Introduction
Breast cancer is the most common malignancy and the
second most common cause of cancer mortality in women worldwide
(1). Metastatic breast cancer (MBC)
is an incurable disease and treatment aims to prolong survival and
to improve or maintain quality of life by palliating
disease-associated symptoms while minimizing the toxicity of
treatment (2). Median survival of
metastatic breast cancer is ~2 years, ranging from a months to
years (3). Patients with
hormone-insensitive disease and the majority of patients that do
not respond to endocrine therapy are candidates for chemotherapy
(4). Unlike in the adjuvant setting,
standards of chemotherapy in metastatic disease are not well
defined.
Combination chemotherapy provides higher response
rates and longer time to progression (TTP), and is usually favored
for patients with a high tumor burden, rapidly progressive disease
or symptomatic visceral disease. Newer taxane-containing
combination regimens, including docetaxel/capecitabine and
paclitaxel/gemcitabine combinations, have been demonstrated to
improve overall survival (OS) compared with single-agent taxanes,
and these regimens are commonly used when a combination therapy is
adopted (2,3). However, the optimal duration of
treatment to disease control with these regimens is unknown. In a
previous meta-analysis, Gennari et al (4) reported that longer first-line
chemotherapy duration was associated with prolonged
progression-free survival (PFS) and marginally longer OS.
Continuing chemotherapy until disease progression ceases is also
reported to improve quality of life measures (5).
Capecitabine is an oral fluoropyrimidine and has
marked activity in MBC. When used as a single agent, capecitabine
provides response rates of 20–30%, and median TTP of 2.8–7.1 months
in the first and subsequent lines of treatment (6,7). The
incidence of neutropenia and alopecia are low; and more common
toxicities, including hand-foot syndrome (HFS), diarrhea and
stomatitis are readily managed with dose modifications (8,9).
Capecitabine may be a suitable option as a single agent for
long-term use due to its tolerability, efficacy, and ease of oral
application.
For patients who achieve a response with a
chemotherapy doublet, clinicians often prefer to continue treatment
with a less intensive regimen. In the clinical setting, continuing
capecitabine or docetaxel/capecitabine combination is a common
practice; however data regarding the efficacy of this approach is
limited. The present study aims to evaluate the efficacy and safety
of first-line therapy with docetaxel plus capecitabine followed by
single-agent capecitabine in human epidermal growth factor receptor
(HER2) negative MBC patients.
Materials and methods
Patient selection
Female patients aged ≥18 years with histologically
or cytologically proven HER2 negative metastatic and/or locally
advanced breast cancer who received no cytotoxic chemotherapy for
MBC were selected for the study. In this retrospective cohort
study, patients who received adjuvant chemotherapy ≥6 months ago or
endocrine therapy for metastatic disease were included. All the
included patients had at ≥1 radiologically measurable or clinically
assessable lesion and an Eastern Cooperative Oncology Group
performance score of ≤2 (range, 0–5) (10). Eligible patients also had normal renal
function and adequate hematological and hepatic function.
Study treatment
Treatment was initiated with docetaxel 75
mg/m2 administered as a 1 h infusion on the first day of
every 21-day cycle plus capecitabine 1,650 mg/m2/day on
days 1–14 followed by a 7-day rest period. Patients who achieved
complete (CR) or partial (PR) responses or stable disease after 6
cycles of combination therapy, received maintenance therapy with
capecitabine 2,000 mg/m2/day on days 1–14 followed by a
7-day rest period until progressive disease or intolerable
toxicity. The defined protocol was administered to eligible
patients in three oncology centers.
Efficacy and safety evaluations
Treatment responses were radiologically evaluated at
12-week intervals on the basis of Response Evaluation Criteria in
Solid Tumors 1.1 guidelines (11).
The best overall response achieved was reported separately for
combination therapy and maintenance therapy. The primary efficacy
endpoint was defined as the interval between the initiation of the
first cycle of maintenance therapy and date of progression or death
from any cause. Secondary endpoints were OS (measured from
beginning of combination therapy to death from any cause) and the
objective response rate (ORR). PFS duration was also calculated
measured from beginning of combination therapy for all patient
population. Toxicity evaluations were made prior to every treatment
cycle and graded according to the Common Terminology Criteria for
Adverse Events version 3 (CTCAEv3).
