Introduction
Prostate cancer (PCa) is the most frequently
diagnosed cancer and the second leading cause of cancer-associated
mortality among men in the United States (1). Current medical management for localized
PCa ranges from close monitoring for indolent disease to radical
treatments, such as radiation therapy (RT) or surgery. RT provides
excellent local control and increased overall survival rates for
PCa. However, a significant number of high-risk patients will fail
therapy, develop resistance and eventually succumb to the disease
(2,3).
With an increased level of knowledge with regard to biomarkers and
their effect on therapeutic response, in the future, physicians may
be able to personalize care based on a patient's biomarkers.
DAB2 interacting protein (DAB2IP), also known as
aspartokinase (ASK1)-interacting protein-1, is a novel member of
the Ras GTPase-activating protein family and is downregulated, with
growth inhibitory and apoptosis enhancing activities, in PCa
(4). Downregulation of DAB2IP, mainly
due to epigenetic regulation, inversely correlates with tumor grade
and predicts PCa progression (5,6). DAB2IP is
a unique scaffold protein that modulates a variety of biological
activities, including cell growth, apoptosis and survival via the
phosphoinositide 3-kinase-Akt, Wnt-epithelial-mesenchymal
transition, ASK-JNK, Ras-mitogen-activated protein kinase, and
nuclear factor-κB pathways in PCa (4,7–9). By knocking down endogenous DAB2IP
levels, PCa cells could gain proliferative potential and become
resistant to stress-induced apoptosis (6). An increasing number of studies have
shown that DAB2IP plays an important role in the radioresistance
and chemoresistance of PCa (1,10). DAB2IP
regulates Clusterin gene expression via cross-talk between
Wnt/β-catenin and insulin-like growth factor-I (IGF-I)/IGF receptor
signaling in metastatic castration-resistant PCa (10). DAB2IP loss has been shown to result in
resistance to ionizing radiation (IR) due to enhanced DSB repair
and apoptosis resistance (1,11). Recently, a novel function of DAB2IP
was shown in suppressing IR-induced and DNA-PKcs-associated
autophagy, and promoting apoptosis in PCa cells (12). However, the regulatory mechanism of
DAB2IP on the radioresistance in PCa has not been well clarified.
Thus, an increased level of knowledge with regard to the molecular
mechanisms of DAB2IP in PCa therapy resistance could aid in the
identification of significant novel therapeutic targets for
advanced disease.
microRNAs (miRNAs/miRs), an abundant class of
~22-nucleotide small non-coding RNAs, post-transcriptionally
regulate gene expression through binding to multiple target mRNAs
(13–15). Extensive PCa miRNA profiling has shown
that a number of miRNAs are differentially expressed between PCa
and adjacent normal tissues, thus contributing to PCa progression
(16,17). Therefore, understanding the molecular
mechanisms by which these miRNAs act in the deregulation of
cellular signaling in PCa cells may assist in the development of
improved therapeutic strategies disease treatment. To date,
however, the mechanism behind the involvement of the
miRNA-dependent DAB2IP pathway in the radioresistance of PCa has
not been investigated.
In the present study, the regulatory effect of
miR-32 on PCa cell survival and apoptosis was determined during
radiotherapy, and the involved pathways were analyzed.
Materials and methods
Cells and specimens
PCa cell lines (DU145 and PC3) and a normal prostate
cell line (RWPE-1) were grown in T medium (Invitrogen Life
Technologies, Carlsbad, CA, USA) with 5% fetal bovine serum
(HyClone, Hudson, NH, USA) at 37°C in a 5% CO2
humidified chamber.
Human PCa tissues, adjacent non-tumor tissues
(located 2.5 cm from the tumor) and normal PCa tissues were
obtained from patients diagnosed with PCa in Department of General
Surgery, Loudi Central Hospital of Hunan (Loudi, China). The
specimens were obtained after surgical resection and immediately
frozen at −80°C until use. The study methodologies conformed to the
standards set by the Declaration of Helsinki. Collection and usage
of all specimens were approved by the Loudi Central Hospital Ethics
Committee (Loudi, China).
miRNAs and transfection
The Homo sapien (has)-miR-32 mimics,
has-miR-32 inhibitor and has-miR-32 scrambled negative control were
obtained from GeneCopoeia, Inc. (Rockville, MD, USA). miR-32 mimics
and miR-32 inhibitor were used to increase and decrease the
expression of miR-32, respectively. miRNA transfection was
performed using Lipofectamine 2000 transfection reagent (11668-019;
Invitrogen Life Technologies). In brief, cells were plated in a
24-well plate and incubated overnight to achieve 80–90% confluence
at the time of transfection. In each well, 5 µl miRNA was added to
50 µl Opti-MEM® (31985070; Gibco Life Technologies, Carlsbad, CA,
USA). Separately, 2 µl Lipofectamine 2000 transfection reagent was
added to 50 µl Opti-MEM and mixed gently. The transfection complex
was added to the cells and incubated for 6 h at 37°C in a 5%
CO2 incubator, after which the serum-containing medium
was replaced.
To confirm the effect of the miRNAs on the
expression of miR-32, reverse transcription-quantitative polymerase
chain reaction (RT-qPCR) was performed to determine the mRNA
expression levels of miR-32 in the PCa cell lines. The RT-qPCR
primers (hsa-miR-32-5p; cat. no. HmiRQP0404) were obtained from
GeneCopoeia, Inc. and the cycling conditions were as follows: Step
1, 95°C for 30 min; and step 2, 40 cycles of 95°C for 15 sec then
58°C for 35 sec. The mRNA copy number results obtained were
recalculated per 1 µg total RNA. The transfected cells were the
expanded and harvested for the following further analyses.
IR treatment
All cells were irradiated in ambient air using a
cesium-137 source (Mark 1–68 irradiator; J.L. Shepherd and
Associates, San Fernando, CA, USA) at 2 Gy at room temperature for
24 h.
