Introduction
AITL is the second most frequent subtype of
peripheral T cell lymphoma in the Western world, accounting for
~25–30% of peripheral T cell lymphoma cases and 2–4% of all cases
of lymphoma (1). It has been reported
that AITL presents with a number of clinical symptoms, including
fever, weight loss, chills, skin rashes, pruritis, lymphadenopathy,
hepatosplenomegaly, anemia, thrombocytopenia and
hypergammaglobulinemia (2). Patients
with AITL, frequently exhibit anemia, thrombocytopenia and
lymphopenia in the peripheral blood. However, it is rare for
patients with AITL to have numerous plasma cells in the peripheral
blood, which is a feature more typical of plasma cell leukemia
(3–5).
The current report presents a case of a patient with AITL, in whom
leukemic change and plasmacytosis were observed in the peripheral
blood, bone marrow and lymph nodes.
Case report
A 78-year-old male was admitted to hospital, due to
systemic lymph node swelling and an elevated number of white blood
cells in comparison with normal levels, which had been detected by
his family doctor 10 days previously. Upon physical examination,
lymph nodes in the cervical region, supraclavicular fossa, axillary
fossa and inguinal fossa were found to be enlarged to 40 mm. The
peripheral white blood cell count was elevated and numerous
plasmacytoid cells without dysmorphic features, in addition to
small-to-medium-sized lymphoid cells with atypical nuclei, were
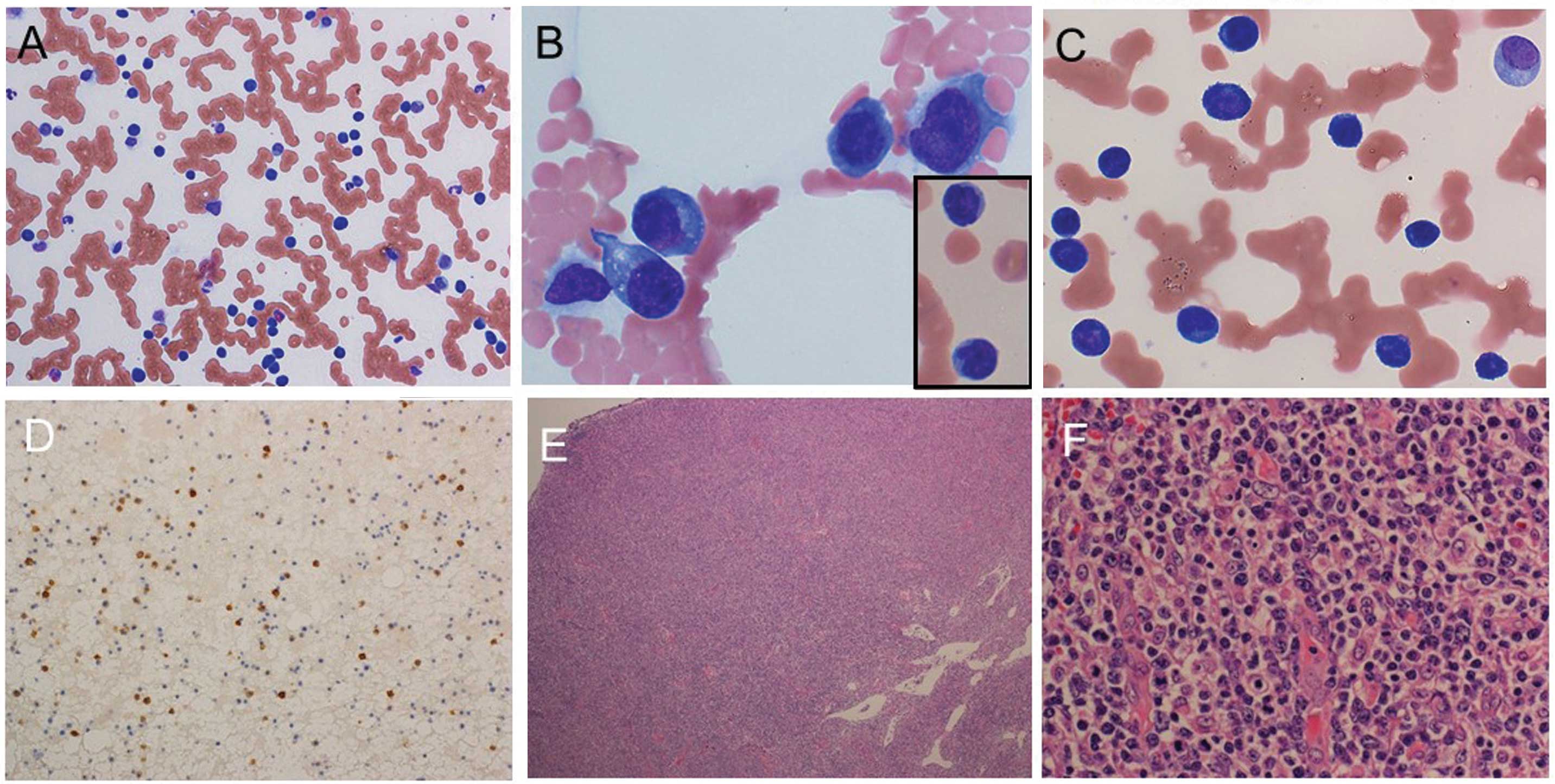
observed in his peripheral blood, as shown in Table I, Fig. 1
and Fig. 2A and B. ‘Other’ in the
leukocyte classification in Table I,
includes plasmacytoid cells (19% of whole white blood cells) and
lymphoid cells with nuclear atypia (22% of whole white blood
cells). Anemia and low platelet numbers were also observed.
