Introduction
Gastric cancer is the fourth most frequent
malignancy and the second most common cause of cancer-associated
mortality worldwide (1). This cancer
is also the second most frequent malignancy in Korea (2). Although patients with early gastric
cancer may be successfully treated by surgical resection, the
majority of patients experience a relapse subsequent to the first
surgical resection (3) or are
initially diagnosed with unresectable, locally advanced or
metastatic disease (4). For these
patients with advanced or metastatic gastric cancer, the objective
of treatment is to relieve symptoms, prevent tumor progression and
prolong the survival time. Therefore, palliative chemotherapy may
play an extremely important role in the treatment of advanced
gastric cancer (AGC).
It has been reported that chemotherapy improves the
survival of AGC patients in comparison to best supportive care, and
it has also been reported that combination chemotherapy is superior
to monotherapy in terms of survival, response rate and symptom
control (5). In general,
fluoropyrimidine, such as 5-fluorouracil (5-FU) or its oral
prodrugs, and platinum, such as cisplatin or oxaliplatin,
combination regimens are widely accepted as the standard first-line
chemotherapy, with a response rate of 25–54% and a median overall
survival time of 8–13 months (5–6). However,
more than one-half of the patients with AGC that receive first-line
chemotherapy do not demonstrate a response, and even in responders
the duration of response may be as short as a few months (6). In addition, the number of patients that
maintain a good general condition following the failure of
first-line chemotherapy has increased due to the improvements in
supportive care. As a result, the number of patients that are good
candidates for subsequent salvage chemotherapy has increased. In
previous years, novel drugs, including docetaxel, paclitaxel and
irinotecan, have been tested in salvage chemotherapy for
pre-treated AGC (7,8). With the availability of these active
cytotoxic agents, numerous patients with refractory or relapsed
gastric cancer subsequent to first-line chemotherapy have received
salvage chemotherapy in routine clinical practice, particularly in
Asia (8).
Although salvage chemotherapy following first-line
treatment may be considered a confirmed option for the treatment of
AGC, the impact of the availability of several active cytotoxic
agents has not yet been assessed in AGC. Therefore, the present
study assessed the impact of the availability of fluoropyrimidines,
platinums, taxanes and irinotecan over the course of treatment on
the survival of patients with AGC.
Materials and methods
Study population
A retrospective chart review was performed on all
patients with newly diagnosed locally advanced or metastatic
gastric cancer that were treated with standard palliative
chemotherapy between March 2002 and November 2012 at the Department
of Internal Medicine, Chungbuk National University Hospital
(Cheongju, North Chungcheong, Republic of Korea). All patients were
consecutive non-selected cases from the Department of Internal
Medicine, Chungbuk National University Hospital and all patients
were treated outside of clinical trials. Patients were included in
the present study if they possessed a histologically-confirmed
diagnosis of adenocarcinoma, newly diagnosed locally advanced or
metastatic gastric cancer, and medical records containing details
of palliative chemotherapy administered. Patients were excluded if
they had not received palliative chemotherapy, had received only
fluoropyrimidine monotherapy or molecular targeted agents during
the course of treatment, had succumbed to AGC during the first
hospitalization, or possessed a history of another malignancy. The
present study was reviewed and approved by the Institutional Review
Board of Chungbuk National University Hospital.
Data collection
The baseline clinical and pathological
characteristics at the time of the diagnosis of locally advanced or
metastatic gastric cancer were reviewed, including the age, gender,
Eastern Cooperative Oncology Group (ECOG) performance status,
location of the primary tumor, histological grading according to
the World Health Organization (WHO) system, timing of metastatic
disease, location of metastasis, number of metastases, baseline
hemoglobin and baseline albumin of the patients. Data from medical
records on the palliative chemotherapeutic agents administered was
also collected, and the patients were divided according to the
availability of active cytotoxic agents over the course of
treatment, regardless of dose or schedule, as follows: Group 1
received two cytotoxic agents, fluoropyrimidine (5-FU, capecitabine
or S-1) and platinum (cisplatin or oxaliplatin); group 2 received
three cytotoxic agents, fluoropyrimidine, platinum and taxane
(docetaxel or paclitaxel) or irinotecan; and group 3 received four
cytotoxic agents, fluoropyrimidine, platinum, taxane and
irinotecan.
Statistical analysis
Overall survival was measured from the date of the
first administration of first-line chemotherapy to the date of
mortality, from any cause, or last follow-up visit. Survival curves
were estimated using the Kaplan-Meier method and the survival
curves of patients were compared using the log-rank test. A
prognostic model for overall survival was constructed using an
assessment of variables by univariate analysis followed by
multivariate analysis, which was performed using a stepwise Cox
proportional hazard regression model. The following variables were
included in the univariate analysis: Age, <65 years vs. ≥65
years; gender, male vs. female; pre-treatment ECOG performance
status, 0–1 vs. 2–3; histological grading according to the WHO
system, good (well- or moderately-differentiated) vs. poor
(poorly-differentiated or signet ring cell carcinoma) vs. no data;
timing of metastatic disease, synchronous vs. metachronous;
presence of peritoneal metastases or malignant ascites, present vs.
absent; number of metastases, ≤1 vs. 2 vs. ≥3; hemoglobin level,
<10 g/dl vs. ≥10 g/dl; and albumin level, <3.5 g/dl vs. ≥3.5
g/dl. Hazard ratios (HRs) of the studied outcomes were calculated
for each parameter estimate, in addition to the 95% confidence
interval (CI). P<0.05 was considered to indicate a statistically
significant difference. All statistical analyses were performed
using SPSS software for Windows, version 15.0 (SPSS, Inc., Chicago,
IL, USA).