Luciferase reporter assay
The cells were lysed in passive lysing buffer and
then analyzed for firefly and Renilla luciferase activities using
the commercial Dual-Luciferase Reporter Assay System (E1910;
Promega Corporation, Madison, WI, USA) according to the
manufacturer's instructions. Firefly luciferase activity was
normalized to the Renilla luciferase activity.
Western blot analysis
Whole cell extracts were prepared with a cell lysis
reagent (Sigma-Aldrich, St. Louis, MO, USA) according to the
manufacturer's instructions, and then the protein was quantified by
a bicinchoninic acid assay (Pierce, Rockford, IL, USA). Next, the
protein samples were separated by 10% SDS-PAGE and detected using
western blot analysis. Mammalian target of rabbit anti-human
polycolonal rapamycin (mTOR), phosphor-mTOR (pmTOR, S2448),
phospho-S6 kinase (pS6K, T389), Light chain 3β (LC3B), Beclin 1 and
DAB2IP antibodies were purchased from Cell Signaling Technology
Inc. (1:1,000 dilution; Danvers, MA, USA). Mouse anti-actin
monoclonal antibody was purchased from Sigma-Aldrich (1:2,000
dilution). Fluorescent dye-conjugated secondary antibodies were
obtained from Invitrogen Life Technologies.
RNA isolation and RT-qPCR
Total RNA was extracted using TRIzol (15596-026;
Invitrogen Life Technologies), according to the manufacturer's
instructions, and then reverse transcribed for quantification using
the TaqMan microRNA Reverse Transcription kit (4366596; Applied
Biosystems Life Technologies, Foster City, CA, USA) according to
the manufacturer's instructions. Mature miRNAs were quantified
using 2-step TaqMan RT-qPCR with the TaqMan microRNA kit. The miRNA
expression level was normalized using U6 small nuclear RNA
(HmiRQP9001) as an internal control, as previously described
(18).
Cell survival and apoptosis assay
The cells were diluted serially to
5.0×105 cells per 60 mm-diameter well and plated into
dishes (area, 2,827.43 mm2) in triplicate. Cells were
pretreated with brefeldin A (BFA) to a final concentration of 4 nM.
After 3 h of incubation at 37°C in a 5% CO2 atmosphere,
the cells were treated with increasing doses of IR, to a total dose
of 2 Gy. After 0 to 48 h, cell viability was estimated by MTT assay
(19). Cell apoptosis was assessed
using the ANXA5-FITC Apoptosis Detection kit (556570; BD
Pharmingen, San Diego, CA, USA) by flow cytometric analysis, as
previously described (4).
Statistical analysis
Each experiment was repeated at least three times.
Data are shown as the mean ± standard deviation, and were analyzed
using SPSS 18.0 (SPSS, Inc., Chicago, IL, USA). Statistical
comparisons between groups were analyzed using the t-test
and two-tailed P<0.05 was considered to indicate a statistically
significant difference.
Results
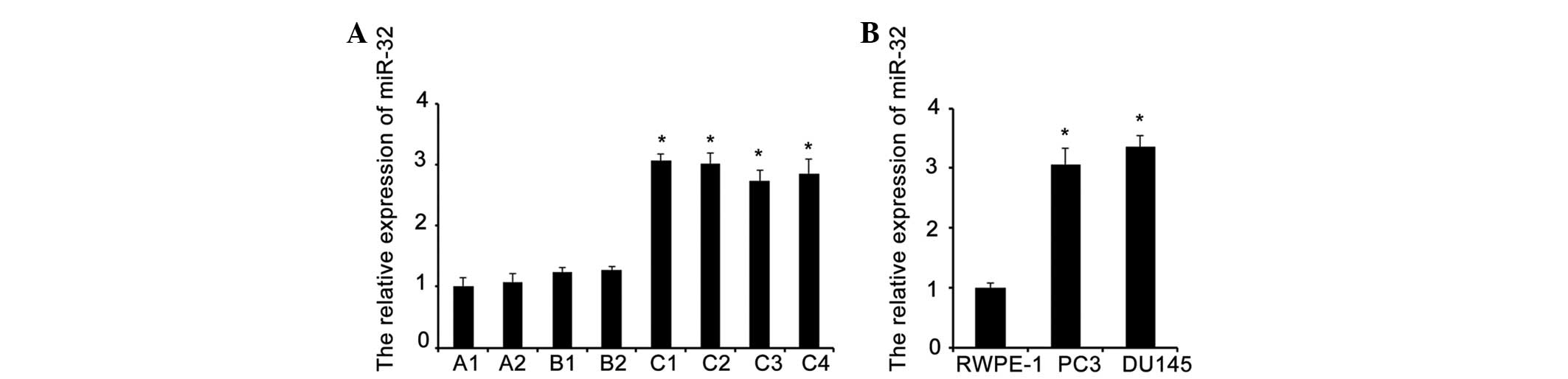
Increased expression of miR-32 in
PCa
As the expression of miR-32 was reported to increase
significantly in PCa tissue in a previous study (16), the present study examined the
expression of miR-32 in normal prostate tissues, human PCa tissues
and adjacent non-tumor tissues, as well as two PCa cell lines (PC3
and DU145) and a normal prostate cell line (RWPE-1), using RT-qPCR.
miR-32 expression was frequently upregulated or overexpressed in
the PCa tissues and cell lines compared with the normal tissues and
RWPE-1 cell line (P=0.004; Fig.
1).
DAB2IP is a target of miR-32 in PCa
cells
Previous studies indicated that DOC-2/DAB2IP is
important in the development of radioresistance in PCa through the
induction of autophagy (10,12). To investigate the diverse and complex
network of upstream signaling pathways of DAB2IP-mediated autophagy
in radioresistance in PCa, the present study screened the candidate
miRNAs that may target DAB2IP using TargetScan (www.targetscan.org) and miRanda (www.microrna.org) software. miR-32 was shown to pair
well with and target the 3′ untranslated region (3′-UTR) of DAB2IP,
with strong evidence provided by the reporter assay and RT-qPCR of
the present study (P<0.009; Fig.
