Introduction
Osteosarcoma predominantly occurs in children and
young adults (1), and is the most
common primary malignant bone tumor worldwide (2). With a global incidence of 8.7 cases per
million children (age, <20 years) per year, osteosarcoma
accounts for ~6% of all childhood cancer (3). Over the previous two decades, advances
have been made in the treatment strategies for patients with of
osteosarcoma, including in surgery and multimodal chemotherapy. As
a consequence, the long-term cure rate for non-metastatic
osteosarcoma following surgery has risen from 25 to 60% (4,5). However,
despite these advances, the survival rate for patients with
osteosarcoma remains low, with novel effective therapeutic
strategies required to target this disease. Molecular therapies
have been proposed for various types of tumor based on the
application of developments in molecular biology. To date, the
experimental results have demonstrated good potential for clinical
application (6). The C-X-C motif
chemokine 12/C-X-C chemokine receptor type 4 (CXCL12/CXCR4)
signaling axis is involved in the development of tumors and the
metastatic spread of various cancer types (7–9), including
osteosarcoma (10). CXCL12 signals
through CXCR4, a seven-transmembrane G protein-coupled receptor
that is expressed by normal osteoblasts and by malignant cells in
osteosarcoma (11,12). Consequently, these proteins have been
proposed as potential biomarkers of tumor behavior (13).
The Wnt/β-catenin signaling pathway is important in
embryogenesis and organ development (14,15), and
has been implicated in the progression and pathogenesis of numerous
types of human cancer (16).
Dysregulation of Wnt/β-catenin expression is responsible for the
invasion and metastasis of osteosarcoma (17), indicating a possible correlation
between the CXCR4/CXCL12 axis and Wnt/β-catenin expression in the
invasion and metastasis of osteosarcoma. Therefore, the present
retrospective study was performed to investigate the in vivo
expression of CXCR4 and β-catenin in human osteosarcoma, and to
analyze the association between the expression of these proteins
and clinical prognosis.
Patients and methods
Patients
All patients or their guardians provided written
informed consent for participation in the present study. In
addition, ethical approval was obtained from the Ethics Committee
of the Fourth Military Medical University (Xi'an, China; approval
ID: 2013109). Fresh osteosarcoma specimens were obtained from 96
patients who underwent tumor resection at Tangdu Hospital of the
Fourth Military Medical University between March 2007 and November
2009. No patients received preoperative chemotherapy or
radiotherapy, however, patients with Enneking stage I, II, III or
IV disease (18) received
postoperative adjuvant chemotherapy [six courses of ifosfamide (2
g/m2 for 5 days/course), methotrexate (8 g/m2
for 1 day/course) and Adriamycin (50 mg/m2 for 1
day/course)]. Of the 96 patients, 44 were female and 52 were male,
with a median age of 18 years (range, 8–49 years). A total of 20
osteochondroma specimens were used as the normal controls,
including 13 male and 7 female patients with a median age of 20
years (range, 12–56 years). Follow-up care was provided for a
minimum of three years. Following resection, formalin-fixed,
paraffin-embedded blocks of the osteosarcoma and osteochondroma
specimens were retrieved from the Department of Pathology of the
Fourth Military Medical University. All samples were evaluated for
diagnosis by two similarly experienced pathologists. In addition,
16 pairs of osteosarcoma and adjacent healthy tissue samples were
obtained from 16 patients who underwent tumor resection at Tangdu
Hospital of the Fourth Military Medical University between July
2013 and December 2013.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Osteosarcoma and adjacent healthy tissue (weight, 25
mg) were harvested, and TRIzol® reagent (Invitrogen Life
Technologies, Carlsbad, CA, USA) was used to extract total RNA.
First-strand complementary (c)DNA was synthesized using the Avian
Myeloblastosis Virus First-Strand cDNA Synthesis kit and oligo(dT)
primers (Sangon Biotech Co., Ltd., Shanghai, China), according to
the manufacturer's instructions. RT-qPCR was performed using
LightCycler®480 software (Roche Diagnostics, Basel, Switzerland)
with the SYBR® Green PCR Master Mix (Applied Biosystems, Foster
City, CA, USA). β-actin was used as the internal housekeeping gene
and relative gene expression was calculated using the cycle
threshold (Ct) method (2−ΔΔCt). The PCR primers were as
follows: Forward, 5′-AATAAAATCTTCCTGCCCACC-3′ and reverse,
5′-CTGTACTTGTCCGTCATGCTTC-3′ for CXCR4; forward,
5′-TGAGCACCTGTTTGCCTGAA-3′ and reverse, 5′-ATGAGCAGCACTCGGACCTT-3′
for β-catenin; and forward, 5′-TAGTTGCGTTACACCCTTTCTTG-3′ and
reverse, 5′-TCACCTTCACCGTTCCAGTTT-3′ for β-actin. All experiments
were independently performed in triplicate at least three
times.
Immunohistochemistry
Hematoxylin and eosin-stained osteosarcoma and
osteochondroma samples were reviewed by two experienced
pathologists to determine the diagnosis and characterize the tumor.
