Introduction
Anorectal melanoma, the third most common site of
mucosal melanoma, is a rare tumor with an incidence rate of 1.7
cases per million in the global population (1). Surgery, including abdominoperineal
resection (APR) or wide local excision (WLE), is the primary
treatment strategy for localized disease; however, these two
surgical approaches result in similarly poor clinical outcomes,
with five-year recurrence rates of 79 and 81% following APR and
WLE, respectively (2). Furthermore,
the five-year overall survival rate following curative resection
ranges from 15 to 22%, with the majority of patients succumbing to
recurrent disease. Recurrence predominantly occurs in the liver,
followed by the lung and brain (3,4).
Gastrointestinal stromal tumors (GISTs),
characterized by the expression of cluster of differentiation (CD)
117 and a high incidence of KIT or platelet-derived growth
factor receptor α (PDGFRA) mutations, are uncommon tumors
with an incidence range of 6–15 cases per million individuals
(5). The majority of GISTs arise in
the stomach and small intestine, and GISTs originating from the
esophagus, colon and extra-gastrointestinal organs, such as the
retroperitoneum and liver, are rare (6). To the best of our knowledge, only five
cases of primary hepatic GIST have previously been reported in the
English literature (7–11). The most common KIT mutation
sites involve exon 11 (66.1%) followed by exon 9 (13%); however,
12% of cases exhibit no detectable KIT mutation and are
therefore categorized as KIT wild-type GISTs (12).
The present study reports the case of a long-term
survivor of anorectal melanoma who, seven years after APR surgery,
developed a solitary rapidly growing tumor in the liver.