Rouleaux formation was noted in the peripheral blood, suggesting
the occurrence of hyperviscosity syndrome, which is likely to have
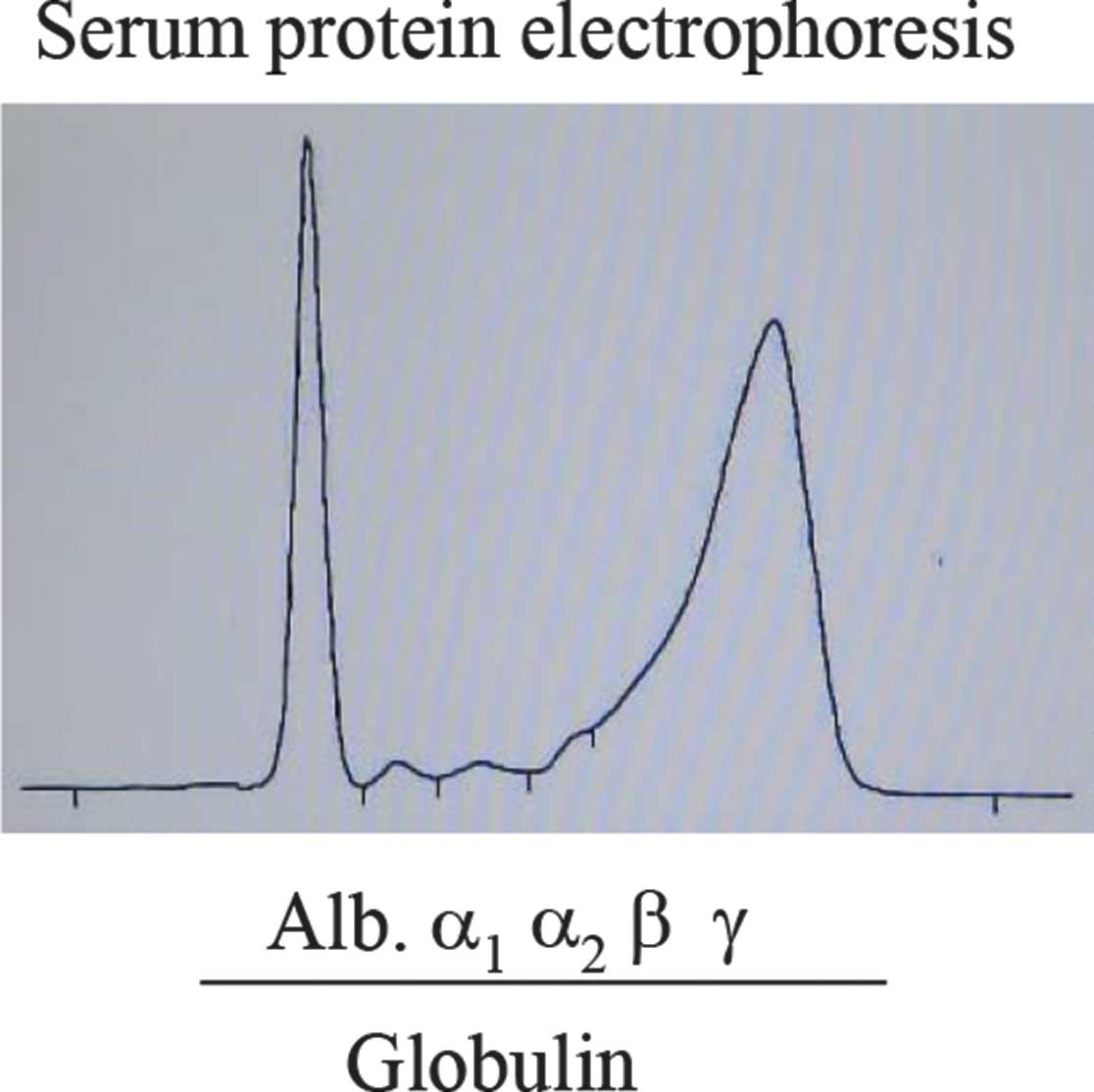
been due to hypergammaglobulinemia (Fig.
2A).
 | Table I.Laboratory results. |
Table I.