The formalin-fixed, paraffin-embedded tissue samples were sectioned
at a thickness of 4 µm prior to heating at 60°C in an oven for ≥60
min. The slides were deparaffinized with xylene, hydrated and
pretreated with phosphate-buffered saline (PBS; pH 7.4).
Subsequently, 3% hydrogen peroxide was used to block endogenous
peroxidase activity for 15 min. Slides were incubated overnight
with primary antibodies [rabbit polyclonal anti-CXCR4 (cat no.
ab2074; Abcam, Cambridge, MA, USA) and rabbit polyclonal
anti-β-catenin (cat no. 9562; Cell Signaling Technology, Inc.,
Boston, MA, USA)] and then with secondary antibodies for 30 min at
room temperature. Streptavidin-peroxidase was applied and EnVision™
and universal 3,3′-diaminobenzidine detection kits (Gene Tech
Biotechnology Co., Ltd., Shanghai, China) was used with an extra
washing step. The slides were counterstained with hematoxylin and
mounted. Immunostaining was compared with osteochondroma samples as
the normal controls. Negative controls were obtained by
substituting the primary antibody with PBS for each protein.
Evaluation of
immunohistochemistry
CXCR4 and β-catenin immunohistochemistry were
examined as previously described (19). Cytoplasmic and membrane immunostaining
were distinguished by examining the slides at ×400 magnification
using a BX51 microscope (Olympus Corporation, Tokyo, Japan). The
extent of immunohistochemical staining was scored as follows: 25%
of cells positively stained, 1; 6–50% of cells positively stained,
2; 51–75% of cells positively stained, 3; and 76–100% of cells
positively stained, 4. The intensity of CXCR4 and β-catenin
expression was scored as negative (0, no staining), weak (1+, only
visible at high magnification), moderate (2+, visible at low
magnification) and strong (3+, striking at low magnification). For
heterogeneous staining, the highest observed level was used for
statistical analysis. All cases were scored by two investigators.
Multiplying the extent and intensity scores was used to calculate
the final immunoreactive score. The IRS of each specimen was
categorized into the following four groups: -, 0; +, 1–3; ++, 4–8;
and +++, 9–12. Scores of 0–3 and 4–12 were designated as negative
and strong expression, respectively. The two investigators reached
a consensus on the expression score in all cases.