Case report
A 58-year-old Taiwanese male was diagnosed with
malignant melanoma of the anorectum and underwent APR at Kaohsiung
Medical University Hospital (Kaosiung, Taiwan) in 2001.
Microscopically, the anorectal tumor was composed of spindle-shaped
neoplastic cells beneath the squamous epithelium with brownish
pigments and adjacent junctional activity. Furthermore, the tumor
was immunohistochemically positive for human melanoma black
(HMB-45) and S-100 (Fig. 2A–D). No
surgical margin or lymph node involvement was apparent, therefore,
stage I anorectal melanoma was diagnosed. The patient did not
receive any adjuvant therapy and was regularly followed up by
physical examination every two weeks at Kaohsiung Medical
University Hospital for five years after surgery.
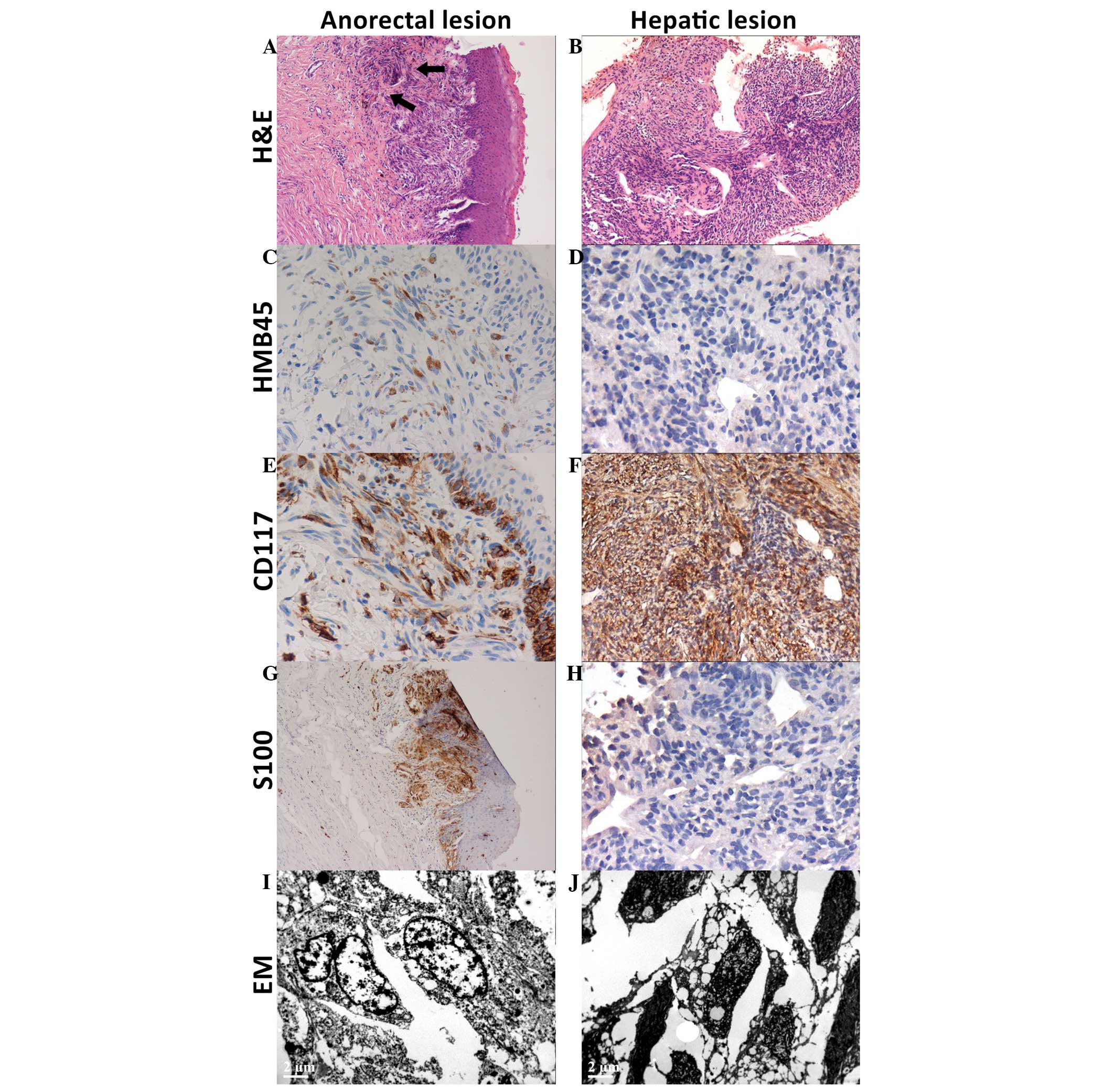 | Figure 2.Histology: (A-H) IHC analysis and (I
and J) EM of the primary anorectal melanoma and second primary
hepatic gastrointestinal stromal tumors. For the anorectal lesion,
(A) H&E staining reveled subepithelial spindle neoplastic cells
with brownish pigments (arrows), which were positive for (C) HMB45,
(E) CD117 and (G) S-100 in IHC analysis, and (I) positive for
atypical melanosome in EM examination. For the hepatic lesion, (B)
H&E staining revealed spindle neoplastic cells arranged in
whorls or intervening fascicles, which were (D) negative for HMB45,
(F) strongly positive for CD117 and (H) negative for S-100 in IHC
staining. (J) EM showed hyperchromatic nuclei and vesicular
cytoplasm. IHC, immunohistochemical; H&E, hematoxylin and
eosin; HMB-45, human melanoma black-45; CD117, cluster of
differentiation 117; EM, electronic microscopy. |
In 2009, after a further two years and at the age of
