Introduction
Gefitinib, an epidermal growth factor receptor
(EGFR) inhibitor, competes with adenosine triphosphate for binding
to the tyrosine kinase binding site of the EGFR (1). Gefitinib then blocks the
phosphoinositide 3-kinase/AKT signaling pathway that is implicated
in the proliferation, invasion and survival of cancer cells
(2). Gefitinib is used to treat
patients with advanced non-small cell lung cancer harboring EGFR
mutations; previous data has established that it is better
tolerated and less toxic than the majority of other
chemotherapeutics (3).
The most common side effects associated with
gefitinib are dermatological adverse reactions, which are observed
in up to 50% of patients (4,5). Other adverse effects include diarrhea,
nausea, stomatitis and an asymptomatic elevation of liver enzymes.
However, the majority of these effects are mild and manageable and
do not require the discontinuation of therapy (6). The most severe adverse effect is
interstitial pneumonia, which occurs in 1–2% of patients and is
fatal in ~1/3 of cases (7). In
addition to these common side effects, gefitinib may occasionally
induce a number of unexpected adverse events.
Intestinal obstruction associated with the use of
gefitinib has not been previously reported. The present study
describes a patient with advanced lung cancer who developed nausea,
abdominal distending pain and frequent vomiting whilst undergoing
treatment with gefitinib for primary and metastatic neoplasms,
which led to a diagnosis of intestinal obstruction. Informed
consent was obtained from the patient and the study was approved by
the Institutional Review Board of Huazhong University of Science
and Technology.
Case report
A 57-year-old man, complaining of a dry cough that
had been apparent for >1 month, was admitted to the Union
Hospital, Tongji Medical College (Wuhan, China) in July 2013.
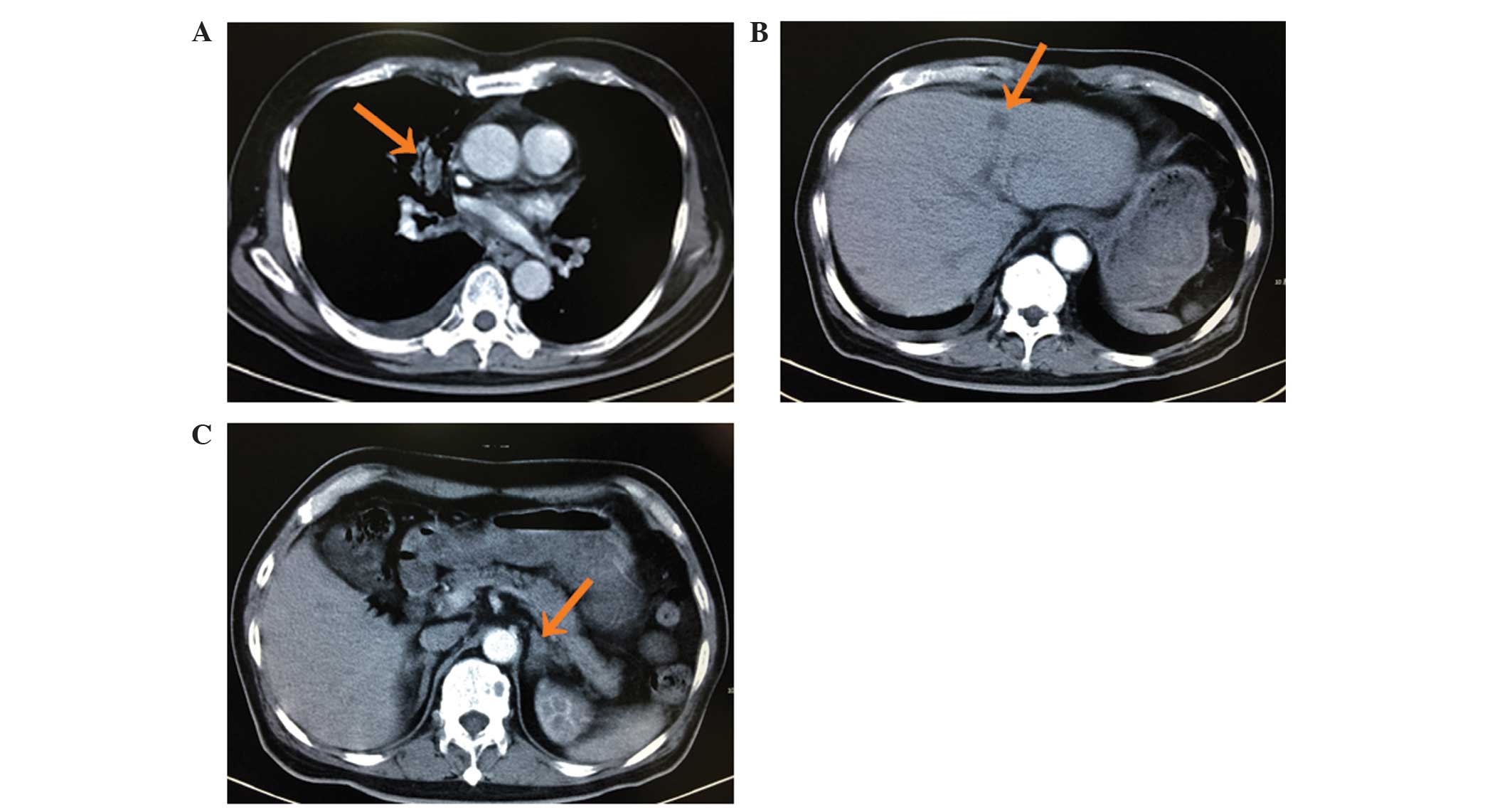
Computed tomography (CT) revealed a lesion in the right upper lobe
of the lung with multiple small nodules scattered throughout the
lungs, multiple masses in the liver, damage to multiple vertebrae