Statistical analysis
A patient was defined as CXCR4- or
β-catenin-positive if all respective evaluated samples exhibited
strong positive CXCR4 or β-catenin protein expression. Thus, a
tumor was negative if all samples from the patient were
immunohistochemically negative. All statistical analyses were
performed using SPSS software (version 19.0; IBM SPSS, Armonk, NY,
USA). To investigate the statistical association between CXCR4 and
β-catenin protein expression in the same sample, Pearson's
χ2 test was used. Correlations between the target
protein expression and clinicopathological features were assessed
using the χ2 test. Furthermore, the Kaplan-Meier product
limit method was used to evaluate survival after surgery and
multivariate survival analysis was performed using the Cox
proportional hazard model. P<0.05 was considered to indicate a
statistically significant difference (two-tailed probability).
Results
Expression of CXCR4 and β-catenin in
osteosarcoma and osteochondroma
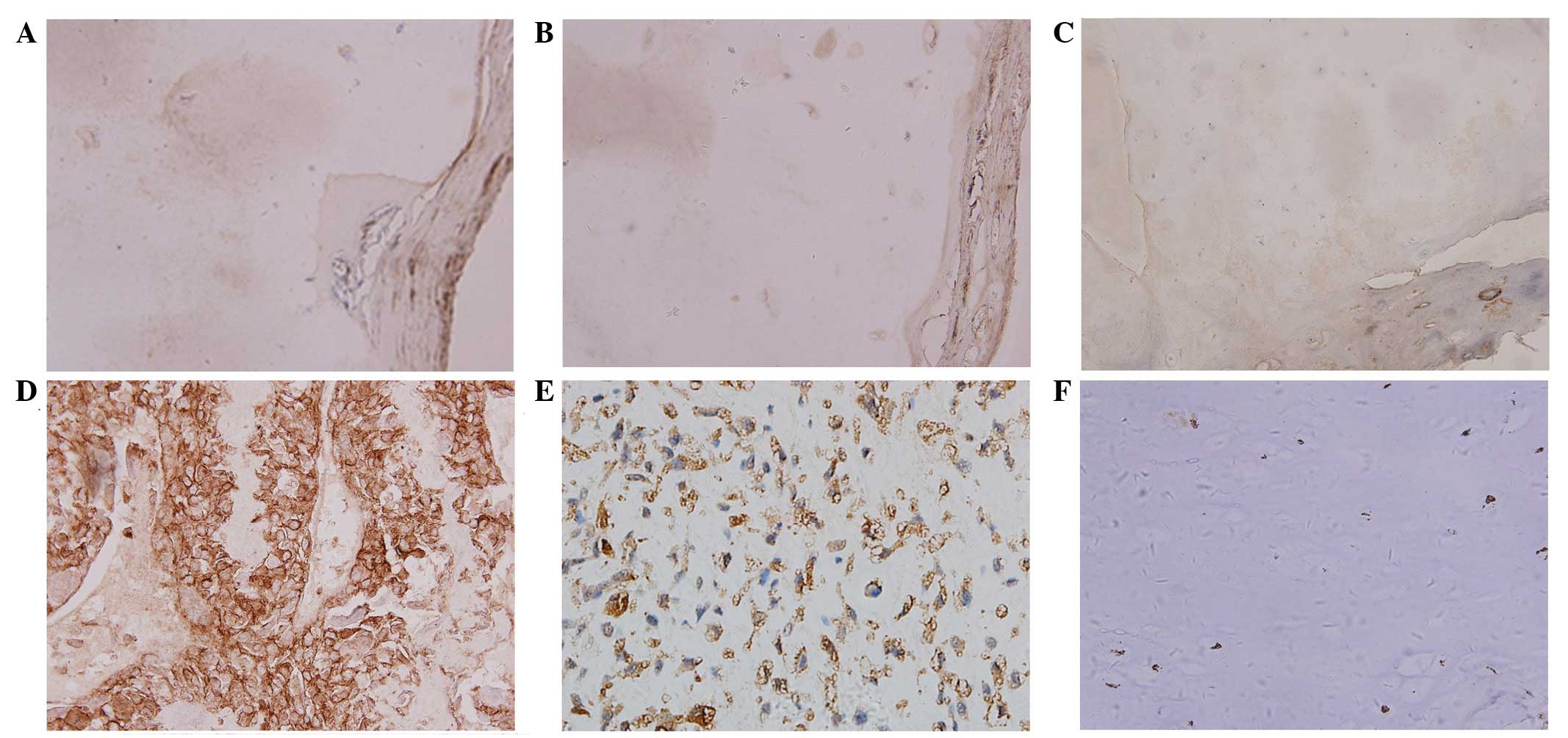
Immunohistochemistry was used to examine CXCR4 and
β-catenin protein expression in samples obtained from osteosarcoma
and osteochondroma patients. The different expression levels of the
two markers are indicated in Fig. 1.
Yellow or brown immunostaining indicated positive CXCR4 expression
and was predominantly identified in the cell membrane and
cytoplasm. Brown yellow or tan immunostaining indicated positive
β-catenin expression and was predominantly identified in the
cytoplasm and nucleus. Positive CXCR4 expression was observed in
four cases (20.00%) and β-catenin in five cases (25.00%) of
osteochondroma (Fig. 1A–C). By
contrast, a greater proportion of osteosarcoma samples exhibited
CXCR4 and β-catenin expression [68.75% (66/96 cases) and 61.46%
(59/96 cases), respectively; Fig.
1D–F; Table I]. The χ2
test demonstrated that the expression of these two markers was
statistically different in osteosarcoma and osteochondroma
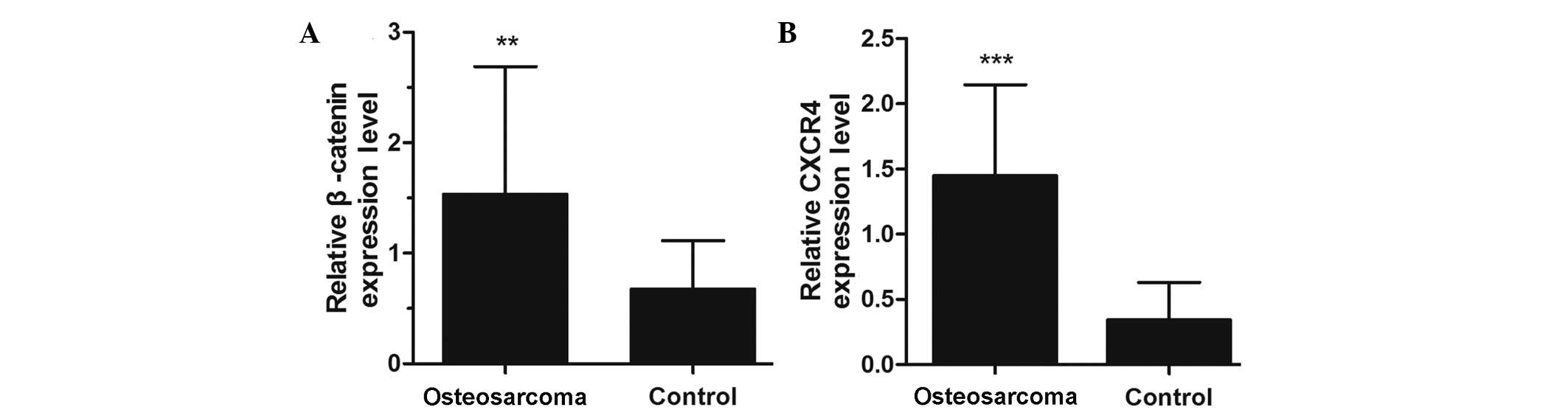
(P<0.05; Table I). RT-qPCR data
also demonstrated significantly increased levels of β-catenin
(P<0.01; Fig. 2A) and CXCR4
(P<0.001; Fig. 2B) mRNA expression
in osteosarcoma compared with the adjacent healthy tissue.
 | Table I.CXCR4 and β-catenin expression in
osteosarcoma and osteochondroma. |
Table I.