Laboratory results.
| Parameter | Result |
|---|
| White blood
cells |
332×102/mm3
(40–80) |
| Myelocytes | 0.5% |
| Band cells | 13.0% (5.0–6.0) |
| Segmented
neutrophils | 30.5% (40–60) |
| Eosinophils | 3.0% (1.0–5.0) |
| Basophils | 0.5% (0–1.0) |
| Monocytes | 6.0% (3.0–5.0) |
| Lymphocytes | 0.5% (30–40) |
| Other | 46.0% |
| Red blood cells |
310×104/mm3
(410–530) |
| Hemoglobin | 9.6 g/dl (13–17) |
| Hematocrit | 28.7% (39–53) |
| Mean cell volume | 92.3 fl (85–102) |
| Mean corpuscular
hemoglobin concentration | 30.9 pg
(28.4–34.6) |
| Mean corpuscular
hemoglobin | 33.4%
(32.5–35.5) |
| Platelets | 5.3 ×
104/mm3 (12–35) |
| INR | 1.63 (1) |
| Activated partial
thrombin time | 51.1 seconds
(25–35) |
| Fibrinogen | 62.7 mg/dl
(150–350) |
| Fibrin degradation
products | 14.1 µg/ml
(<10) |
| D-dimer | 9.2 µg/ml
(<1) |
| Total protein | 10.6 g/dl
(6.7–8.0) |
| Albumin | 2.0 g/dl
(3.4–4.9) |
| Albumin fraction of
protein | 25.0% |
| α1 globulin fraction
of protein | 1.6% |
| α2 globulin fraction
of protein | 2.4% |
| β fraction of
protein | 2.8% |
| γ fraction of
protein | 68.2% |
| Total bilirubin | 1.6 mg/dl
(0.2–1.0) |
| Aspartate
aminotransferase | 67 IU/l (8–30) |
| Alkaline
phosphatase | 284 IU/l
(102–302) |
| Lactate
dehydrogenase | 1,031 IU/l
(106–211) |
| γ-glutamyl
transpepsidase | 33 IU/l (8–64) |
| Blood urea
nitrogen | 22 mg/dl (8–20) |
| Creatinine | 1.06 mg/dl
(0.4–1.2) |
| Glycosylated
hemoglobin | 5.9% (4.4–5.9) |
| C-reactive
protein | 2.4 mg/dl
(0–0.5) |
| Na | 131 mEq/l
(134–145) |
| K | 4.0 mEq/l
(3.6–5.0) |
| Cl | 100 mg/dl
(98–110) |
| Ca | 8.4 mg/dl
(8.0–10.2) |
| IgG | 6,112 mg/dl
(870–1700) |
| IgA | 1,039 mg/dl
(100–410) |
| IgM | 176 mg/dl
(35–220) |
| IgG4 | 84.8 mg/dl
(4.8–105) |
| β2
microglobulin | 13.42 mg/dl
(0.9–2.1) |
| Interleukin-2
receptor | 8,220 U/ml
(145–519) |
| Interleukin-6 | 10.8 pg/ml
(<4) |
Biochemical examination demonstrated a coagulation
abnormality, suggesting disseminated intravascular coagulation,
with high levels of γ-globulin without M-peak, immunoglobulin G
(IgG), IgA, lactate dehydrogenase, C-reactive protein,
β2-microglobulin, interleukin 2-receptor (IL-2R) and IL-6.
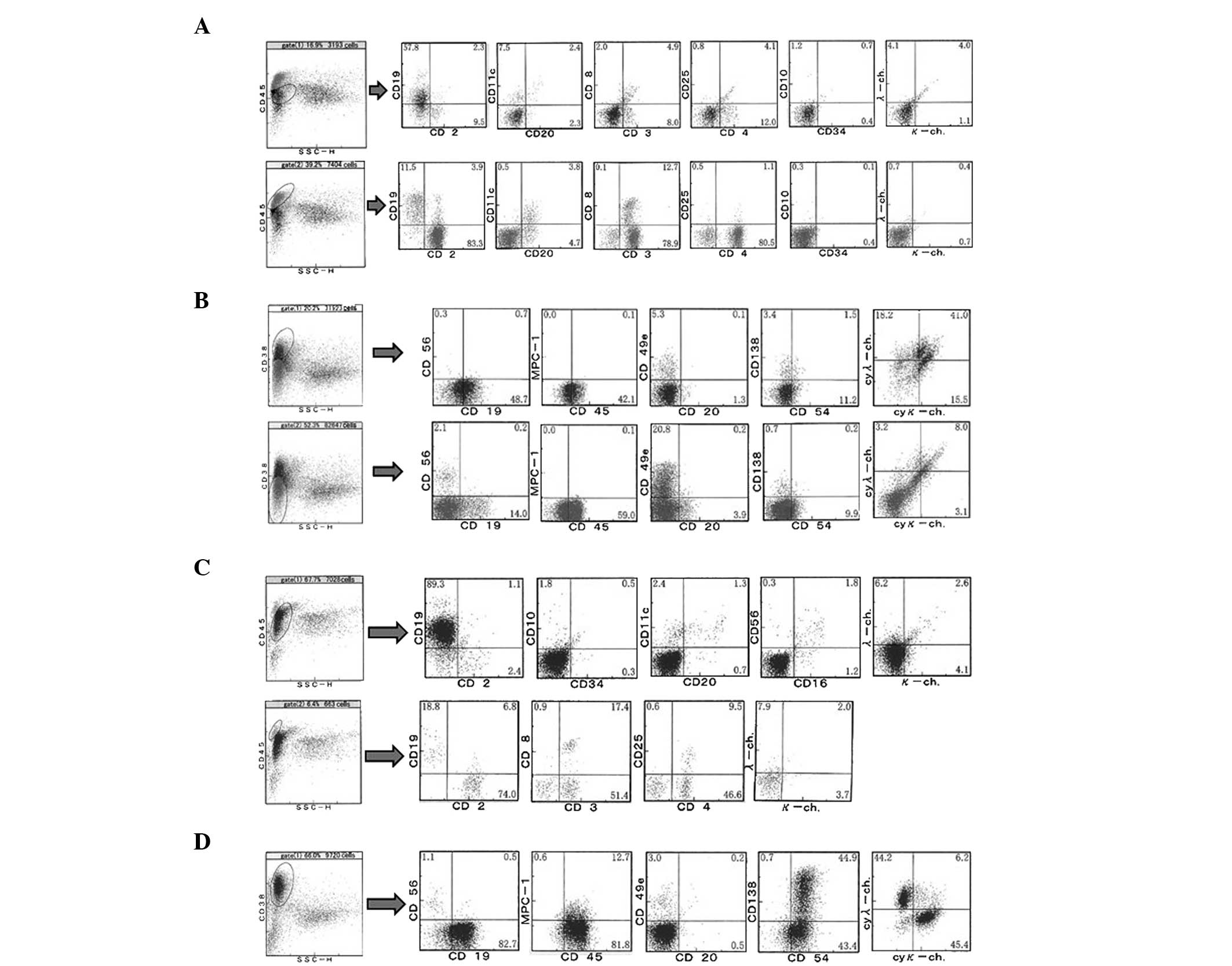
Flow cytometric analyses of the patient's peripheral
blood demonstrated the CD45low population, including