2A). To further analyze the targeted regulation of miR-32 on
DAB2IP in the IR-treated PCa cells, the miR-32 level was
manipulated by miR-32 mimic and inhibitor transfection. The miR-32
level was significantly elevated in the miR-32 mimic group, but
significantly decreased in the miR-32 inhibitor group of the PCa
PC3 and DU145 cell lines (P=0.007; Fig.
2B). The miR-32 mimic transfection significantly reduced the
DAB2IP expression at the mRNA (Fig.
2C) and protein (Fig. 2D) levels
in the PC3 and DU145 cells (P=0.007). These results confirmed that
miR-32 regulated DAB2IP by targeting its 3′-UTR and suppressing its
translation.
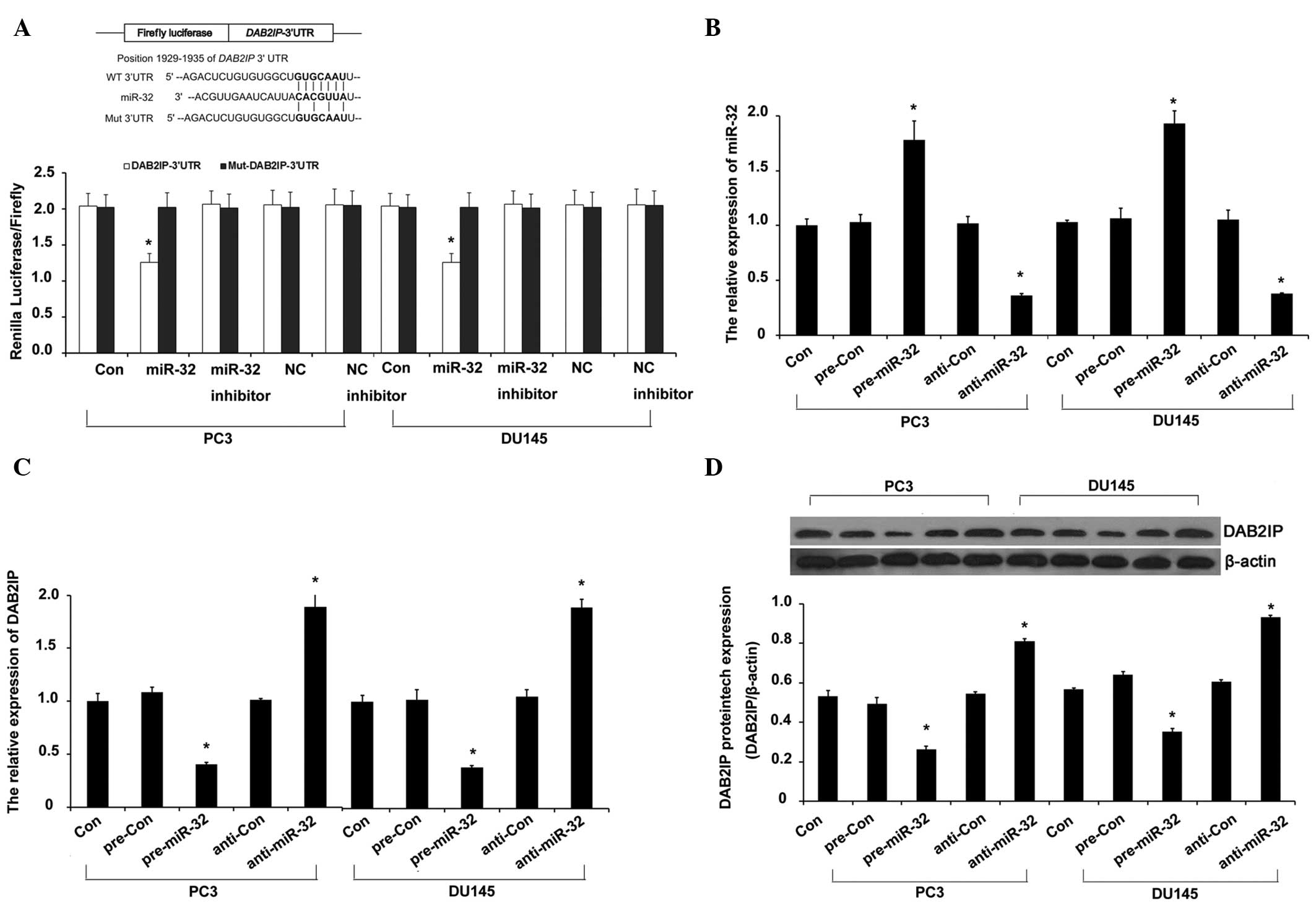 | Figure 2.miR-32 targets DAB2IP. (A) Schema
representing the functional interaction between miR-32 and the seed
sequence (bold) in the 3′-UTR of DAB2IP, as predicted by
TargetScan. Luciferase assay of PC3 and DU145 cotransfected with
reporter constructs containing DAB2IP 3′-UTRs with (DAB2IP-3′-UTR)
or without (Mut-DAB2IP-3′-UTR) miR-32 binding sites and miR-32
mimic, miR-32 inhibitor or scrambled control miRNA for 72 h. (B and
C) The level of miR-32 was assayed by TaqMan reverse
transcription-quantitative polymerase chain reaction (RT-qPCR). In
parallel, the mRNA level of DAB2IP was assayed by qRT-PCR. (D) The
level of DAB2IP protein expression was assayed by western blotting.
PC3 and DU145 cells were transfected with inhibitor or mimic of
miR-32 or inhibitor control or mimic control (100 nM) for 72 h.