CXCR4 and β-catenin expression in
osteosarcoma and osteochondroma.
|
|
| CXCR4, n |
| β-catenin, n |
|
|---|
|
|
|
|
|
|
|
|---|
| Group | Cases, n | ﹢ | - | P-value | ﹢ | - | P-value |
|---|
| Osteosarcoma | 96 | 66 | 30 |
| 59 | 37 |
|
| Osteochondroma | 20 | 4 | 16 | 0.000 | 5 | 15 | 0.006 |
CXCR4 and β-catenin expression in
osteosarcoma patients correlates with clinicopathological
features
As indicated in Table
II, overall CXCR4 and β-catenin immunostaining were
significantly associated with Enneking stage and metastasis
(P<0.05). However, the expression of CXCR4 and β-catenin were
not significantly associated with gender, age, histological subtype
or tumor site.
 | Table II.Correlation between CXCR4 and
β-catenin expression, and clinicopathological data in patients with
osteosarcoma. |
Table II.
Correlation between CXCR4 and
β-catenin expression, and clinicopathological data in patients with
osteosarcoma.
|
| β-catenin, n |
| CXCR4, n |
|
|---|
|
|
|
|
|
|
|---|
| Variable | - | + | P-value | - | + | P-value |
|---|
| Total | 37 | 59 |
| 30 | 66 |
|
| Gender |
|
| 0.215 |
|
| 0.124 |
|
Male | 17 | 35 |
| 20 | 32 |
|
|
Female | 20 | 24 |
| 10 | 34 |
|
| Age, years |
|
| 0.199 |
|
| 0.497 |
|
<20 | 20 | 40 |
| 17 | 43 |
|
|
≥20 | 17 | 19 |
| 13 | 23 |
|
| Histology |
|
| 0.200 |
|
| 0.507 |
|
Osteoblastic | 17 | 25 |
| 12 | 30 |
|
|
Chondroblastic | 8 | 10 |
| 6 | 12 |
|
|
Fibroblastic | 8 | 9 |
| 8 | 9 |
|
|
Telangiectantic | 2 | 10 |
| 3 | 9 |
|
|
Mixed | 1 | 6 |
| 1 | 6 |
|
| Primary site |
|
| 0.531 |
|
| 0.189 |
|
Femur | 21 | 25 |
| 18 | 28 |
|
|
Tibia | 6 | 17 |
| 4 | 19 |
|
|
Humerus | 5 | 9 |
| 6 | 8 |
|
|
Fibula | 2 | 3 |
| 0 | 5 |
|
|
Ilium | 3 | 3 |
| 1 | 5 |
|
|
Other | 0 | 2 |
| 1 | 1 |
|
| Distant
metastasis |
|
| 0.001 |
|
| 0.000 |
|
Yes | 13 | 42 |
| 5 | 50 |
|
| No | 24 | 17 |
| 25 | 16 |
|
| Enneking stage |
|
| 0.047 |
|
| 0.016 |
|
I/IIA | 9 | 12 |
| 11 | 10 |
|
|
IIB | 12 | 8 |
| 8 | 12 |
|
|
III | 16 | 39 |
| 11 | 44 |
|
Correlation between CXCR4 and
β-catenin protein expression, and patient survival
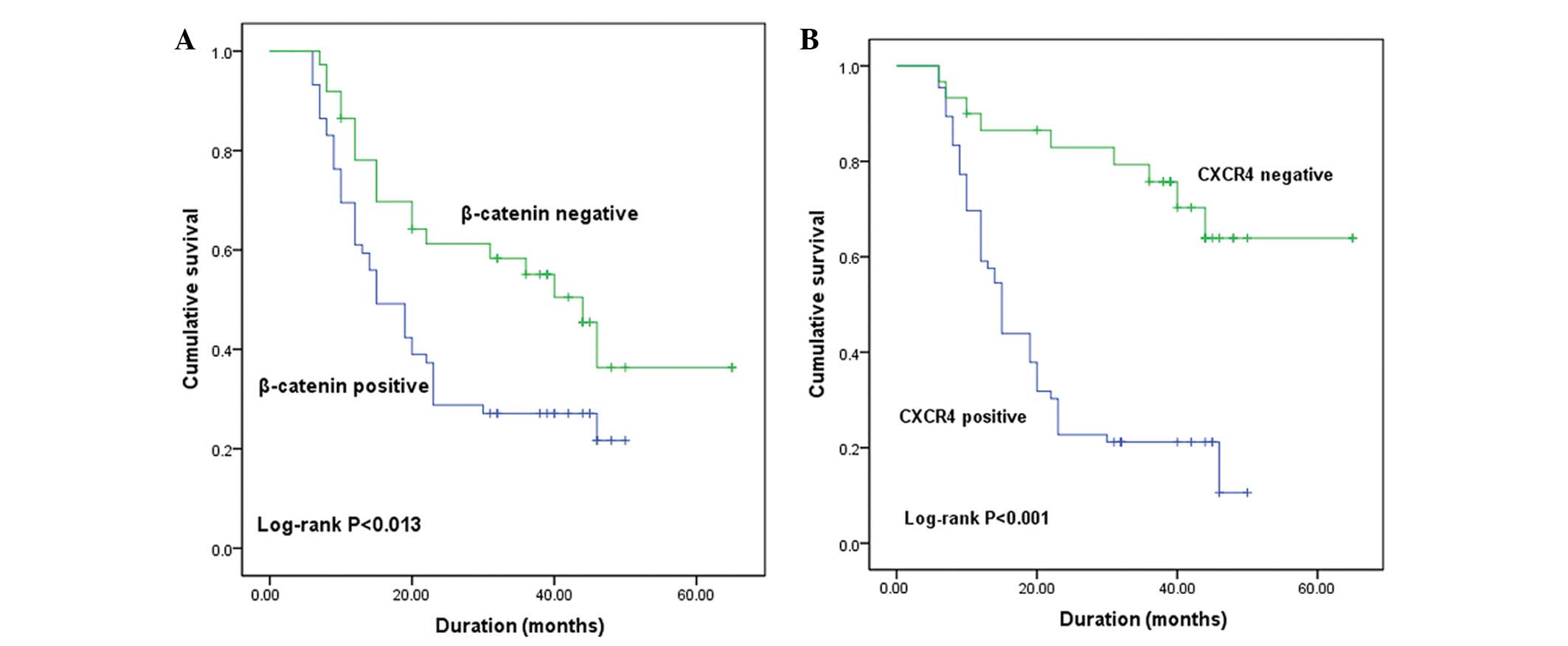
During the follow-up period, 66 patients with
osteosarcoma succumbed to the disease. Survival curves correlating
immunohistochemical staining patterns with Kaplan-Meier survival
are presented in Fig. 3. β-catenin