blastic cells, to comprise 16.9% of nuclear cells, while the
CD45high lymphoid cell population comprised 39.2%, as
shown in Fig. 3A. Usually, B cells in
the peripheral blood express both CD19 and CD20. However, of the
CD45low population, there were lots of
CD19+CD20− cells and these cells did not
express the Ig light chain, indicating that the population included
plasma cells or extremely immature B lineage cells. In addition,
there was a marked elevation in the number of CD38+
cells without MCP-1 expression, which formed ~20% of the peripheral
blood nuclear cells, as shown in Fig.
2B, suggesting an increase in immature plasma cells in the
peripheral blood. These results were consistent with the complete
blood counts (CBC; Table I), which
showed that 19% of the whole white blood cells were plasmacytoid
cells.
Within the CD45high population, the
number of CD4+CD3+ cells made up ~30% of
peripheral nuclear cells, which were negative for CD10. The CD4:CD8
ratio was ~6:1, and the majority of the CD4+ cells did
not express CD25. As noted, the percentage of small-to-medium-sized
lymphoid cells with atypical nuclei had increased to ~22% in the
peripheral blood, suggesting that cells exhibiting this increase
may be CD4+CD10−CD25− T cell
lymphoma/leukemia cells.
Enhanced computerized tomography demonstrated
splenomegaly and splenic infarction (data not shown).
Upon examination of the bone marrow, the
CD45low population, including blastic cells, was 67.7%,
and 89.3% of these were CD19+. However, the
CD19+ cells expressed neither CD20 nor surface Ig light
chain (Fig. 3C). As shown in Fig. 3D, CD38+ cells made up 66%
of the bone marrow nuclear cells. Of CD38+ cells, 13.3%
were MPC-1+, 45.6% were CD138+ and 95.8% were
intracytoplasmic Ig light chain-positive, suggesting an elevation
of plasma cells, particularly of immature plasma cells, in the bone
marrow. These plasma cells did not exhibit clonal proliferation,
since the κ:λ ratio in the Ig light chain was ~1:1. In the
lymphocyte gate, ~70% of nuclear cells were T cells, and >2/3 of
the T cells were CD4+. In the May-Giemsa stained smear
specimens of the bone marrow, small-to-medium-sized lymphoid cells
with nuclear atypia were found (data not shown). The cells were
similar to the small to medium sized lymphoid cells with nuclear
atypia observed in the peripheral blood.