Error bars represent the standard deviation from the mean.
*P<0.01 vs. control. miRNA, microRNA; DAB2IP, DAB2 interacting
protein; NC, negative control of miR-32 group; NC inhibitor,
negative control of miR-32 inhibitor group; UTR, untranslated
region; con, control; pre-miR-32, miR-32 mimic; pre-con, mimic
control; anti-miR-32, miR-32 inhibitor; anti-con, inhibitor
control. |
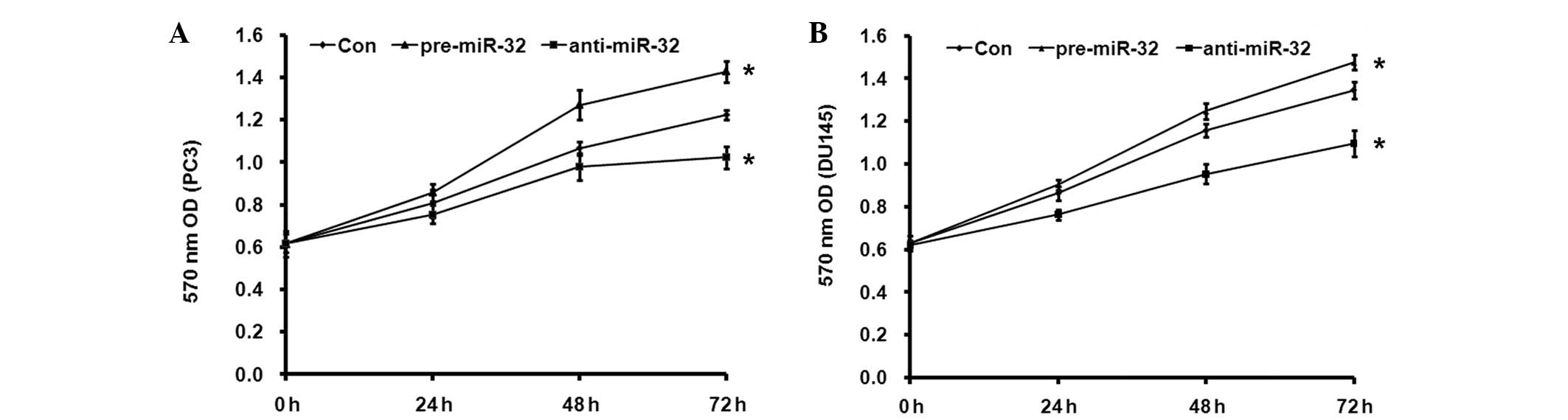
miR-32 contributes to the
radioresistance of PCa cells
To test the effect of miR-32 on the radiosensitivity
of PCa cells, PC3 and DU145 cells were employed. As shown in
Fig. 3, the silencing of endogenous
miR-32 resulted in a significant radiation-sensitizing effect in
the PC3 and DU145 cells (P=0.008). By contrast, the overexpression
of miR-32 increased the radiation resistance of the PC3 and DU145
cells (P=0.008; Fig. 3). These in
vitro results indicated that miR-32 can increase the resistance
of PC3 and DU145 cells to radiation.