and adrenal thickening (Fig. 1).
These findings were suggestive of lung cancer with intrapulmonary,
liver, bone and adrenal metastasis. A CT-guided needle biopsy of
the pulmonary mass was performed on August 5, 2013. Pathological
analysis of the specimen revealed adenocarcinoma of the lung. An
EGFR gene mutation test identified a deletion in exon 19; however,
no abnormalities were identified in exons 18, 20 or 21. On August
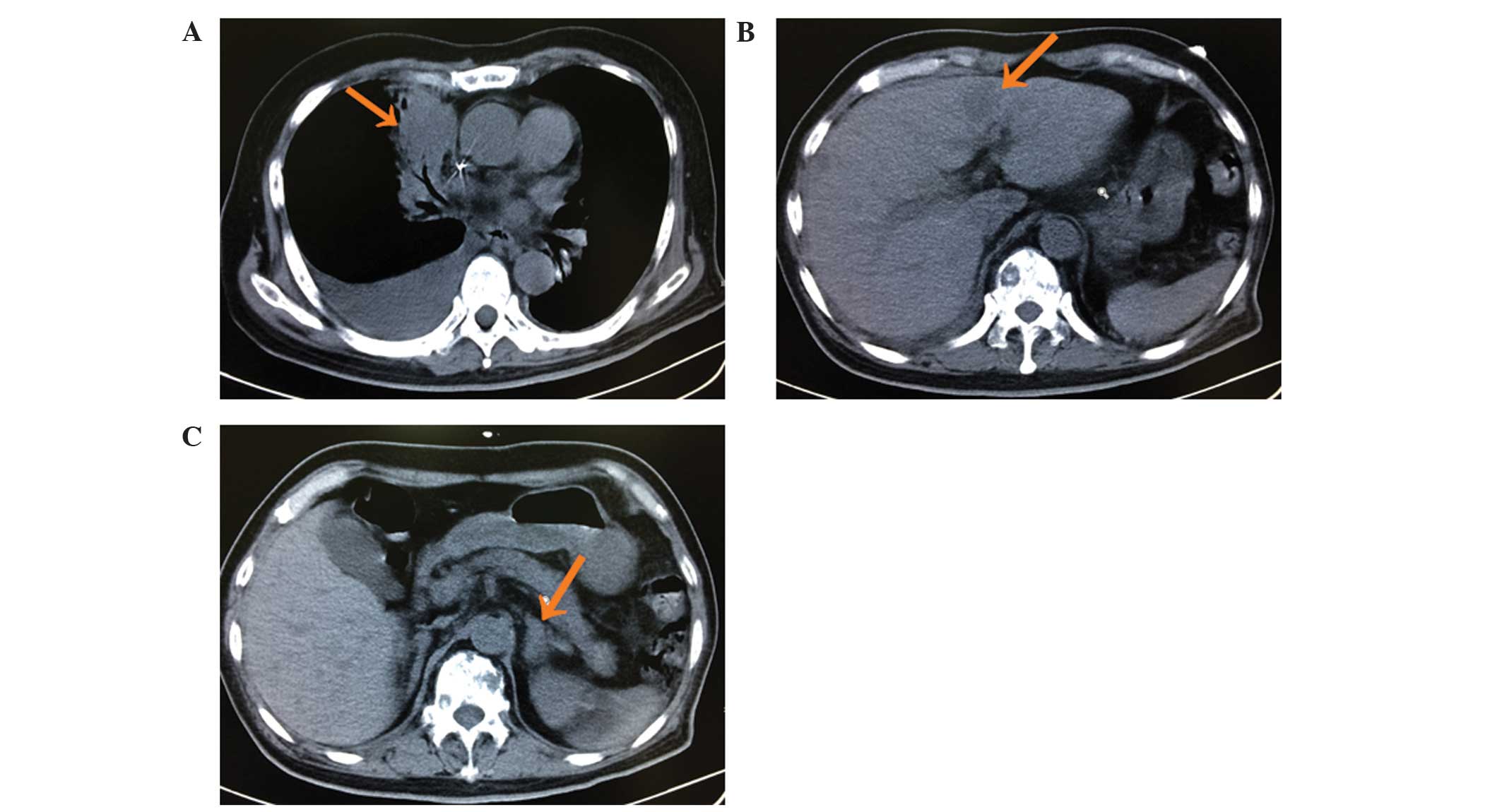
26, 2013, the patient began chemotherapy with pemetrexed (500
mg/m2, 21 days/cycle, three cycles); however, following
three cycles of treatment the disease progressed (Fig. 2). On November 4, 2013, daily treatment
with 250 mg/day oral gefitinib was initiated. The symptom of cough
was much improved following four days of gefitinib treatment.
However, 10 days following the initiation of the treatment with
gefitinib, the patient developed nausea, abdominal distending pain
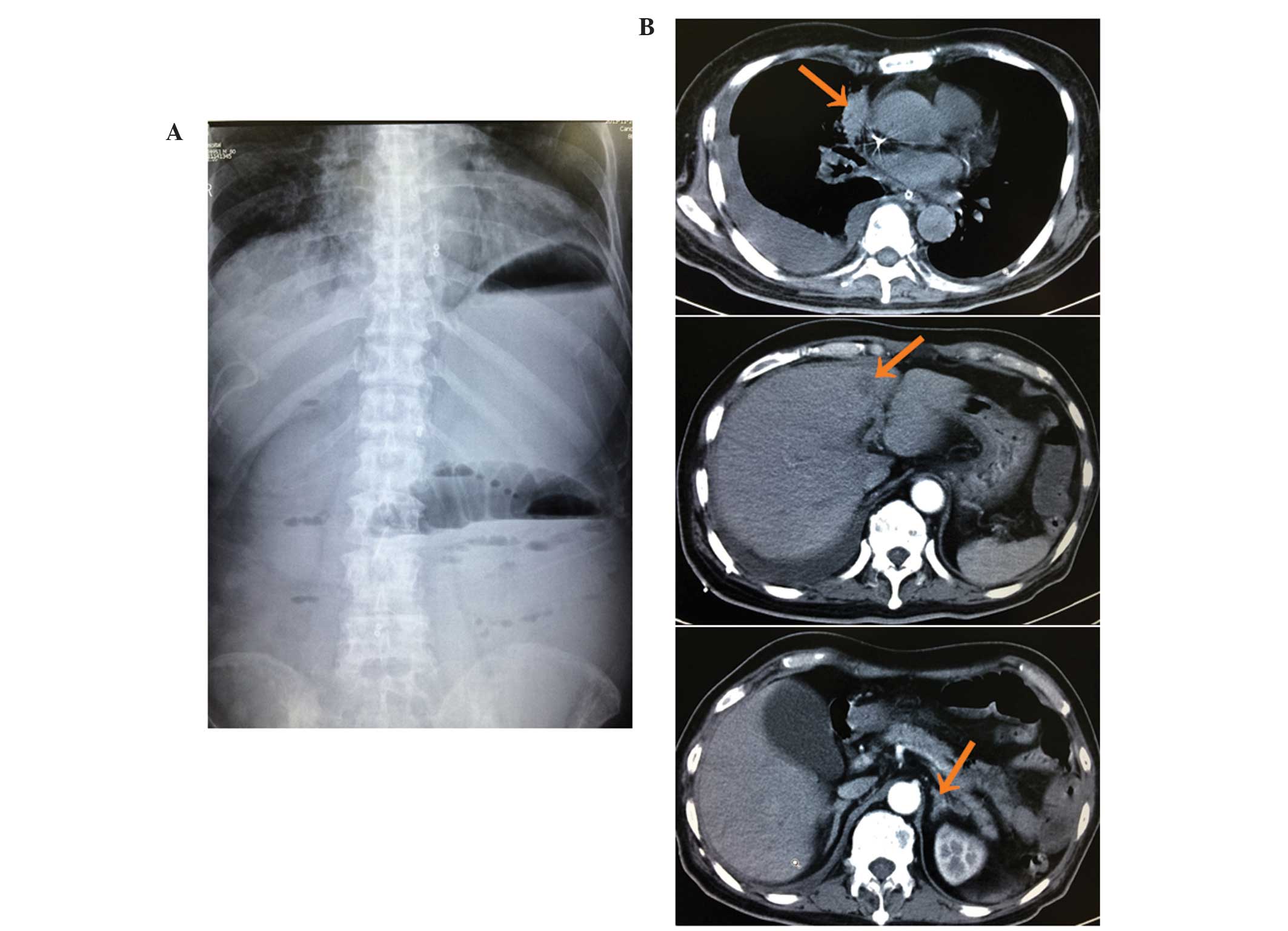
and frequent vomiting. An abdominal plain film, which was performed
12 days following the initiation of the gefitinib, identified an
intestinal obstruction (Fig. 3A). In
addition, CT revealed smaller lung and liver masses and thinner
adrenals than previously observed, but no gastrointestinal mass
(Fig. 3B). Therefore, it was
hypothesized that the intestinal obstruction had been induced by
gefitinib. Gefitinib treatment was immediately stopped and the
patient received gastrointestinal decompression, parenteral
alimentation, antibiotics (cefoperazone sodium and sulbactam
sodium; 3 g/day for 1 week), anti-peptic enzymes (octreotide
injection; 300 µg/day for 1 week) and acid suppressants (omeprazole
sodium; 40 mg/day for 1 week). Following 1 week of discontinuation
of gefitinib, the symptoms of intestinal obstruction disappeared.
Based on the patient's recovery, gefitinib treatment was
reinitiated under close observation. The dosage of gefitinib
administered to the patient was adjusted to 250 mg taken once every
2 days and intestinal obstruction did not recur.