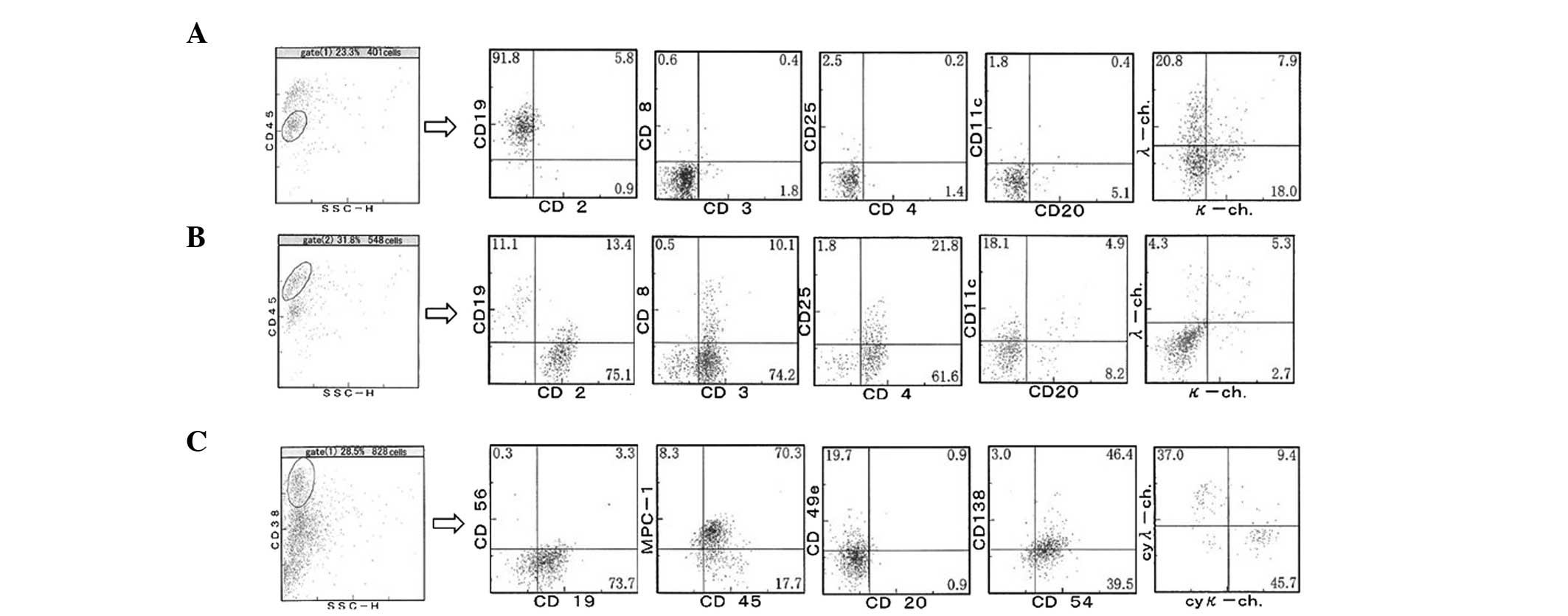
Subsequently, biopsies of an enlarged inguinal lymph
node were taken. The normal architecture of the lymph node had been
replaced by variously-sized, although predominantly medium-sized,
lymphoid cells with clear cytoplasm, as shown in Fig. 2E and F. High endothelial venules were
prominent (Fig. 2F). Upon flow
cytometric analysis, 91.8% of the CD45low population,
including blastic cells, were found to be CD19+, while
the majority of CD19+ cells were CD20−, and
half of CD19+ cells expressed Ig light chain, although
the pattern of expression of light chains did not indicate clonal
expansion. In the gate of CD45high, a lymphocyte gate,
84.3% of cells were CD3+, and nearly all were
CD4+ (Fig. 4B). As shown
in Fig. 4C, even in the lymph node,
the percentage of CD38+ cells had risen to 28.5%. These
cells did not express CD20, while 77% expressed CD19, and ~50% of
expressed CD138. Unlike the peripheral blood and bone marrow
samples, the CD38+ cells in the lymph node sample
expressed MPC-1 and CD45, suggesting an increase in mature plasma
cells in the lymph node. The majority of cells expressed
cytoplasmic Ig light chain, although the κ:λ ratio did not indicate
clonal expansion.
Immunohistological analyses demonstrated a diffuse
increase in CD138+ cells and CD3+ cells (T
cells) within the lymph node specimen (Fig. 5). Among T cells, the number of
CD4+ cells was markedly increased. Numerous
Maf-1+ cells were observed in the lymph node, in
addition to a diffuse distribution of Epstein-Barr virus
(EBV)-encoded small RNA-positive cells.