Statistical analyses
Descriptive data are expressed as frequency and
central tendency measures. Survival durations were estimated using
the Kaplan-Meier method, and the log-rank test was used to compare
survival durations of patient subgroups. All P-values reported were
two-sided and P<0.05 was considered to indicate a statistically
significant difference. Statistical analyses were performed using
the SPSS software, version 17 (SPSS, Inc., Chicago, IL, USA.
Results
Patient characteristics
Between June 2009 and June 2012, 55 patients were
enrolled in the study and received docetaxel plus capecitabine
regimen. During combination chemotherapy one patient died, one
patient was lost to follow-up and one patient developed intolerable
hand-foot syndrome. Response assessments were made for 52 patients.
After 6 cycles of combination chemotherapy 29 patients had PR,
three patients had CR and 16 patients had stable disease. The ORR
for combination chemotherapy was 61.6% (Table I). A total of 4 patients had
progressive disease, one patient died, one patient was lost to
follow-up, and one patient had intolerable HFS and did not receive
maintenance therapy. Forty-eight patients proceeded to maintenance
phase and received ≥1 cycle of single-agent capecitabine. The
median age of the patient group was 52 years (range 28–70 years). A
total of 32 patients (66.7%) were postmenopausal and 37 (77.1%) had
estrogen and progesterone receptor (hormone receptor) positive
disease. The number of metastatic sites was one site in 20
patients, two sites in 19 patients and three or more sites in 9
patients. The most common metastatic sites were bone (75%), lymph
nodes (33.3%), lungs (27.1%) and liver (14.6%). Twenty-five
patients (52.1%) had at least one visceral metastatic site, and
metastases were limited to bone, lymph nodes or soft tissue in 23
patients (47.9%). Seventeen patients (35.4%) received previous
endocrine therapy for MBC and 29 patients (60.4%) had received
endocrine therapy in adjuvant and/or metastatic setting. Adjuvant
chemotherapy was administered to 28 patients (58.3%). All adjuvant
regimens included an anthracycline, and 24 patients (50%) received
a taxane in the adjuvant setting. The baseline characteristics of
the patient population are presented in Table II.
 | Table I.Objective responses with combination
therapy (n=52). |
Table I.
Objective responses with combination
therapy (n=52).
|
| n | % |
|---|
| Partial response | 29 | 55.8 |
| Complete
response | 3 | 5.8 |
| Stable disease | 16 | 30.7 |
| Progressive
disease | 4 | 7.7 |
 | Table II.Characteristics of patients who
received maintenance treatment. |
Table II.
Characteristics of patients who
received maintenance treatment.
| Parameter | n | % |
|---|
| Total | 48 | 100 |
| Median age
(range) | 52 (28–70) | – |
| Menopausal
status |
|
|
|
Premenopausal | 16 | 33.3 |
|
Postmenopausal | 32 | 66.7 |
| Hormone receptor |
|
|
|
Positive | 37 | 77.1 |
|
Negative | 11 | 22.9 |
| Metastatic site |
|
|
|
Visceral | 25 | 52.1 |
|
Non-visceral | 23 | 47.9 |
| No. of tumor
sites |
|
|
| 1 | 20 | 41.7 |
| 2 | 19 | 39.6 |
| ≥3 | 9 | 18.8 |
| Previous endocrine
therapy |
|
|
|
Adjuvant | 18 | 37.5 |
|
Metastatic | 17 | 35.4 |
| Adjuvant
chemotherapy | 28 | 58.3 |
| Anthracycline
exposure | 28 | 58.3 |
| Taxane exposure | 24 | 50.0 |
Efficacy
The median follow-up duration following the
initiation of maintenance capecitabine treatment was 17.6 months.