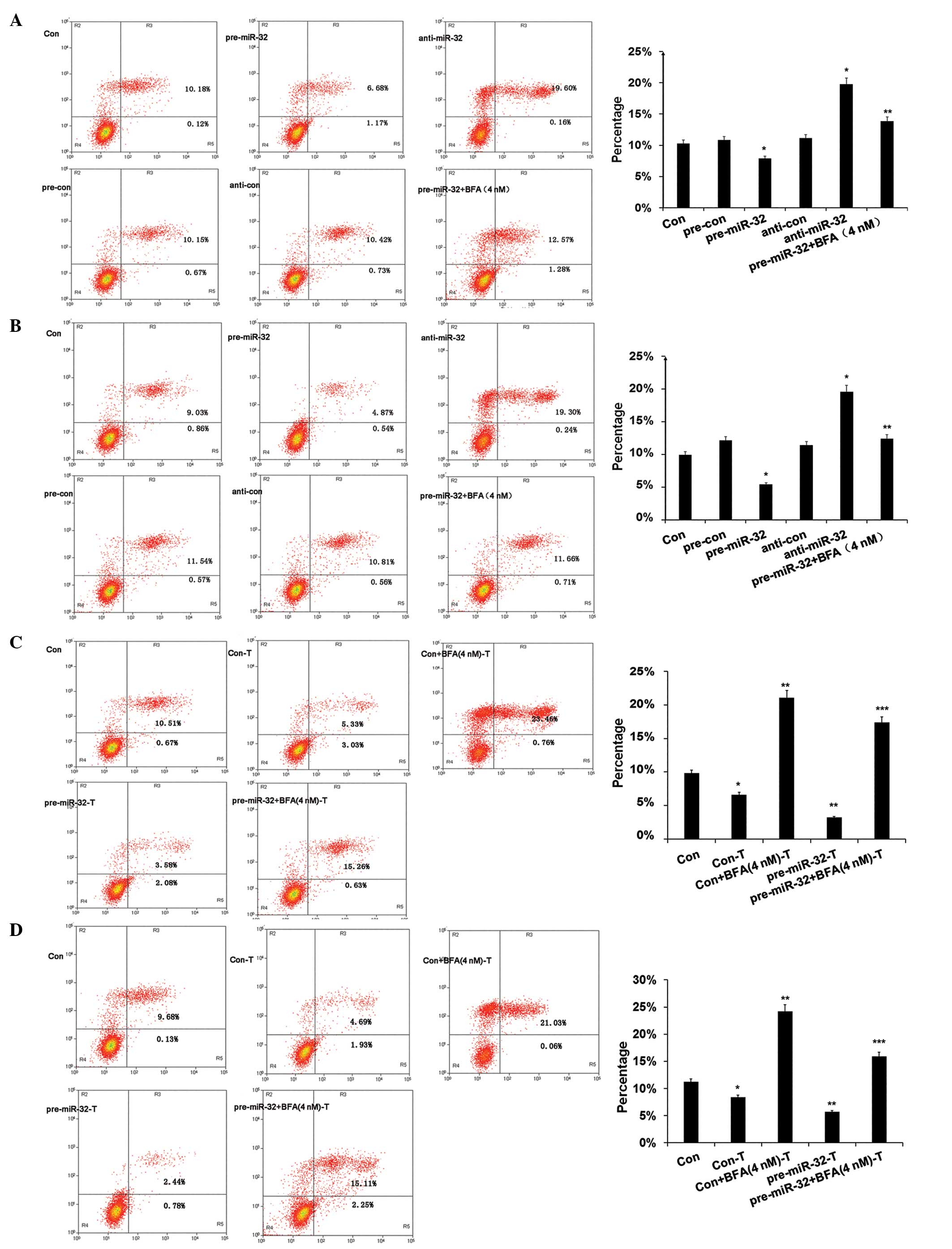
miR-32 inhibits apoptosis by modifying
DAB2IP expression
In previous studies, DAB2IP-knockdown cells were
resistant to radiation-induced apoptosis in PCa (1,12). The
present study investigated whether miR-32 mimic treatment and the
suppression of DAB2IP expression by endogenous DAB2IP knocked down
the resistance of PCa to radiotherapy. PCa cells were treated with
2 Gy IR for 24 h. As shown in Fig.
4A, cell apoptosis was significantly upregulated in the miR-32
deficient group (anti-miR-32) and downregulated in the miR-32
overexpression group (pre-miR-32; P=0.006). The PCa cells were
further subjected to IR combined with an apoptosis promoter, BFA.
Flow cytometry assay clearly showed that BFA treatment restored
radiation-induced cell death when miR-32-overexpressing PCa cells
and BFA-treated miR-32-overexpressing PCa cells were compared
(P=0.004). Similar to miR-32 overexpression, DAB2IP-knockdown
treatment reduced apoptosis (P=0.009; Fig. 4C). BFA (4 nM) could restore cell
apoptosis in the DAB2IP-deficient cells. On the basis of these
results, we propose that the increased resistance to IR in
miR-32-overexpressing and DAB2IP-knockdown PCa cells may be
partially due to the inhibition of apoptosis.
miR-32 induces autophagy via the
mTOR-S6K pathway
Given that DAB2IP acts as an autophagy inhibitor in
radioresistant PCa cells, the present study investigated the
function of miR-32 as a regulator of PCa autophagy. Beclin1 and
LC3B are autophagy-related markers and are critical for regulating
autophagy. LC3B exists in a cytosolic form, LC3B-I, and a form that
is conjugated to phosphatidylethanolamine, LC3B-II (20,21).
Increased LC3B-II levels are closely associated with the number of
autophagosomes and serve as a good indicator of autophagosome
formation (22). To investigate the
role of miR-32 in autophagy, the expression levels of the
microtubule-associated proteins Beclin1 and LC3B were determined.
As shown in Fig. 5A, silenced miR-32
impaired the IR-mediated induction of LC3B-II in the PCa cells. In
addition, DAB2IP-knockdown decreased the expression of LC3B-I and
increased the expression of LC3B-II, which were revised by miR-32
inhibitor (P=0.004 and P=0.007, respectively; Fig. 5B). Moreover, the higher expression of
Beclin 1 protein was noted in the miR-32 overexpressing and
DAB2IP-knockdown PCa cells (P=0.006 and P=0.005, respectively;
Fig. 5C).
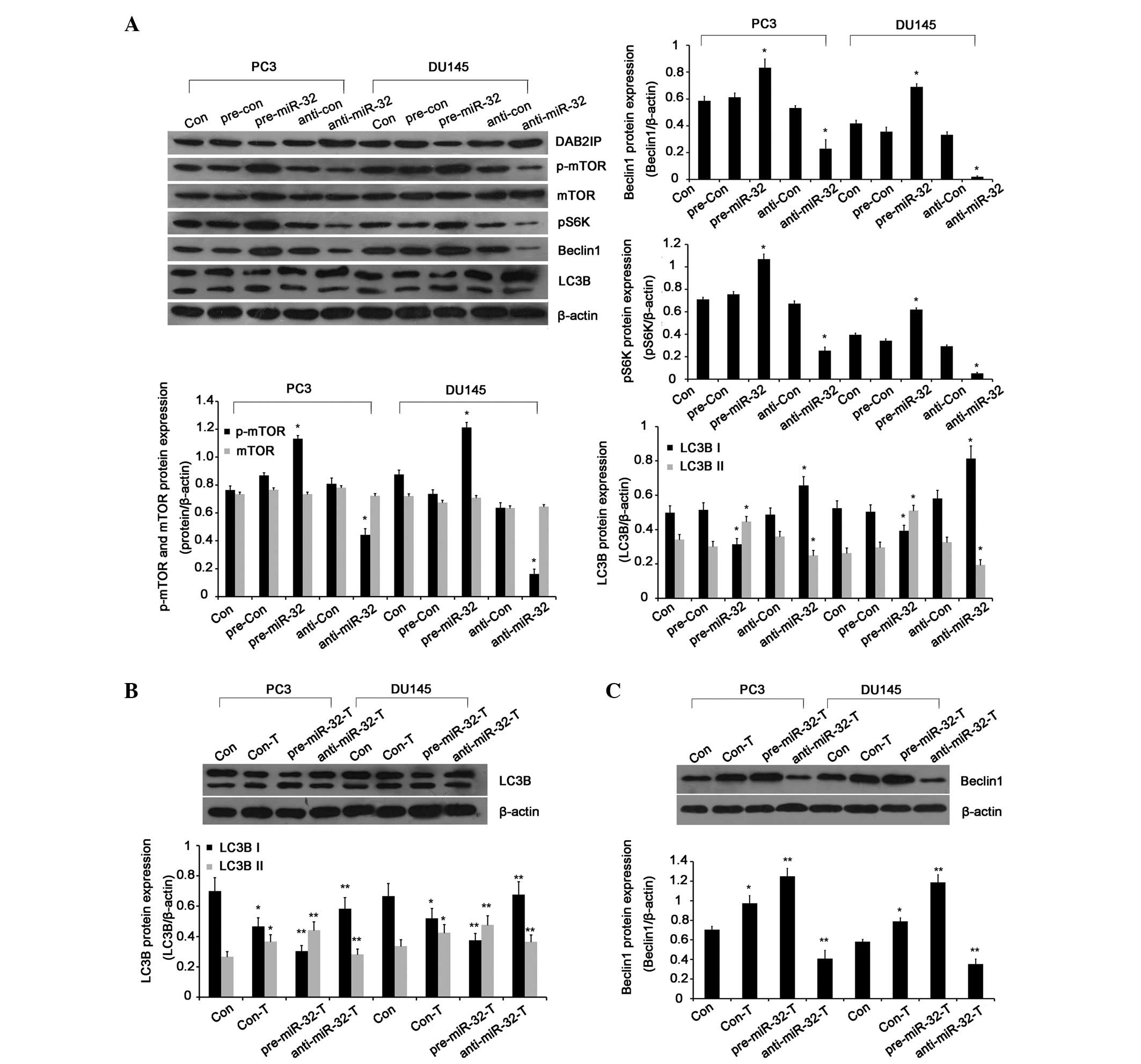 | Figure 5.miR-32 induces autophagy via the
mTOR-S6K Pathway. (A) The phosphorylation of mTOR and S6K and the
expression of autophagy-associated Beclin 1 and LC3B were
determined by Western blot analysis at 24 h post-IR (2 Gy).
*P<0.01 vs. control. DAB2IP regulates autophagy-associated
proteins (B) LC3B and (C) Beclin 1. *P<0.01 vs. control. Error
bars represent the standard deviation from the mean. **P<0.01
vs. Con-T group. miR, microRNA, p-, phosphorylated; mTOR, mammalian
target of rapamycin; S6K, S6 kinase; LC3B, light chain 3β; T,
TALEN; pre-miR-32, miR-32 mimic; pre-con, mimic control;
anti-miR-32, miR-32 inhibitor; anti-con, inhibitor control. |
It has been reported that the Akt-mTOR pathway
negatively regulates autophagy (23,24). To
investigate how miR-32 regulated IR-induced autophagy in the PCa
cells, the phosphorylation of mTOR was measured in the two cell
lines. Fig. 5A shows the marked
inhibition of phosphorylated mTOR in miR-32-silenced cells
(P=0.007). Furthermore, studies have shown that S6K is a critical
downstream effector of the mTOR signaling pathway (25). In the present study, increased