65 years, the patient presented to the Department of General
Surgery at Kaohsiung Medical University Hospital with a two-month
history of general malaise, loss of appetite and progressively
worsening dull pain over the epigastrium. An appropriate physical
examination revealed no abnormalities, with the exception of the
APR surgical scar on the abdominal wall. However, a follow-up
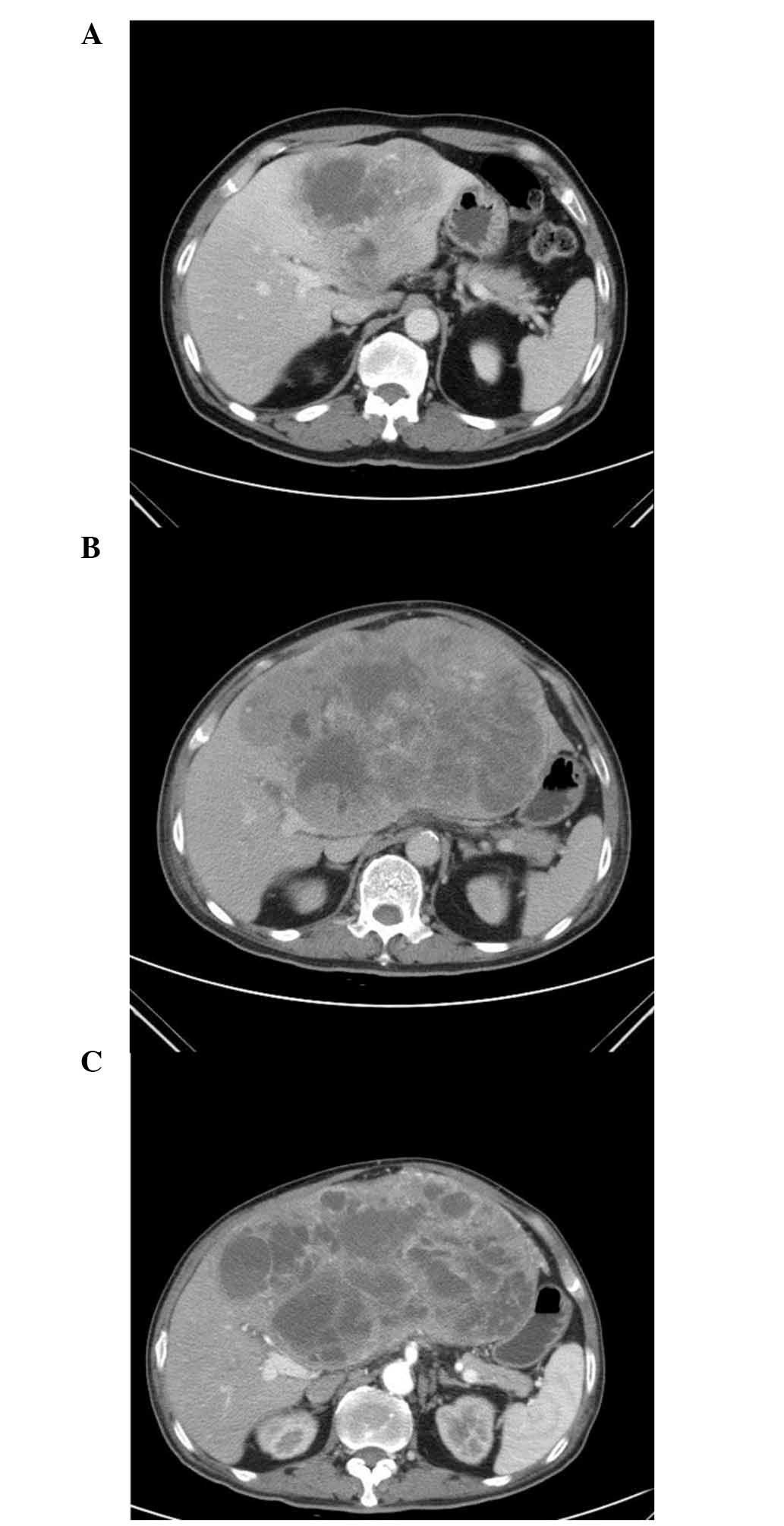
contrast-enhanced computed tomography (CT) scan revealed a 12×11-cm
solitary tumor within the left lobe of the liver (Fig. 1A). Serum α-fetoprotein,
carcinoembryonic antigen and carbohydrate antigen 19-9 levels were
all within normal limits. Subsequently, a percutaneous CT-guided
biopsy of the liver tumor was performed. Microscopically, the liver
tumor was composed of spindle cells arranged in interlacing
fascicles with hyperchromatic, round or oval to spindle pleomorphic
nuclei. Immunohistochemical (IHC) staining revealed that the
neoplastic cells were diffusely and strongly positive for CD117,
but negative for HMB-45, S-100 melan-A and CD34. Furthermore, the
mitotic activity of the tumor cells was five mitoses per 10
high-power fields. Retrospectively, positive CD117 staining was
also observed in the anorectal melanoma and the basal layer
squamous epithelium. The results of the histological and IHC
staining of the anorectal melanoma are shown in Fig. 2A–D, and the liver tumor in Fig. 2F–I. In addition, polymerase chain
reaction followed by direct sequencing was performed using DNA
extracted from formalin-fixed, paraffin-embedded (FFPE) tissue,
revealing that the anorectal melanoma and hepatic tumor were
negative for BRAFV600E (data not shown).