The median number of maintenance capecitabine cycles was 6.5 (range
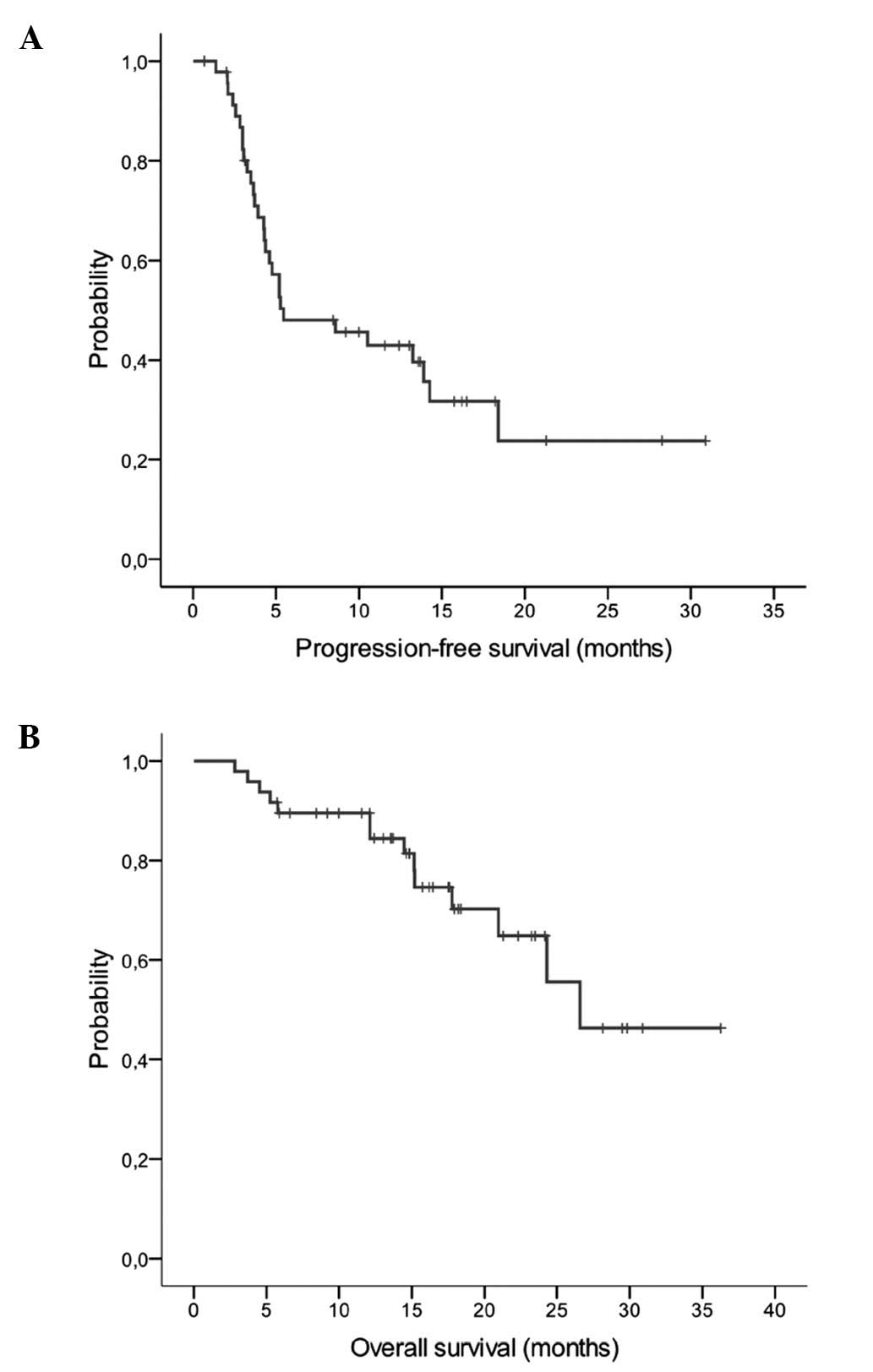
1–28, total 441). The median PFS with single agent capecitabine was
5.5 months (95% CI, 0–11.4) with 29 events (Fig. 1A). In the maintenance phase, 4
patients (8.3%) had improved responses with single-agent
capecitabine; 2 patients prior PR further improved and 2 patients
improved from PR to CR. Thirty-two patients (66.7%) had stable
disease. Twenty-one patients received maintenance therapy for ≥6
months, 15 patients for ≥12 months and 5 patients for ≥18 months.
At the time of analyses 5 patients were still receiving maintenance
therapy. Hormone receptor positive patients (13.2 vs. 3.7 months,
P<0.001) and patients with no visceral metastases (14.3 vs. 4.3
months, P<0.016) had significantly longer PFS durations
(Table III). Fourteen patients died
and the median OS after the initiation of maintenance therapy was
26.6 months (95% CI, 21.8–30.1; Fig.