phosphorylation of S6K was observed in the miR-32-overexpressing
PCa cells and decreased phosphorylation of S6K was observed in the
miR-32-silenced PCa cells (P=0.006; Fig.
5A). Although mTOR-S6K activation is known to suppress
autophagy in mammalian cells, emerging studies have indicated that,
in certain situations, the mTOR-S6K pathway positively regulates
autophagy (26–28). Together, the findings indicate that
the miR-32 may promote IR-induced autophagy through the mTOR-S6K
pathway.
Discussion
PCa, the second most commonly occurring cause of
cancer-related mortality among men in the majority of countries, is
a complex and multifactorial disease (29). Current medical management for
localized PCa ranges from close monitoring for indolent disease to
treatments such as radiotherapy, chemotherapy or surgery. Treatment
with radiation therapy has the advantages of being non-invasive and
well tolerated. However, radiotherapy resistance is also a common
occurrence and contributes to the failure in blocking disease
progression (30). Accumulating
studies have evaluated the resistance mechanisms and biological
factors that are involved (31,32).
Autophagy is an intracellular self-protective mechanism that
functions by preventing the toxic accumulation of damaged
components and by recycling these components to sustain metabolic
homoeostasis (33,34). Upregulated autophagy has been
identified in a wide variety of cancer cells that undergo metabolic
and therapeutic stress, and the process contributes to the
resistance to chemotherapy by a range of tumor types (35,36). The
blocking of autophagy in cancer cells is emerging as a novel
approach to enhance the sensitivity of therapy in cancers (37). A previous study confirmed the
important role of DAB2IP in the autophagy inhibition of PCa cells
with IR-treatment in vitro (12). DAB2IP, a potential tumor suppressor
gene, is often downregulated in PCa primarily due to altered
epigenetic regulation of its promoter (38). Recent studies have indicated that the
loss of DAB2IP expression in PCa cells greatly increases radiation
resistance in vitro (1), and
the overexpression of DAB2IP suppresses IR-induced autophagy and
promotes apoptosis in PCa cells (12). However, the regulation of DAB2IP,
particularly by miRNAs, remains largely unknown. miRNAs have been
detected in association with cancer diagnosis and prognosis as
promising biomarkers and regulators of tumor proliferation,
invasion, migration and apoptosis. miR-218, as a tumor-suppresser,
inhibits cancer cell migration and invasion via the targeting of
LASP1 in PCa (39). miR-20a promotes
the invasion and migration of PCa via the targeting of ABL2
(40). miR-494-3p targets CXCR4 in
order to the proliferation, invasion and migration of PCa (41). miR-124 exhibits anti-proliferative and
anti-aggressive effects on PCa cells via the PACE4 pathway
(42). These miRNAs often function as
oncogenes by repressing tumor suppressors or function as
suppressors by negatively regulating oncogenes, indicating the
potential effects on the prognosis and clinical application to PCa
therapy. The present study established for the first time the
important role played by miR-32 in inhibiting the expression of the
DAB2IP tumor suppressors in PCa. It was demonstrated that miR-32
was well paired with the 3′-UTR of DAB2IP. Functional analysis
demonstrated that PCa cells post-DAB2IP blockage by miR-32 were
more resistant to IR treatment, with increased cell proliferation
and reduced cell apoptosis.