Discussion
The patient in the present study was diagnosed with
adenocarcinoma of lung and had no history of gastrointestinal
disease. Prior to treatment with gefitinib there were no symptoms
of intestinal obstruction. However, 10 days subsequent to the
initiation of gefitinib treatment, the patient developed nausea,
abdominal distension and frequent vomiting. An abdominal plain film
revealed an intestinal obstruction. CT identified no masses in the
gastrointestinal tract and established that the therapeutic
evaluation of gefitinib treatment was that of partial remission.
Following the immediate discontinuation of gefitinib and the
symptomatic treatment of the intestinal obstruction, the patient
recovered. The dose of gefitinib was subsequently reduced to 250 mg
once every 2 days and the intestinal obstruction did not recur.
Therefore, it was concluded that the intestinal obstruction was
associated with gefitinib treatment.
The most common adverse effects associated with the
use of gefitinib are acneiform skin rashes, diarrhea and nausea;
however, these are usually mild and manageable (6). Mucositis/stomatitis is another common
non-hematological toxicity that has frequently been observed in
patients receiving treatment with gefitinib (8). The development of therapeutic approaches
without severe adverse side effects, as well as continuous
management of the therapeutic regime, are required to ensure that
patients receive treatment strategies that do not adversely affect
survival or quality of life. Management of these dermatological
adverse effects rarely requires discontinuation of targeted therapy
and can typically be managed symptomatically (9).
Intestinal obstruction associated with the use of
gefitinib has not been previously reported and the mechanisms
underlying it remain to be elucidated. However, the findings of the
present study suggested that careful monitoring of gastrointestinal
symptoms is important throughout the course of gefitinib treatment.
When intestinal obstruction occurs during the course of
gefitinib-based treatment, cessation of therapy should be conducted
and, following the recovery of intestinal obstruction, a lower dose
of gefitinib prescribed.
References
|
1
|
Shepherd FA, Dancey J, Ramlau R, et al:
Prospective randomized trial of docetaxel versus best supportive
care in patients with non-small-cell lung cancer previously treated
with platinum-based chemotherapy. J Clin Oncol. 18:2095–2103.
2000.PubMed/NCBI
|
|
2
|
Ettinger DS: Clinical implications of EGFR
expression in the development and progression of solid tumors:
Focus on non-small cell lung cancer. Oncologist. 11:358–373. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Haaland B, Tan PS, de Castro G Jr and
Lopes G: Meta-analysis of first-line therapies in advanced
non-small-cell lung cancer harboring EGFR-activating mutations. J
Thorac Oncol. 9:805–811. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Faivre S, Delbaldo C, Vera K, et al:
Safety, pharmacokinetic and antitumor activity of SU11248, a novel
oral multitarget tyrosine kinase inhibitor, in patients with
cancer. J Clin Oncol. 24:25–35. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hartmann JT and Kanz L: Sunitinib and
periodic hair depigmentation due to temporary c-KIT inhibition.
Arch Dermatol. 144:1525–1526. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hotta K, Kiura K, Takigawa N, et al:
Comparison of the incidence and pattern of interstitial lung
disease during erlotinib and gefitinib treatment in Japanese
patients with non-small cell lung cancer: The Okayama Lung Cancer
Study Group experience. J Thorac Oncol. 5:179–184. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nakagawa M, Nishimura T, Teramukai S, et
al: Interstitial lung disease in gefitinib-treated Japanese
patients with non-small cell lung cancer - a retrospective
analysis: JMTO LC03-02. BMC Res Notes. 2:1572009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Passaro A, Di Maio M, Del Signore E, Gori
B and de Marinis F: Management of nonhematologic toxicities
associated with different EGFR-TKIs in advanced NSCLC: A comparison
analysis. Clin Lung Cancer. 15:307–312. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Madke B, Gole P, Kumar P and Khopkar U:
Dermatological side effects of epidermal growth factor receptor
inhibitors: ‘PRIDE’ complex. Indian J Dermatol. 59:271–274. 2014.
View Article : Google Scholar : PubMed/NCBI
|