferation-associated human nuclear antigen defined by the
monoclonal antibody Ki-67. J Immunol. 133:1710–1715.
1984.PubMed/NCBI
|
|
4
|
Gerdes J, Li L, Schlueter C, et al:
Immunobiochemical and molecular biologic characterization of the
cell proliferation-associated nuclear antigen that is defined by
monoclonal antibody Ki-67. Am J Pathol. 138:867–873.
1991.PubMed/NCBI
|
|
5
|
de Azambuja E, Cardoso F, de Castro G Jr,
et al: Ki-67 as prognostic marker in early breast cancer: a
meta-analysis of published studies involving 12,155 patients. Br J
Cancer. 96:1504–1513. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kontzoglou K, Palla V, Karaolanis G, et
al: Correlation between Ki67 and breast cancer prognosis. Oncology.
84:219–225. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jones RL, Salter J, A'Hern R, et al:
Relationship between oestrogen receptor status and proliferation in
predicting response and long-term outcome to neoadjuvant
chemotherapy for breast cancer. Breast Cancer Res Treat.
119:315–323. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ellis MJ, Coop A, Singh B, et al:
Letrozole inhibits tumor proliferation more effectively than
tamoxifen independent of HER1/2 expression status. Cancer Res.
63:6523–6531. 2003.PubMed/NCBI
|
|
9
|
Dowsett M, Smith IE, Ebbs SR, et al IMPACT
Trialists: Short-term changes in Ki-67 during neoadjuvant treatment
of primary breast cancer with anastrozole or tamoxifen alone or
combined correlate with recurrence-free survival. Clin Cancer Res.
11:951s–958s. 2005.PubMed/NCBI
|
|
10
|
Dowsett M, Nielsen TO, A'Hern R, et al
International Ki-67 in Breast Cancer Working Group: Assessment of
Ki67 in breast cancer: recommendations from the International Ki67
in Breast Cancer Working Group. J Natl Cancer Inst. 103:1656–1664.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cheang MC, Chia SK, Voduc D, et al: Ki67
index, HER2 status, and prognosis of patients with luminal B breast
cancer. J Natl Cancer Inst. 101:736–750. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Goldhirsch A, Wood WC, Coates AS, Gelber
RD, Thürlimann B and Senn HJ: Role: Panel MembersStrategies for
subtypes - dealing with the diversity of breast cancer: highlights
of the St. Gallen International Expert Consensus on the Primary
Therapy of Early Breast Cancer 2011. Ann Oncol. 22:1736–1747. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Haroon S, Hashmi AA, Khurshid A, et al:
Ki67 index in breast cancer: correlation with other prognostic
markers and potential in pakistani patients. Asian Pac J Cancer
Prev. 14:4353–4358. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pinder SE, Wencyk P, Sibbering DM, et al:
Assessment of the new proliferation marker MIB1 in breast carcinoma
using image analysis: associations with other prognostic factors
and survival. Br J Cancer. 71:146–149. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Midulla C, De Iorio P, Nagar C, et al:
Immunohistochemical expression of p53, nm23-HI, Ki67 and DNA
ploidy: correlation with lymph node status and other clinical
pathologic parameters in breast cancer. Anticancer Res.
19:4033–4037. 1999.PubMed/NCBI
|
|
16
|
No authors listed. The World Health
Organization. Histological typing of breast tumors. Neoplasma.
30:113–123. 1983.PubMed/NCBI
|
|
17
|
Robbins P, Pinder S, de Klerk N, et al:
Histological grading of breast carcinomas: a study of interobserver
agreement. Hum Pathol. 26:873–879. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Edge SB, Byrd DR, Compton CC, et al: AJCC
Cancer Staging Manual. 7th. Springer; New York, NY, USA: 2009
|
|
19
|
Hammond ME, Hayes DF, Dowsett M, et al:
American Society of Clinical Oncology/College Of American
Pathologists guideline recommendations for immunohistochemical
testing of estrogen and progesterone receptors in breast cancer. J
Clin Oncol. 28:2784–2795. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wolff AC, Hammond ME, Hicks DG, et al
American Society of Clinical Oncology; College of American
Pathologists: Recommendations for human epidermal growth factor
receptor 2 testing in breast cancer: American Society of Clinical
Oncology/College of American Pathologists clinical practise
guideline update. J Clin Oncol. 31:3997–4013. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fasching PA, Heusinger K, Haeberle L, et
al: Ki67, chemotherapy response, and prognosis in breast cancer
patients receiving neoadjuvant treatment. BMC Cancer. 11:4862011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Heusinger K, Jud SM, Haeberle L, et al:
Association of mammographic density with the proliferation marker
Ki-67 in a cohort of patients with invasive breast cancer. Breast
Cancer Res Treat. 135:885–892. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ferguson NL, Bell J, Heidel R, et al:
Prognostic value of breast cancer subtypes, Ki-67 proliferation
index, age, and pathologic tumor characteristics on breast cancer
survival in Caucasian women. Breast J. 19:22–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Reyal F, Hajage D, Savignoni A, et al:
Long-term prognostic performance of Ki67 rate in early stage,
pT1-pT2, pN0, invasive breast carcinoma. PLoS One. 8:e559012013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yerushalmi R, Woods R, Ravdin PM, Hayes MM
and Gelmon KA: Ki67 in breast cancer: prognostic and predictive
potential. Lancet Oncol. 11:174–183. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kontzoglou K, Palla V, Karaolanis G, et
al: Correlation between Ki67 and breast cancer prognosis. Oncology.
84:219–225. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mitrović O, Čokić V, Đikić D, et al:
Correlation between ER, PR, HER-2, Bcl-2, p53, proliferative and
apoptotic indexes with HER-2 gene amplification and TOP2A gene
amplification and deletion in four molecular subtypes of breast
cancer. Target Oncol. 9:367–379. 2014. View Article : Google Scholar : PubMed/NCBI
|