To confirm the diagnosis, transmission electron
microscopy (TEM) of the FFPE-derived tumors was performed to
determine the ultrastructure of the two tumors. Analysis of the
anorectal tumor showed a number of electron-dense granules around
the nucleus, which were considered to be atypical melanosomes, and
inconspicuous microtubules within the cisternal space of the
cytoplasm (Fig. 2E). These features
were considered to correspond with the diagnosis of melanoma. Due
to damage of the fine structures of the small paraffin-embedded
tissue during formalin fixation and subsequent xylene treatment,
artifacts were present in the sample and a collapsed cytoplasmic
structure was observed. Thus, the quality of the liver TEM image
was poor. Therefore, no villous cytoplasmic processes or dispersed
intermediate filaments, which are considered to be characteristic
ultrastructure features of GISTs, could be identified (13). However, hyperchromatic nuclei and a
vesicular cytoplasm without melanosomes were present (Fig. 2J), indicating that the liver tumor may
have a different histology from the prior melanoma.
Despite testing negative for KIT and
PDGFRA mutations, the liver tumor was diagnosed as primary
GIST of the liver based on its IHC profiling (diffusely strongly
CD117-positive, and negative for HMB-45 and S-100), and TEM
features when compared with anorectal melanoma, as well as the lack
of other intra-abdominal tumors identified on abdominal CT imaging.
Due to the presence of left portal vein invasion, the hepatic GIST
was considered as unresectable, therefore, oral imatinib was
administered at a dose of 400 mg/day for two months. However, a
follow-up CT scan after two months of imatinib therapy demonstrated
that the tumor had progressed to 22×13 cm in size (Fig. 1B). While awaiting the approval of
sunitinib from regulatory authorities, systemic chemotherapy
consisting of 50 mg/m2 doxorubicin and 50
mg/m2 cisplatin was administered every three weeks for
five months. This regimen resulted in symptomatic improvement
manifesting as a decrease in dull epigastric pain and tumor size by
palpation after the first cycle of treatment. Thus, this
chemotherapeutic treatment strategy was continued. A follow-up CT
scan, conducted two months later, demonstrated that the tumor had
stabilized with minimal regression to a size of 19×11 cm (Fig. 1C). The chemotherapy was continued for
an additional three months, substituting with 37.5 mg/day sunitinib
after disease progression. However, the patient did not respond to
sunitinib and succumbed to the disease one month later.
Discussion
The current study describes the case of a unique
patient who was found to have a solitary hypervascular liver tumor
that was subsequently diagnosed as a second primary GIST of the
liver by IHC. The patient had undergone APR for a stage I anorectal
melanoma seven years prior. Clinically, it is not possible to
distinguish between a metastatic melanoma and a metastatic or
primary GIST in the liver, as the two types of tumor can be
hypervascular in the early arterial phase of a CT scan, and grow
rapidly. Considering the history of anorectal melanoma and the
absence of a KIT/PDGFRA mutation during genotyping,
the diagnosis of GIST in the present patient may be disputable.