(Fig. 3A) and CXCR4 (Fig. 3B) expression were significant
predictors of overall survival (P<0.05; log-rank test).
Specifically, survival analysis revealed that a shorter survival
time was significantly correlated with patients who demonstrated
abnormal (positive) CXCR4 and β-catenin expression. Furthermore,
multivariate analysis using the Cox proportional hazard model
indicated a significant correlation between overall survival, and
CXCR4-positive expression, β-catenin-positive expression, late
Enneking stage and the presence of metastases (P=0.000, P=0.018,
P=0.000 and P=0.001, respectively). However, age and gender
demonstrated no significant association with patient survival
(P=0.115 and P=0.457, respectively; Table III).
 | Table III.Multivariate analysis of overall
survival in patients with osteosarcoma. |
Table III.
Multivariate analysis of overall
survival in patients with osteosarcoma.
| Variable | P-value | RR | 95% CI |
|---|
| Gender | 0.457 | 0.827 | 0.502–1.364 |
| Age | 0.115 | 0.976 | 0.946–1.006 |
| Metastasis
status | 0.001 | 2.487 | 1.443–4.286 |
| Clinical stage | 0.000 | 1.847 | 1.319–2.586 |
| CXCR4
expression | 0.000 | 0.301 | 0.156–0.581 |
| β-catenin
expression | 0.018 | 0.304 | 0.304–0.895 |
Correlation between CXCR4 and
β-catenin protein expression in osteosarcoma
Correlation analysis was performed to determine
whether CXCR4 and β-catenin markers were associated with each other
in osteosarcoma. The results revealed that β-catenin was positively
correlated with CXCR4 expression (r=0.339; P=0.001; Table IV).
 | Table IV.Correlation analysis of CXCR4 and
β-catenin expression levels. |
Table IV.
Correlation analysis of CXCR4 and
β-catenin expression levels.
|
| β-catenin
expression, n |
|---|
|
|
|
|---|
| CXCR4
expression | + | - |
|---|
| + | 1 | 5 |
| - | 8 | 22 |
Discussion
Osteosarcoma is the most frequent type of primary
bone cancer and typically occurs during childhood or adolescence
(2). Osteosarcoma exhibits high local
aggressiveness and a high propensity for metastasis, 90% of which
is to the lungs (20,21). Furthermore, osteosarcoma metastasis
promotes and regulates migratory tumor cells to generate metastatic
lesions at distant sites (22), with
malignant progression typically resulting in poor prognosis for
patients.