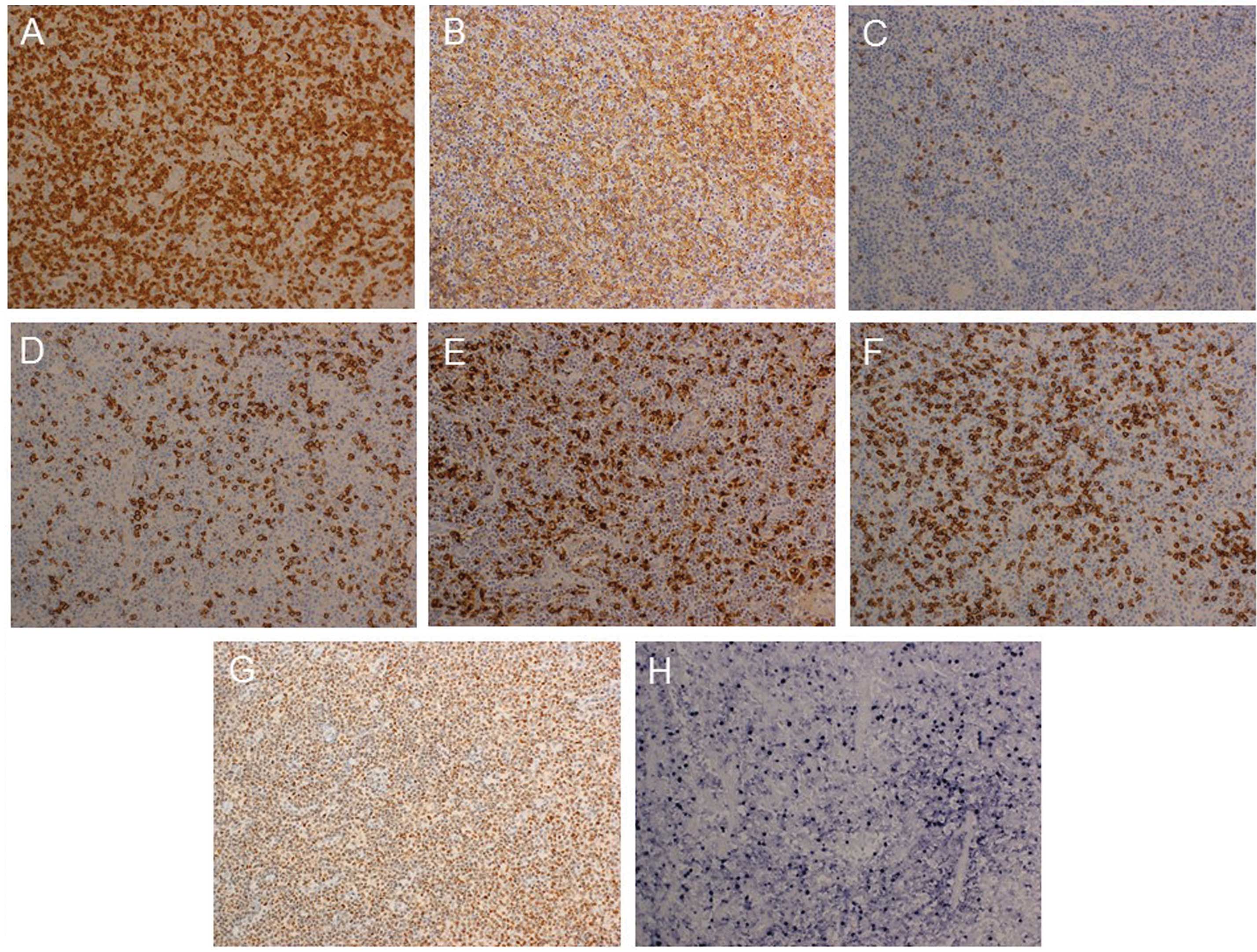 | Figure 5.Immunohistological analyses and in
situ hybridization for EBER within the lymph node. (A), (B),
(C), (D), (E), (F) and (G) Expression of CD3, CD4, CD8, CD20, CD68,
CD138 and c-MAF-1 in the lymph node speciment, respectively
(original magnification of the objective lens, x20). Positive cells
appear brown. (H) Expression of EBER (original magnification of the
objective lens, x20). Positive cells appear navy-blue. EBER,
Epstein-Barr virus-encoded small RNA. |
In order to examine the clonal rearrangement of T
cell receptor (TCR) and Ig, a PCR assay was conducted, as described
in the European BIOMED-2 collaborative study (6). PCR indicated the presence of clonal
rearrangements of TCR and Ig (data not shown).
Based on the histological features of the lymph
node, the patient's symptoms, the increase in B-lineage cells
without neoplastic light chain expression, the increase in
CD4+ T cells with clear cytoplasm expressing Maf-1, and
the presence of EBV-infected lymphoid cells, the patient was
diagnosed with AITL with leukemic change. Following diagnosis, the
patient died unexpectedly. No autopsy was permitted, and the exact
cause of death therefore remains unclear, although hyperviscosity
of the blood may have been a contributing factor. The family of the
patient provided informed consent for the publication of this
report.