1B). The treatment regimen including the combination phase
provided a median PFS of 9.8 months (95% CI, 8.4–11.1) for all
patient groups (n=55).
 | Table III.PFS durations in patient
subgroups. |
Table III.
PFS durations in patient
subgroups.
| Subgroup | Median PFS
(months) | 95% CI | P-value |
|---|
| Menopausal
status |
|
|
|
| Pre-
(n=16) | 10.5 | 0.9–20.1 |
0.923 |
| Post-
(n=32) | 5.4 |
0–10.9 |
|
| Hormone receptor |
|
|
|
| Positive
(n=37) | 13.2 | 6.1–20.4 | <0.001 |
| Negative
(n=11) | 3.7 | 2.5–4.80 |
|
| No. of metastatic
sites |
|
|
|
| 1
(n=20) | 13.2 |
3–23.5 |
0.230 |
| ≥2
(n=28) | 4.8 | 3.3–6.20 |
|
| Metastatic site |
|
|
|
|
Non-visceral (n=23) | 14.3 |
8–20.5 |
0.016 |
| Visceral
(n=25) | 4.3 | 3.3–5.30 |
|
| Previous taxane
exposure |
|
|
|
| No
(n=24) | 10.5 | 0–22 |
0.065 |
| Yes
(n=24) | 4.4 | 3.3–5.40 |
|
| Previous
anthracycline exposure |
|
|
|
| No
(n=20) | 5.8 | 0–13.8 |
0.271 |
| Yes
(n=28) | 5.2 | 0–10.7 |
|
Safety
Adverse events were assessed in 36 patients. The
most common toxicities (>10%) were HFS, neutropenia and fatigue
with combination therapy and HFS with maintenance therapy. Febrile
neutropenia, grade 3/4 neutropenia and grade 1/2 fatigue were more
common during combination therapy (Table
IV). Capecitabine was discontinued in 5 patients due to
intolerable toxicity during maintenance therapy (3 HFS, 1 edema and
1 abnormal vision). During combination therapy dose reductions of
capecitabine were performed in 9 patients (16.4%) and reduction of
docetaxel were performed in 11 patients (20%). Seven patients
(14.6%) required a dose reduction during maintenance therapy. No
treatment-associated mortality occurred.
 | Table IV.Treatment-associated adverse
events. |
Table IV.
Treatment-associated adverse
events.
|
|
Docetaxel/capecitabine | Capecitabine
maintenance |
|---|
|
|
|
|
|---|
| Event | Grade 1/2 (%) | Grade 3/4 (%) | Grade 1/2 (%) | Grade 3/4 (%) |
|---|
| Neutropenia | 2 (5.6) | 5 (13.9) | 2 (5.6) | 1 (2.8) |
| Febrile
neutropenia | 0 | 3 (8.3) | 0 | 0 |
| Hand foot
syndrome | 8 (22.2) | 9 (25) | 7 (19.4) | 5 (13.9) |
| Fatigue | 8 (22.2) | 1 (2.8) | 3 (8.3) | 0 |
| Stomatitis | 3 (8.3) | 1 (2.8) | 2 (5.6) | 0 |
Discussion
The results of the present study indicate that
maintenance therapy with single agent capecitabine following
first-line treatment with 6 cycles of docetaxel plus capecitabine
therapy is an effective treatment option for treatment of patients
with HER2 negative MBC. The maintenance therapy provided a median
PFS duration of 5.5 months and treatment regimens that included
combination therapy provided a median PFS duration of 9.8 months.
Efficacy outcomes of this regimen are in the range of data reported
for docetaxel and capecitabine combination. In the first-line
setting this combination is reported to provide a PFS duration of
8.5–10 months and an ORR of 39–74% (12,13).
Although a control arm was not included in the present study, the
TTP following 6 cycles of taxane with combination therapy without
maintenance was 3.8 months in a previous study (14). Objective responses were preserved or
improved in 75% of patients. Toxicity profiles were compatible with
a previous study (3). Capecitabine
maintenance resulted in reduced neutropenia, febrile neutropenia
and fatigue compared with combination treatment. During maintenance
therapy incidence of grade 3/4 neutropenia was only 2.8% and no
febrile neutropenia was observed. The main toxicity event
associated with capecitabine therapy was HFS; 13.9% of patients
developed grade 3/4 HFS during maintenance therapy.