A previous study showed that DAB2IP mediated the
radiosensitization of PCa cells partially through the inhibition of
autophagy (12). DAB2IP was involved
in the autophagy pathway and overexpression of this gene attenuated
IR-induced autophagy (12). In the
present study, it was observed that LC3B and Beclin 1 were
upregulated in the PCa cells with silenced DAB2IP and overexpressed
miR-32. The mTOR-S6K pathway is postulated to be a negative
regulator of mammalian autophagy (19). The mTOR-S6K pathway was recorded as
inactivated in PCa cells with downregulated DAB2IP expression
(12). Activated mTOR induced mTOR
complex 1 substrate S6K phosphorylation, which resulted in the
induction of the functional protein translational machinery
(43). Consistent with this study,
the present results showed that the phosphorylation of S6K was
increased in the miR-32-overexpressed PCa cells. Therefore, we
speculate that miR-32 enhanced the radioresistance of the PCa cells
by promoting DAB2IP-related autophagy via the mTOR-S6K pathway.
In conclusion, the present study demonstrated that
miR-32 directly targeted DAB2IP in PCa, and induced
DAB2IP-deficient radioresistant human PCa cells. Moreover, the
findings demonstrated the critical role of miR-32 in inhibiting the
mTOR-S6K pathway and suppressing autophagy by targeting DAB2IP. On
the basis of these results, miR-32 appears to be a novel tumor
promoter and plays an important role in radiotherapy resistance
during the treatment of PCa.
References
|
1
|
Kong Z, Xie D, Boike T, Raghavan P, Burma
S, Chen DJ, Habib AA, Chakraborty A, Hsieh JT and Saha D:
Downregulation of human DAB2IP gene expression in prostate cancer
cells results in resistance to ionizing radiation. Cancer Res.
70:2829–2839. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hanks GE, Pajak TF, Porter A, et al: Phase
III trial of long-term adjuvant androgen deprivation after
neoadjuvant hormonal cytoreduction and radiotherapy in locally
advanced carcinoma of the prostate: The radiation therapy oncology
group protocol 92–02. J Clin Oncol. 21:3972–3978. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Roach M III: Radiotherapy plus adjuvant
goserelin improves survival in men with poor prognosis prostate
cancer. Cancer Treat Rev. 31:582–586. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xie D, Gore C, Zhou J, Pong RC, Zhang H,
Yu L, Vessella RL, Min W and Hsieh JT: DAB2IP coordinates both
PI3K-Akt and ASK1 pathways for cell survival and apoptosis. Proc
Natl Acad Sci USA. 106:19878–19883. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen H, Toyooka S, Gazdar AF and Hsieh JT:
Epigenetic regulation of a novel tumor suppressor gene (hDAB2IP) in
prostate cancer cell lines. J Biol Chem. 278:3121–30. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wu K, Liu J, Tseng SF, et al: The role of
DAB2IP in androgen receptor activation during prostate cancer
progression. Oncogene. 33:1954–1963. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xie D, Gore C, Liu J, et al: Role of
DAB2IP in modulating epithelial-to-mesenchymal transition and
prostate cancer metastasis. Proc Natl Acad Sci USA. 107:2485–2490.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Min J, Zaslavsky A, Fedele G, et al: An
oncogene-tumor suppressor cascade drives metastatic prostate cancer
by coordinately activating Ras and nuclear factor-kappaB. Nat Med.
16:286–294. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang Z, Tseng CP, Pong RC, et al: The
mechanism of growth-inhibitory effect of DOC-2/DAB2 in prostate
cancer. Characterization of a novel GTPase-activating protein
associated with N-terminal domain of DOC-2/DAB2. J Biol Chem.
277:12622–12631. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu K, Xie D, Zou Y, et al: The mechanism
of DAB2IP in chemoresistance of prostate cancer cells. Clin Cancer
Res. 19:4740–4749. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kong Z, Raghavan P, Xie D, et al:
Epothilone B confers radiation dose enhancement in DAB2IP gene
knock-down radioresistant prostate cancer cells. Int J Radiat Oncol
Biol Phys. 78:1210–1218. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yu L, Tumati V, Tseng SF, et al: DAB2IP
regulates autophagy in prostate cancer in response to combined
treatment of radiation and a DNA-PKcs inhibitor. Neoplasia (New
York, NY). 14:1203–1212. 2012. View Article : Google Scholar
|
|
13
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Calin GA and Croce CM: MicroRNA signatures
in human cancers. Nat Rev Cancer. 6:857–866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tutar L, Tutar E and Tutar Y: MicroRNAs
and cancer; an overview. Curr Pharm Biotechnol. 15:430–437. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Walter BA, Valera VA, Pinto PA and Merino
MJ: Comprehensive microRNA profiling of prostate cancer. J Cancer.
4:350–357. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Leung CM, Li SC, Chen TW, et al:
Comprehensive microRNA profiling of prostate cancer cells after
ionizing radiation treatment. Oncol Rep. 31:1067–1078.
2014.PubMed/NCBI
|
|
18
|
Liu K, Huang J, Xie M, et al: MIR34A
regulates autophagy and apoptosis by targeting HMGB1 in the
retinoblastoma cell. Autophagy. 10:442–452. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu B, Che W, Xue J, et al: SIRT4 prevents
hypoxia-induced apoptosis in H9c2 cardiomyoblast cells. Cell
Physiol Biochem. 32:655–662. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tanida I, Ueno T and Kominami E: LC3
conjugation system in mammalian autophagy. Int J Biochem Cell Biol.