Discussion
The current report discusses the case of a patient
with CD10− AITL with leukemic change, plasmacytosis
mimicking plasma cell leukemia and polyclonal
hypergammaglobulinemia.
Examination of a lymph node biopsy demonstrated a
histology typical of AITL, including completely effaced nodal
architecture and the infiltration of medium-sized lymphocytes with
clear cytoplasm, in addition to an inflammatory background.
Furthermore, increased numbers of plasma cells and lymphoid cells
with atypical nuclei were observed in the peripheral blood.
Plasma cell leukemia is defined as circulating
peripheral blood plasma cells exceeding 2×109/l or 20%
of peripheral white blood cells (7).
In addition, the clonality of these plasma cells may be
demonstrated by serum protein electrophoresis, flow cytometric
analyses and/or Ig rearrangement. In the present case,
6.308×109/l and 19% of peripheral white blood cells were
plasmacytoid cells. The serum γ-globulin was significantly
elevated, while serum protein electrophoresis and flow cytometric
analyses did not demonstrate any clonal proliferation of B-lineage
cells.
The presence of plasmacytoid cells in the peripheral
blood is occasionally observed during reactive processes, such as
bacterial and viral infections, such as parvovirus B19, hepatitis
or EBV; autoimmune disease, such as rheumatoid arthritis, systemic
lupus erythematosus or Sjögren's syndrome; and serum sickness.
However, in these conditions, the plasmacytoid cell counts are
usually not notably elevated (8–16).
A number of cases of AITL with increased
plasmacytoid cells in the peripheral blood, which is typical of
plasma cell leukemia, have been reported (3–5). In these
reports, the plasmacytoid cell counts in the peripheral blood were
markedly elevated, although they did not exhibit clonal expansion.
These reports were in accordance with the findings in the present
case. In the case reported here, small-to-medium-sized lymphoid
cells with atypical nuclei were also observed in the peripheral
blood, and flow cytometric analyses demonstrated elevated CD4 T
cell counts in the lymphocyte gate, suggesting leukemic changes,
typical of AITL. Sakai et al (4) described a case of a patient with AITL,
with plasmacytosis in the peripheral blood and leukemic changes,
which is similar to the findings in the present case. Baseggio
et al (17) attempted to
detect T cells expressing CD10 in the peripheral blood of patients
with AITL. In each of the 6 cases examined, the authors observed
the presence of T cells expressing CD10 in the peripheral blood
(mean percentage, 17%; range, 5–58%), while T cells in the control
group were CD10−, suggesting that lymphoma cells appear
in the peripheral blood of patients with AITL to varying
degrees.
In the present case, CD10 was negative, while c-Maf
was positive, in lymphoma cells. A previous study reported that
CD10 was detected in 39% of cases of AITL, suggesting that CD10 may
a useful diagnostic tool in AITL, although it is neither
particularly sensitive, nor specific to this disease (18). Furthermore, Murakami et al
(19) reported that c-Maf may also be
a useful marker of AITL. They reported that c-Maf expression was
observed in 23 of 31 cases of AITL; 3 of 11 cases of adult T-cell
leukemia/lymphoma; 4 of 19 cases of peripheral T-cell lymphoma,
unspecified; 0 of 11 cases of mycosis fungoides; 0 of 11 cases of
anaplastic large cell lymphoma; and 1 of 10 cases of extranodal
NK/T-cell lymphoma, nasal type. Therefore, c-Maf appears to be
relatively specifically expressed in AITL.
As previously described, AITL patients tend to be
diagnosed at an advanced stage of disease. In the patient reported
here, lymphoid cells with atypical nuclei were observed in the
lymph node biopsy, and the peripheral blood and bone marrow at
presentation. Furthermore, splenomegaly with infarction was
detected, suggesting involvement of the spleen. These results
suggest that the patient was at stage IV in the Ann Arbor staging
system.
The current case report discussed the case of a
patient with AITL, presenting with hypergammaglobulinemia,
plasmacytosis, leukemic change, and clonal rearrangement of Ig and
TCR. A diagnosis of AITL should be considered when encountering
patients with polyclonal hypergammaglobulinemia and/or
plasmacytosis.
Acknowledgements
The authors would like to thank Ms. K. Ando
(Department of Stem Cell Disorders, Kansai Medical University,
Hirakata, Japan) and Mr. Hilary Eastwick-Field (Department of Stem
Cell Disorders, Kansai Medical University, Hirakata, Japan) for
assistance with the preparation of this manuscript. The authors
would also like to thank Mr. K. Nagaoka, Ms. H. Ogaki, Mr. T. Kuge,
Mr. H. Takenaka and Ms. S. Eriguchi (Toyooka Hospital) for their
expert technical assistance.