A series of complex processes dependent on multiple
factors results in the metastasis of a malignant tumor. The
well-known role of chemokines in recruiting multiple cell types
has, thus far, led the cancer field to focus on the concentration
gradients of chemokines and chemokine receptors produced by
metastatic sites. Such concentration gradients are known to attract
tumor cells to distant locations (23); this evidence is important for
explaining why different cancers spread to distinct metastatic
sites. Additionally, chemokine receptors appear to be important in
the homing of metastatic tumor cells (24,25).
CXCR4 is a major chemokine receptor and is expressed
in multiple types of cancer, such as breast and prostate (26,27).
Previous studies demonstrated that the application of
CXCR4-neutralizing antibodies or small interfering RNA targeting
the CXCR4 gene may inhibit metastasis in vivo and in
vitro (28,29). According to the current body of
knowledge, CXCR4 is involved in various cancer-related processes,
including its development and metastasis (30,31).
Therefore, understanding the function of CXCR4 may provide new
insights for the development of novel therapeutic strategies for
the treatment of cancer.
Inhibition of CXCR4 effectively blocked cancer
progression in vitro through the traditional Wnt pathway in
a previous study (32). CXCR4 was
expressed in 20.00% of the osteochondroma samples collected in the
current study, whereas CXCR4 was expressed in 68.75% of the
osteosarcoma samples. These findings are in agreement with a study
by Laverdiere et al (33),
which demonstrated that CXCR4 expression correlates with metastasis
and poor prognosis in patients with osteosarcoma. Additionally,
numerous studies identified that the expression of CXCR4
significantly correlates with metastasis in multiple tumor types,
including prostate cancer melanoma, breast cancer and
rhabdomyosarcoma (34–36). Furthermore, Müller et al
(27) reported that CXCR4 expression
is a key factor in regulating breast cancer metastasis. It was
revealed that breast neoplasms expressed high levels of CXCR4,
whereas healthy breast tissues expressed low levels.
CXCR4 is a CXCL12 ligand that signals through the
CXCL4/CXCR12 axis in a variety of mammals (37). The AMD3100 antagonist is known to
block the CXCL4/CXCR12 interaction, resulting in the enhanced
mobilization of progenitor cells from bone marrow to peripheral
blood (38). Additionally, a number
of studies have identified that CXCL12/CXCR4-induced chemotaxis
regulates the metastasis of malignant solid tumors (39,40). The
current data indicates a correlation between increased CXCR4
expression and a poor prognosis, supporting the possibility of
CXCR4 inhibition as a therapeutic target for patients with
osteosarcoma. Previously, an inhibiting peptide or a blocking
anti-CXCR4 monoclonal antibody were used to specifically inhibit
metastasis to the lungs in breast cancer models (27). In addition, non-small cell lung cancer
cells with knocked down CXCR4 expression produced larger and more
distant tumors compared with wild-type cells, indicating that CXCR4
mediates the metastatic behavior of non-small cell lung cancer
(41). Thus, the aforementioned
studies provide evidence for the suitability of CXCR4 expression as
a prognostic marker and potential therapeutic target in patients
with osteosarcoma.
β-catenin is a key protein in the canonical
Wnt/β-catenin signaling pathway. Upon activation of the Wnt
signaling pathway, β-catenin accumulates in the cytoplasm and is
able to translocate to the nucleus, where it engages the DNA-bound
T-cell factor transcription factor (42). Previous studies have proposed that
abnormal expression of β-catenin may be associated with tumor
progression, metastasis and poor prognosis in different cancer
types (43,44). Additionally, membrane and cytoplasmic
staining indicated that activation of the Wnt/β-catenin signaling
pathway is involved in osteosarcoma progression (45).
In the present study, cytoplasmic immunostaining was
observed in the majority of osteosarcoma cases (66/96) and the
expression of β-catenin was significantly increased in osteosarcoma
compared with osteochondroma samples. Furthermore, it was observed
that cytoplasmic β-catenin expression was upregulated and
membrane-associated β-catenin expression was downregulated in
advanced stage tumors. Correlation analysis indicated that aberrant
β-catenin expression was significantly associated with metastasis
and decreased patient survival. Furthermore, an absence of
β-catenin expression significantly correlated with increased
patient survival. Aberrant β-catenin and CXCR4 expression were
simultaneously observed in 53.1% of the samples. In addition, the
current data revealed that CXCR4 and β-catenin mRNA expression were
significantly higher in osteosarcoma compared with adjacent healthy
tissue. To evaluate these proteins as biomarkers, Spearman
correlation coefficient analysis was performed, revealing a
significant association between CXCR4 and β-catenin expression.
In conclusion, the present study demonstrated that
strong CXCR4 and β-catenin expression were associated with advanced
stage disease. Kaplan-Meier survival curves indicated significant
differences in clinical prognosis between the β-catenin-positive
and β-catenin-negative groups. Furthermore, statistical analysis
revealed CXCR4 and β-catenin expression as a predictor of overall
survival. Additionally, the present study identified high
expression of at least one of the synergistically-regulated mRNAs
(CXCR4 or β-catenin) in all osteosarcoma patients. Collectively,
the current results indicate that CXCR4 and β-catenin expression
may be used as biomarkers to predict prognosis in patients with
osteosarcoma and allow for novel therapeutic strategies to be
developed.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81272441).