36:2503–2518. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sou YS, Tanida I, Komatsu M, Ueno T and
Kominami E: Phosphatidylserine in addition to
phosphatidylethanolamine is an in vitro target of the mammalian
Atg8 modifiers, LC3, GABARAP and GATE-16. J Biol Chem.
281:3017–3024. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rouschop KM, van den Beucken T, Dubois L,
et al: The unfolded protein response protects human tumor cells
during hypoxia through regulation of the autophagy genes MAP1LC3B
and ATG5. J Clin Invest. 120:127–141. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kondo Y, Kanzawa T, Sawaya R and Kondo S:
The role of autophagy in cancer development and response to
therapy. Nat Rev Cancer. 5:726–734. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jung CH, Kim H, Ahn J, Jung SK, Um MY, Son
KH, Kim TW and Ha TY: Anthricin isolated from Anthriscus
sylvestris (L.) Hoffm. Inhibits the growth of breast cancer cells
by inhibiting Akt/mTOR signaling and its apoptotic effects are
enhanced by autophagy inhibition. Evid Based Complement
Alternat Med. 2013:3852192013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zoncu R, Efeyan A and Sabatini DM: mTOR:
From growth signal integration to cancer, diabetes and ageing. Nat
Rev Mol Cell Biol. 12:21–35. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zeng X and Kinsella TJ: Mammalian target
of rapamycin and S6 kinase 1 positively regulate
6-thioguanine-induced autophagy. Cancer Res. 68:2384–2390. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Scott RC, Schuldiner O and Neufeld TP:
Role and regulation of starvation-induced autophagy in the
Drosophila fat body. Dev Cell. 7:167–178. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Klionsky DJ, Meijer AJ and Codogno P:
Autophagy and p70S6 kinase. Autophagy. 1:59–60. 2005.discussion
60-1. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ambs S, Prueitt RL, Yi M, Hudson RS, Howe
TM, Petrocca F, Wallace TA, Liu CG, Volinia S, Calin GA, et al:
Genomic profiling of microRNA and messenger RNA reveals deregulated
microRNA expression in prostate cancer. Cancer Res. 68:6162–6170.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Paller CJ, Antonarakis ES, Eisenberger MA
and Carducci MA: Management of patients with biochemical recurrence
after local therapy for prostate cancer. Hematol Oncol Clin North
Am. 27:1205–1219. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Orth M, Lauber K, Niyazi M, Friedl AA, Li
M, Maihöfer C, Schüttrumpf L, Ernst A, Niemöller OM and Belka C:
Current concepts in clinical radiation oncology. Radiat Environ
Biophys. 53:1–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kaliberov SA and Buchsbaum DJ: Chapter
seven - Cancer treatment with gene therapy and radiation therapy.
Adv Cancer Res. 115:221–263. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Huber TB, Edelstein CL, Hartleben B, Inoki
K, Jiang M, Koya D, Kume S, Lieberthal W, Pallet N, Quiroga A, et
al: Emerging role of autophagy in kidney function, diseases and
aging. Autophagy. 8:1009–1031. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hale AN, Ledbetter DJ, Gawriluk TR and
Rucker EB: 3rd: Autophagy: Regulation and role in development.
Autophagy. 9:951–972. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Massoner P, Thomm T, Mack B, Untergasser
G, Martowicz A, Bobowski K, Klocker H, Gires O and Puhr M: EpCAM is
overexpressed in local and metastatic prostate cancer, suppressed
by chemotherapy and modulated by MET-associated miRNA-200c/205. Br
J Cancer. 111:955–964. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Schmukler E, Shai B, Ehrlich M and
Pinkas-Kramarski R: Neuregulin promotes incomplete autophagy of
prostate cancer cells that is independent of mTOR pathway
inhibition. PLoS One. 7:e368282012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Livesey KM, Tang D, Zeh HJ and Lotze MT:
Autophagy inhibition in combination cancer treatment. Curr Opin
Investig Drugs. 10:1269–1279. 2009.PubMed/NCBI
|
|
38
|
Chen H, Tu SW and Hsieh JT:
Down-regulation of human DAB2IP gene expression mediated by
polycomb Ezh2 complex and histone deacetylase in prostate cancer. J
Biol Chem. 280:22437–22444. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Nishikawa R, Goto Y, Sakamoto S, Chiyomaru
T, Enokida H, Kojima S, Kinoshita T, Yamamoto N, Nakagawa M, Naya
Y, et al: Tumor-suppressive microRNA-218 inhibits cancer cell
migration and invasion via targeting of LASP1 in prostate cancer.
Cancer Sci. 105:802–811. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Qiang XF, Zhang ZW, Liu Q, Sun N, Pan LL,
Shen J, Li T, Yun C, Li H and Shi LH: miR-20a promotes prostate
cancer invasion and migration through targeting ABL2. J Cell
Biochem. 115:1269–1276. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Shen PF, Chen XQ, Liao YC, Chen N, Zhou Q,
Wei Q, Li X, Wang J and Zeng H: MicroRNA-494-3p targets CXCR4 to
suppress the proliferation, invasion and migration of prostate
cancer. Prostate. 74:756–767. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kang S, Zhao Y, Hu K, Xu C, Wang L, Liu J,
Yao A, Zhang H and Cao F: miR-124 exhibits antiproliferative and
antiaggressive effects on prostate cancer cells through PACE4
pathway. Prostate. 74:1095–1106. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Surviladze Z, Sterk RT, DeHaro SA and
Ozbun MA: Cellular entry of human papillomavirus type 16 involves
activation of the phosphatidylinositol 3-kinase/Akt/mTOR pathway
and inhibition of autophagy. J Virol. 87:2508–2517. 2013.
View Article : Google Scholar : PubMed/NCBI
|