References
|
1
|
Vose J, Armitage J and Weisenburger D:
International T-Cell Lymphoma Project: International peripheral
T-cell and natural killer/T-cell lymphoma study: Pathology findings
and clinical outcomes. J Clin Oncol. 26:4124–4130. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Iannitto E, Ferreri AJ, Minardi V, Tripodo
C and Kreipe HH: Angioimmunoblastic T-cell lymphoma. Crit Rev Oncol
Hematol. 68:264–271. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yamane A, Awaya N, Shimizu T, Ikeda Y and
Okamoto S: Angioimmunoblastic T-cell lymphoma with polyclonal
proliferation of plasma cells in peripheral blood and marrow. Acta
Haematol. 117:74–77. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sakai H, Tanaka H, Katsurada T, Yoshida Y,
Okamoto E and Ohno H: Angioimmunoblastic T-cell lymphoma initially
presenting with replacement of bone marrow and peripheral
plasmacytosis. Intern Med. 46:419–424. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ahsanuddin AN, Brynes RK and Li S:
Peripheral blood polyclonal plasmacytosis mimicking plasma cell
leukemia in patients with angioimmunoblastic T-cell lymphoma:
Report of 3 cases and review of the literature. Int J Clin Exp
Pathol. 4:416–420. 2011.PubMed/NCBI
|
|
6
|
van Dongen JJ, Langerak AW, Brüggemann M,
Evans PA, Hummel M, Lavender FL, Delabesse E, Davi F, Schuuring E,
García-Sanz R, et al: Design and standardization of PCR primers and
protocols for detection of clonal immunoglobulin and T-cell
receptor gene recombinations in suspect lymphoproliferations:
Report of the BIOMED-2 Concerted Action BMH4-CT98-3936. Leukemia.
17:2257–2317. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kyle RA, Maldonado JE and Bayrd ED: Plasma
cell leukemia. Report on 17 cases. Arch Intern Med. 133:813–818.
1974. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gawoski JM and Ooi WW: Dengue fever
mimicking plasma cell leukemia. Arch Pathol Lab Med. 127:1026–1027.
2003.PubMed/NCBI
|
|
9
|
Li L, Hsu P, Patel K, Saffari Y, Ashley I
and Brody J: Polyclonal plasma cell proliferation with marked
hypergammaglobulinemia and multiple autoantibodies. Ann Clin Lab
Sci. 36:479–484. 2006.PubMed/NCBI
|
|
10
|
Koduri PR and Naides SJ: Transient blood
plasmacytosis in parvovirus B19 infection: A report of two cases.
Ann Hematol. 72:49–51. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wada T, Maeba H, Ikawa Y, Hashida Y,
Okumura A, Shibata F, Tone Y, Inoue M, Koizumi S, Takatori H, et
al: Reactive peripheral blood plasmacytosis in a patient with acute
hepatitis A. Int J Hematol. 85:191–194. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shtalrid M, Shvidel L and Vorst E:
Polyclonal reactive peripheral blood plasmacytosis mimicking plasma
cell leukemia in a patient with Staphylococcal sepsis. Leuk
Lymphoma. 44:379–380. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mori I, Parizot C, Dorgham K, Demeret S,
Amoura Z, Bolgert F and Gorochov G: Prominent plasmacytosis
following intravenous immunoglobulin correlates with clinical
improvement in Guillain-Barre syndrome. PLoS One. 3:e21092008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Touzeau C, Pellat-Deceunynck C, Gastinne
T, Accard F, Jego G, Avet-Loiseau H, Robillard N, Harousseau JL,
Bataille R and Moreau P: Reactive plasmacytoses can mimick plasma
cell leukemia: Therapeutical implications. Leuk Lymphoma.
48:207–208. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Komiya I, Saito Y and Kuriya S: Peripheral
blood plasmacytosis in a patient with infectious mononucleosis-like
illness. Eur J Haematol. 46:61–62. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Thai KT, Wismeijer JA, Zumpolle C, de Jong
MD, Kersten MJ and de Vries PJ: High incidence of peripheral blood
plasmacytosis in patients with dengue virus infection. Clin
Microbiol Infect. 17:1823–1828. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Baseggio L, Berger F, Morel D,
Delfau-Larue MH, Goedert G, Salles G, Magaud JP and Felman P:
Identification of circulating CD10 positive T cells in
angioimmunoblastic T-cell lymphoma. Leukemia. 20:296–303. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Went P, Agostinelli C, Gallamini A,
Piccaluga PP, Ascani S, Sabattini E, Bacci F, Falini B, Motta T,
Paulli M, et al: Marker expression in peripheral T-cell lymphoma: A
proposed clinical-pathologic prognostic score. J Clin Oncol.
24:2472–2479. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Murakami YI, Yatabe Y, Sakaguchi T, Sasaki
E, Yamashita Y, Morito N, Yoh K, Fujioka Y, Matsuno F, Hata H, et
al: c-Maf expression in angioimmunoblastic T-cell lymphoma. Am J
Surg Path. 31:1695–1702. 2007. View Article : Google Scholar : PubMed/NCBI
|