References
|
1
|
Ferrari S, Mercuri M and Bacci G: Comment
on ‘Prognostic factors in high-grade osteosarcoma of the
extremities or trunk: An analysis of 1,702 patients treated on
neoadjuvant Cooperative Osteosarcoma Study Group protocols’. J Clin
Oncol. 20:2910–2911. 2002.PubMed/NCBI
|
|
2
|
Kansara M, Teng MW, Smyth MJ and Thomas
DM: Translational biology of osteosarcoma. Nat Rev Cancer.
14:722–735. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mirabello L, Troisi RJ and Savage SA:
Osteosarcoma incidence and survival rates from 1973 to 2004: Data
from the Surveillance, Epidemiology, and End Results Program.
Cancer. 115:1531–1543. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wittenburg LA, Bisson L, Rose BJ, Korch C
and Thamm DH: The histone deacetylase inhibitor valproic acid
sensitizes human and canine osteosarcoma to doxorubicin. Cancer
Chemother Pharmacol. 67:83–92. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Koshkina NV, RaoBindal K and Kleinerman
ES: Effect of the histone deacetylase inhibitor SNDX-275 on Fas
signaling in osteosarcoma cells and the feasibility of its topical
application for the treatment of osteosarcoma lung metastases.
Cancer. 117:3457–3467. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Levine AJ: p53, the cellular gatekeeper
for growth and division. Cell. 88:323–331. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang LP, Jin J, Lv FF, Cao J, Zhang J,
Wang BY, Shao ZM, Hu XC and Wang ZH: Norepinephrine attenuates
CXCR4 expression and the corresponding invasion of MDA-MB-231
breast cancer cells via β2-adrenergic receptors. Eur Rev Med
Pharmacol Sci. 19:1170–1181. 2015.PubMed/NCBI
|
|
8
|
Song T, Dou C, Jia Y, Tu K and Zheng X:
TIMP-1 activated carcinoma-associated fibroblasts inhibit tumor
apoptosis by activating SDF1/CXCR4 signaling in hepatocellular
carcinoma. Oncotarget. 6:12061–12079. 2015.PubMed/NCBI
|
|
9
|
Singla AK, Downey CM, Bebb GD and Jirik
FR: Characterization of a murine model of metastatic human
non-small cell lung cancer and effect of CXCR4 inhibition on the
growth of metastases. Oncoscience. 2:263–271. 2015.PubMed/NCBI
|
|
10
|
Perissinotto E, Cavalloni G, Leone F, et
al: Involvement of chemokine receptor 4/stromal cell-derived factor
1 system during osteosarcoma tumor progression. Clin Cancer Res.
11:490–497. 2005.PubMed/NCBI
|
|
11
|
Jung Y, Wang J, Schneider A, et al:
Regulation of SDF-1 (CXCL12) production by osteoblasts; a possible
mechanism for stem cell homing. Bone. 38:497–508. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Taichman RS, Cooper C, Keller ET, Pienta
KJ, Taichman NS and McCauley LK: Use of the stromal cell-derived
factor-1/CXCR4 pathway in prostate cancer metastasis to bone.
Cancer Res. 62:1832–1837. 2002.PubMed/NCBI
|
|
13
|
Sun X, Cheng G, Hao M, et al:
CXCL12/CXCR4/CXCR7 chemokine axis and cancer progression. Cancer
Metastasis Rev. 29:709–722. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Glass DA II and Karsenty G: vivo analysis
of Wnt signaling in bone. Endocrinology. 148:2630–2634. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wodarz A and Nusse R: Mechanisms of Wnt
signaling in development. Annu Rev Cell Dev Biol. 14:59–88. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Thomas DM: Wnts, bone and cancer. J
Pathol. 220:1–4. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Haydon RC, Deyrup A, Ishikawa A, et al:
Cytoplasmic and/or nuclear accumulation of the beta-catenin protein
is a frequent event in human osteosarcoma. Int J Cancer.
102:338–342. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jawad MU and Scully SP: In brief:
Classifications in brief: Enneking classification: Benign and
malignant tumors of the musculoskeletal system. Clin Orthop Relat
Res. 468:2000–2002. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hoogland AM, Jenster G, van Weerden WM, et
al: ERG immunohistochemistry is not predictive for PSA recurrence,
local recurrence or overall survival after radical prostatectomy
for prostate cancer. Mod Pathol. 25:471–479. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Osborne TS and Khanna C: A review of the
association between osteosarcoma metastasis and protein
translation. J Comp Pathol. 146:132–142. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xu WT, Bian ZY, Fan QM, Li G and Tang TT:
Human mesenchymal stem cells (hMSCs) target osteosarcoma and
promote its growth and pulmonary metastasis. Cancer Lett.
281:32–41. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nurwidya F, Takahashi F, Murakami A and
Takahashi K: Epithelial mesenchymal transition in drug resistance
and metastasis of lung cancer. Cancer Res Treat. 44:151–156. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lazennec G and Richmond A: Chemokines and
chemokine receptors: New insights into cancer-related inflammation.