However, if the patient did not have a history of mucosal melanoma,
the tumor could be easily diagnosed as GIST based on the results of
the CD117 staining, irrespective of its KIT/PDGFRA
genotyping. Therefore, a final diagnosis of hepatic GIST was
determined, based on the diffusely strong CD117 staining,
negativity for HMB-45 and S100 (two positive markers noted in his
anorectal melanoma) and the difference in ultrastructure between
the two tumors, as noted by performing TEM.
Initially, the diagnosis of GIST was considered to
be more favorable for the current patient compared with those
exhibiting metastatic melanoma, with disease-specific median
survival periods of 19.0 and 13.3 months, respectively (14). However, following the approval of
imatinib in 2002, results indicated that metastatic or unresectable
GIST was more treatable compared with metastatic melanoma, with a
notable increase in survival to 38.4–60 months (according to the
kinase genotype) (15). Imatinib
markedly improved the survival of GIST patients, regardless of the
KIT/PDGFR mutation status. Despite imatinib typically
producing a poorer response in wild-type GIST compared with exon
11-mutated GIST, imatinib does provide a benefit to patients with
wild-type GIST, exhibiting a disease control rate of >60%
(15). Furthermore, in patients with
wild-type GIST, Asian populations appear more likely to benefit
from imatinib treatment compared with Western populations. In the
CALGB 150105 study, clinical benefit rates were 78–95% in the Asian
population and 64% in the Western population (15). Additionally, five-year survival rates
of 60–70% and 40–45% were noted for Asian and Western populations,
respectively (15–17). Although the differences may have been
caused by the small-size bias of Asian studies, the possibility of
higher pharmacokinetic parameters in Asian populations following
fixed-dose imatinib treatment should also be considered. Despite
the increased benefit in Asian populations, the current patient did
not receive any benefit from imatinib treatment and the tumor
progressed rapidly within two months, which indicates that the
nature of primary hepatic GIST may be different from those arising
in the alimentary tract.
To the best of our knowledge, to date, only five
cases of primary hepatic GIST have been previously reported
(Table I) (7–11). Among
these five cases, two cases received imatinib therapy (8,9). One of
the imatinib-treated cases lacked mutation analysis data, however,
the patient exhibited a good response to imatinib, resulting in a
disease-free status for three years (8). The other imatinib-treated case had no
mutation of KIT exon 11 (only KIT exon 11 was
analyzed) and responded well to imatinib, with a long-term survival
period of nine years (9). As only a
small number of cases of primary hepatic GIST have been reported
thus far, the correlation between the KIT genotype and
response to imatinib, a tyrosine kinase inhibitor, remains
inconclusive; thus, additional studies are required to address the
issue.
 | Table I.Five cases of primary gastrointestinal
stromal tumors of the liver. |
Table I.
Five cases of primary gastrointestinal
stromal tumors of the liver.
|
|
|
|
|
| Immunohistochemical
staining |
|
|
|---|
|
|
|
|
|
|
|
|
|
|---|
| Author
(Reference) | Cell type | Age, years | Gender | Tumor size, cm | CD117 | CD34 | Vimentin | S100 | Mitotic index |
KIT/PDGFRA status |
|---|
| Hu et al
(7) | Spindle | 79 | Female | 15 | + | + | + | – | 4/10 | Not performed |
| De Chiara et
al (8) | Spindle | 37 | Male | 18 | + | – | + | – | 20/50 | Not performed |
| Ochiai et al
(9) | Mixed | 30 | Male | Largea | + | + |
| – | 75/50 | No KIT exon 11
mutationb |
| Luo et al
(10) | Spindle | 17 | Male | 5.1 | + | + | + | – |
| Not performed |
| Yamamoto et al
(11) | Epithelioid | 70 | Male | 20 | – | + | + | – | 1/50 | PDGFRA, exon
12 |
| Current case | Spindle | 65 | Male | 12 | + | – | + | – | 5/10 | Wild-type
KIT/PDGFRA |
In conclusion, despite the poor response to imatinib
of the primary hepatic KIT/PDGFRA wild-type GIST in
the current patient, the present study highlights the importance of
considering a second primary malignancy and performing
immunohistochemical analysis upon the occurrence of a newly
developed lesions in long-term remission cancer survivors. With the
emergence of modern molecular technologies, such as next-generation
sequencing, it may be possible to precisely determine genetic
alterations that exist between the two tumors. This may aid in
determining the nature of the second tumor (delayed recurrence
versus second primary), as well as providing useful information to
guide therapeutic treatment of the disease.