To the best of our knowledge, the efficacy of
capecitabine maintenance therapy has only been evaluated in one
previous study (15). Patients were
administered single agent capecitabine after a positive response to
capecitabine plus docetaxel or vinorelbine in the first or
second-line setting and the median TTP with maintenance was 4.4
months. In this study 81.4% of the patient group maintained the
response to combination regimen by maintenance, 1.7% demonstrated
an improvement from PR to CR in maintenance setting.
In management of MBC, newer combination regimens may
offer a survival rate advantage, however increased toxicity may
limit tolerability. When disease control is achieved, possible
options include, discontinuing treatment until progression or
continuing with the same or a less intensive regimen including
hormonal therapy. For hormone receptor-positive patients,
maintenance with endocrine therapy, such as tamoxifen, anastrazole,
letrozole or exemestane, is preferred by a number of clinicians.
Although available data is limited, a study by Bertelli et
al (16), supports this approach.
Letrozole provided a median TTP of 18.5 months in 58 postmenopausal
patients who attained disease control with first-line chemotherapy.
The benefit of maintenance therapy with modern chemotherapy
regimens is still a matter of debate. Three trials with different
designs assessed the efficacy of maintenance chemotherapy following
taxane-containing combination chemotherapy in HER2 negative
patients. In MANTA1 study, maintenance with paclitaxel after
anthracycline-paclitaxel combination provided no benefit in terms
of PFS and OS compared with control group (17). However, it should be noted that around
60% of the patients had received concurrent endocrine therapy in
both arms. In GEICAM 2001-01 study, maintenance with pegylated
liposomal doxorubicin significantly prolonged TTP (8.4 vs. 5.1
months), but not OS compared with observation and patients were not
allowed to receive hormonal therapy in both arms (18). In a recent study by the Korean Cancer
Study Group (KCSG), patients received 6 cycles of paclitaxel plus
gemcitabine and those who achieved disease control were randomized
to receive maintenance with the same regimen until progression or
observation (14); again, endocrine
therapy was not allowed. In this study, patients in the maintenance
arm received additional 6 cycles (median) of therapy, and the
results demonstrated a superior OS (32.3 vs. 23.5 months) and PFS
with maintenance therapy; however the incidence of grade 3/4
neutropenia was also increased (61%).
In conclusion, present data about the benefit of
maintenance therapy is inconclusive, and decision of maintenance
therapy should be based on the disease characteristics and patient
preferences (19). The results of the
present study demonstrate that single-agent capecitabine after
docetaxel and capecitabine combination is safe, efficient and
feasible, and may be considered as an option when maintenance
therapy is preferred.
References
|
1
|
McPherson K, Steel CM and Dixon JM: Breast
cancer-epidemiology, risk factors, and genetics. BMJ. 321:624–628.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Albain KS, Nag SM, CalderilloRuiz G,
Jordaan JP, Llombart AC, Pluzanska A, Rolski J, Melemed AS,
ReyesVidal JM, Sekhon JS, et al: Gemcitabine plus Paclitaxel versus
Paclitaxel monotherapy in patients with metastatic breast cancer
and prior anthracycline treatment. J Clin Oncol. 26:3950–3957.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
O'Shaughnessy J, Miles D, Vukelja S,
Moiseyenko V, Ayoub JP, Cervantes G, Fumoleau P, Jones S, Lui WY,
Mauriac L, et al: Superior survival with capecitabine plus
docetaxel combination therapy in anthracycline-pretreated patients
with advanced breast cancer: Phase III trial results. J Clin Oncol.
20:2812–2823. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gennari A, Stockler M, Puntoni M, Sormani
M, Nanni O, Amadori D, Wilcken N, D'Amico M, DeCensi A and Bruzzi
P: Duration of chemotherapy for metastatic breast cancer: A
systematic review and meta-analysis of randomized clinical trials.