Trends Mol Med. 16:133–144. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Balkwill F: Cancer and the chemokine
network. Nat Rev Cancer. 4:540–550. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kryczek I, Wei S, Keller E, Liu R and Zou
W: Stroma-derived factor (SDF-1/CXCL12) and human tumor
pathogenesis. Am J Physiol Cell Physiol. 292:C987–995. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sun YX, Wang J, Shelburne CE, et al:
Expression of CXCR4 and CXCL12 (SDF-1) in human prostate cancers
(PCa) in vivo. J Cell Biochem. 89:462–473. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Müller A, Homey B, Soto H, et al:
Involvement of chemokine receptors in breast cancer metastasis.
Nature. 410:50–56. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Du YF, Shi Y, Xing YF and Zeng FQ:
Establishment of CXCR4-small interfering RNA retrovirus vector
driven by human prostate-specific antigen promoter and its
biological effects on prostate cancer in vitro and in vivo. J
Cancer Res Clin Oncol. 134:1255–1264. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jeong WJ, Choi IJ, Park MW, An SY, Jeon
EH, Paik JH, Sung MW and Ahn SH: CXCR4 antagonist inhibits
perineural invasion of adenoid cystic carcinoma. J Clin Pathol.
67:992–998. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Guan G, Zhang Y, Lu Y, Liu L, Shi D, Wen
Y, Yang L, Ma Q, Liu T, Zhu X, et al: The HIF-1α/CXCR4 pathway
supports hypoxia-induced metastasis of human osteosarcoma cells.
Cancer Lett. 357:254–264. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang P, Dong L, Yan K, Long H, Yang TT,
Dong MQ, Zhou Y, Fan QY and Ma BA: CXCR4-mediated osteosarcoma
growth and pulmonary metastasis is promoted by mesenchymal stem
cells through VEGF. Oncol Rep. 30:1753–1761. 2013.PubMed/NCBI
|
|
32
|
Wang Z, Ma Q, Li P, Sha H, Li X and Xu J:
Aberrant expression of CXCR4 and β-catenin in pancreatic cancer.
Anticancer Res. 33:4103–4110. 2013.PubMed/NCBI
|
|
33
|
Laverdiere C, Hoang BH, Yang R, et al:
Messenger RNA expression levels of CXCR4 correlate with metastatic
behavior and outcome in patients with osteosarcoma. Clin Cancer
Res. 11:2561–2567. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Libura J, Drukala J, Majka M, et al:
CXCR4-SDF-1 signaling is active in rhabdomyosarcoma cells and
regulates locomotion, chemotaxis, and adhesion. Blood.
100:2597–2606. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Murakami T, Maki W, Cardones AR, et al:
Expression of CXC chemokine receptor-4 enhances the pulmonary
metastatic potential of murine B16 melanoma cells. Cancer Res.
62:7328–7334. 2002.PubMed/NCBI
|
|
36
|
Smith MC, Luker KE, Garbow JR, et al:
CXCR4 regulates growth of both primary and metastatic breast
cancer. Cancer Res. 64:8604–8612. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zlotnik A and Yoshie O: The chemokine
superfamily revisited. Immunity. 36:705–716. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Larochelle A, Krouse A, Metzger M, et al:
AMD3100 mobilizes hematopoietic stem cells with long-term
repopulating capacity in nonhuman primates. Blood. 107:3772–3778.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Guergnon J and Combadière C: Role of
chemokines polymorphisms in diseases. Immunol Lett. 145:15–22.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Teicher BA and Fricker SP: CXCL12
(SDF-1)/CXCR4 pathway in cancer. Clin Cancer Res. 16:2927–2931.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Choi YH, Burdick MD, Strieter BA, Mehrad B
and Strieter RM: CXCR4, but not CXCR7, discriminates metastatic
behavior in non-small cell lung cancer cells. Mol Cancer Res.
12:38–47. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Molenaar M, van de Wetering M, Oosterwegel
M, et al: XTcf-3 transcription factor mediates β-catenin-induced
axis formation in Xenopus embryos. Cell. 86:391–399. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hsu HP, Shan YS, Jin YT, Lai MD and Lin
PW: Loss of E-cadherin and β-catenin is correlated with poor
prognosis of ampullary neoplasms. J Surg Oncol. 101:356–362.
2010.PubMed/NCBI
|
|
44
|
Wang L, Cheng H, Liu Y, et al: Prognostic
value of nuclear β-catenin overexpression at invasive front in
colorectal cancer for synchronous liver metastasis. Ann Surg Oncol.
18:1553–1559. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kansara M, Tsang M, Kodjabachian L, et al:
Wnt inhibitory factor 1 is epigenetically silenced in human
osteosarcoma, and targeted disruption accelerates
osteosarcomagenesis in mice. J Clin Invest. 119:837–851. 2009.
View Article : Google Scholar : PubMed/NCBI
|