References
|
1
|
Weinstock MA: Epidemiology and prognosis
of anorectal melanoma. Gastroenterology. 104:174–178.
1993.PubMed/NCBI
|
|
2
|
Yeh JJ, Shia J, Hwu WJ, et al: The role of
abdominoperineal resection as surgical therapy for anorectal
melanoma. Ann Surg. 244:1012–1017. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Homsi J and Garrett C: Melanoma of the
anal canal: a case series. Dis Colon Rectum. 50:1004–1010. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Aytac B, Adim SB, Yerci O and Yilmazlar T:
Anorectal malignant melanomas: experience of Uludag University.
Kaohsiung J Med Sci. 26:658–662. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ho MY and Blanke CD: Gastrointestinal
stromal tumors: disease and treatment update. Gastroenterology.
140:1372–1376.e2. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Reith JD, Goldblum JR, Lyles RH and Weiss
SW: Extragastrointestinal (soft tissue) stromal tumors: an analysis
of 48 cases with emphasis on histologic predictors of outcome. Mod
Pathol. 13:577–585. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hu X, Forster J and Damjanov I: Primary
malignant gastrointestinal stromal tumor of the liver. Arch Pathol
Lab Med. 127:1606–1608. 2003.PubMed/NCBI
|
|
8
|
DeChiara A, De Rosa V, Lastoria S, et al:
Primary gastrointestinal stromal tumor of the liver with lung
metastases successfully treated with STI-571 (imatinib mesylate).
Front Biosci. 11:498–501. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ochiai T, Sonoyama T, Kikuchi S, et al:
Primary large gastrointestinal stromal tumor of the liver: report
of a case. Surg Today. 39:633–636. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Luo XL, Liu D, Yang JJ, Zheng MW, Zhang J
and Zhou XD: Primary gastrointestinal stromal tumor of the liver: a
case report. World J Gastroenterol. 15:3704–3707. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yamamoto H, Miyamoto Y, Nishihara Y, et
al: Primary gastrointestinal stromal tumor of the liver with PDGFRA
gene mutation. Hum Pathol. 41:605–609. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Corless CL, Fletcher JA and Heinrich MC:
Biology of gastrointestinal stromal tumors. J Clin Oncol.
22:3813–3825. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Park SH, Kim MK, Kim H, et al:
Ultrastructural studies of gastrointestinal stromal tumors. J
Korean Med Sci. 19:234–244. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Balch CM, Buzaid AC, Soong SJ, et al:
Final version of the American Joint Committee on Cancer staging
system for cutaneous melanoma. J Clin Oncol. 19:3635–3648.
2001.PubMed/NCBI
|
|
15
|
Heinrich MC, Owzar K, Corless CL, et al:
Correlation of kinase genotype and clinical outcome in the North
American Intergroup Phase III Trial of imatinib mesylate for
treatment of advanced gastrointestinal stromal tumor: CALGB 150105
Study by Cancer and Leukemia Group B and Southwest Oncology Group.
J Clin Oncol. 26:5360–5367. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yeh CN, Chen TW, Lee HL, et al: Kinase
mutations and imatinib mesylate response for 64 Taiwanese with
advanced GIST: preliminary experience from Chang Gung Memorial
Hospital. Ann Surg Oncol. 14:1123–1128. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim TW, Ryu MH, Lee H, et al: Kinase
mutations and efficacy of imatinib in Korean patients with advanced
gastrointestinal stromal tumors. Oncologist. 14:540–547. 2009.
View Article : Google Scholar : PubMed/NCBI
|