J Clin Oncol. 29:2144–2149. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Coates A, Gebski V, Bishop JF, Jeal PN,
Woods RL, Snyder R, Tattersall MH, Byrne M, Harvey V, Gill G, et
al: Improving the quality of life during chemotherapy for advanced
breast cancer. A comparison of intermittent and continuous
treatment strategies. N Engl J Med. 317:1490–1495. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
O'Shaughnessy JA, Kaufmann M, Siedentopf
F, Dalivoust P, Debled M, Robert NJ and Harbeck N: Capecitabine
monotherapy: Review of studies in first-line HER-2-negative
metastatic breast cancer. Oncologist. 17:476–484. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Blum JL, Barrios CH, Feldman N, Verma S,
McKenna EF, Lee LF, Scotto N and Gralow J: Pooled analysis of
individual patient data from capecitabine monotherapy clinical
trials in locally advanced or metastatic breast cancer. Breast
Cancer Res Treat. 136:777–788. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rossi D, Alessandroni P, Catalano V,
Giordani P, Fedeli SL, Fedeli A, Baldelli AM, Casadei V, Ceccolini
M and Catalano G: Safety profile and activity of lower capecitabine
dose in patients with metastatic breast cancer. Clin Breast
Cancer7. 857–860. 2007. View Article : Google Scholar
|
|
9
|
Hennessy BT, Gauthier AM, Michaud LB,
Hortobagyi G and Valero V: Lower dose capecitabine has a more
favorable therapeutic index in metastatic breast cancer:
Retrospective analysis of patients treated at M. D. Anderson Cancer
Center and a review of capecitabine toxicity in the literature. Ann
Oncol. 16:1289–1296. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the Eastern Cooperative Oncology Group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vici P, Giotta F, Di Lauro L, Sergi D,
Vizza E, Mariani L, Latorre A, Pizzuti L, D'Amico C, Giannarelli D,
et al: A multicenter phase II randomized trial of
docetaxel/gemcitabine versus docetaxel/capecitabine as first-line
treatment for advanced breast cancer: A Gruppo Oncologico Italia
Meridionale study. Oncology. 81:230–236. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Soto C, Torrecillas L, Reyes S, Ramirez M,
Perez L, Cervantes G, Gonzalez F, Tellez E, Cortes P and Benitez H:
Capecitabine (X) and taxanes in patients (pts) with
anthracycline-pretreated metastatic breast cancer (MBC): Sequential
vs. combined therapy results from a MOSG randomized phase III
trial. J Clin Oncol (Meeting abstracts). 24:5702006.
|
|
14
|
Park YH, Jung KH, Im SA, Sohn JH, Ro J,
Ahn JH, Kim SB, Nam BH, Oh Y, Han SW, et al: Phase III,
multicenter, randomized trial of maintenance chemotherapy versus
observation in patients with metastatic breast cancer after
achieving disease control with six cycles of gemcitabine plus
paclitaxel as first-line chemotherapy: KCSG-BR07-02. J Clin Oncol.
31:1732–1739. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang H, Jiang Z, Wang T, Zhang S, Bian L,
Cao Y, Wu S and Song S: Single-agent capecitabine maintenance
therapy after response to capecitabine-based combination
chemotherapy in patients with metastatic breast cancer. Anticancer
Drugs. 23:718–723. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bertelli G, Garrone O, Bertolotti L,
Occelli M, Conforti S, Marzano N, Febbraro A, Carlini P, Liossi C,
Del Mastro L, et al: Maintenance hormone therapy with letrozole
after first-line chemotherapy for advanced breast cancer. Oncology.
68:364–370. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gennari A, Amadori D, De Lena M, Nanni O,
Bruzzi P, Lorusso V, Manzione L and Conte PF: Lack of benefit of
maintenance paclitaxel in first-line chemotherapy in metastatic
breast cancer. J Clin Oncol. 24:3912–3918. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Alba E, RuizBorrego M, Margelí M,
Rodríguez-Lescure A, Sánchez-Rovira P, Ruiz A, Mel-Lorenzo Jr,
Ramos-Vázquez M, Ribelles N, Calvo E, et al: Maintenance treatment
with pegylated liposomal doxorubicin versus observation following
induction chemotherapy for metastatic breast cancer: GEICAM 2001-01
study. Breast Cancer Res Treat. 122:169–176. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Carrick S, Parker S, Thornton CE, Ghersi
D, Simes J and Wilcken N: Single agent versus combination
chemotherapy for metastatic breast cancer. Cochrane Database Syst
Rev. Apr 15–2009.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|