Results
Baseline patient characteristics
Of the 262 patients with newly-diagnosed locally
advanced or metastatic gastric cancer that received palliative
chemotherapy between March 2002 and November 2012 at the Department
of Internal Medicine, Chungbuk National University Hospital, 216
patients were included in the final analysis. The baseline
clinicopathological characteristics of the patients are reported in
Table I. The median age was 57 years
(range, 18–79 years), and 161 patients (74.5%) were male. In total,
196 patients (90.7%) demonstrated good performance status (ECOG
status, 0–1). The majority of the patients (97.7%) possessed
metastatic disease, and only five patients (2.3%) possessed locally
advanced disease. The most common metastatic sites were the
peritoneum (56.9%), liver (35.6%) and distant lymph nodes
(31.0%).
 | Table I.Patient characteristics (n=216). |
Table I.
Patient characteristics (n=216).
| Characteristics | Value, n (%) |
|---|
| Age |
|
| Median,
years (range) | 57.0 (18–79) |
| <65
years | 126 (58.3) |
| ≥65
years | 90
(41.7) |
| Gender |
|
| Male | 161 (74.5) |
|
Female | 55
(25.5) |
| ECOG performance
status |
|
| 0–1 | 196 (90.7) |
| 2–3 | 20 (9.3) |
| Location of primary
tumor |
|
| EGJ and
cardia | 25
(11.6) |
| Fundus
and body | 87
(40.3) |
|
Antrum | 104 (48.1) |
| Histological
grade |
|
| Good | 72
(33.3) |
| Poor | 122 (56.5) |
| No
data | 22
(10.2) |
| Timing of locally
advanced or metastatic disease |
|
|
Synchronous | 139 (64.4) |
|
Metachronous | 77
(35.6) |
| Extent of
disease |
|
| Locally
advanced | 5
(2.3) |
|
Metastatic | 211 (97.7) |
|
Liver | 77
(35.6) |
|
Peritoneum | 123 (56.9) |
| Distant
lymph nodes | 67
(31.0) |
| Bone | 22
(10.2) |
| Lung | 13 (6.0) |
| Number of
metastases |
|
| ≤1 | 98
(45.4) |
| 2 | 89
(41.2) |
| ≥3 | 29
(13.4) |
| Baseline
hemoglobin |
|
| Median,
g/dl (range) | 11.9 (3.5–16.8) |
| <10.0
g/dl | 59
(27.3) |
| ≥10.0
g/dl | 157 (72.7) |
| Baseline albumin |
|
| Median,
g/dl (range) | 3.9 (2.4–5.0) |
| <3.5
g/dl | 42
(19.4) |
| ≥3.5
g/dl | 174 (80.6) |
Treatment regimens
The characteristics of the cytotoxic agents used are
listed in Table II. The most
commonly used cytotoxic agents were 5-FU in 171 patients (79.2%),
cisplatin in 140 patients (64.8%) and oxaliplatin in 117 patients
(54.2%). Of the patients enrolled, 92 patients (42.6%) were treated
with fluoropyrimidine and platinum, classed as group 1, 75 patients
(34.7%) were treated with fluoropyrimidine, platinum and taxane or
irinotecan, classed as group 2, and 49 patients (22.7%) were
treated with fluoropyrimidine, platinum, taxane and irinotecan,
classed as group 3, over the course of palliative treatment.
 | Table II.Characteristics of palliative
chemotherapy (n=216). |
Table II.
Characteristics of palliative
chemotherapy (n=216).
|
Characteristics | Value, n (%) |
|---|
| Cytotoxic
agents |
|
|
Fluoropyrimidines |
|
|
5-FU | 171 (79.2) |
|
Capecitabine | 70
(32.4) |
|
S-1 | 51
(23.6) |
|
Platinum |
|
|
Cisplatin | 140 (64.8) |
|
Oxaliplatin | 117 (54.2) |
|
Taxanes |
|
|
Docetaxel | 55
(25.5) |
|
Paclitaxel | 45
(20.8) |
|
Irinotecan | 74
(34.3) |
| Availability of
active cytotoxic agents |
|
| Group
1 | 92
(42.6) |
| Group
2 | 75
(34.7) |
| Group
3 | 49
(22.7) |
Univariate and multivariate
analysis
The median overall survival time for all patients
was 9.3 months (95% CI, 8.4–10.5 months). The results of the
univariate and multivariate analyses for overall survival are
summarized in Table III. Univariate
analysis revealed that the ECOG performance status (0–1 vs. 2–3)
and the availability of the active cytotoxic agents (group 1 vs.
group 2 vs. group 3) had prognostic significance. The median
overall survival time was significantly longer in patients with an
ECOG performance status of 0–1 compared with in patients with an
ECOG performance status of 2–3 (9.9 vs. 4.7 months, respectively;
HR for mortality, 3.52; 95% CI, 1.57–7.86; P<0.0001). The median
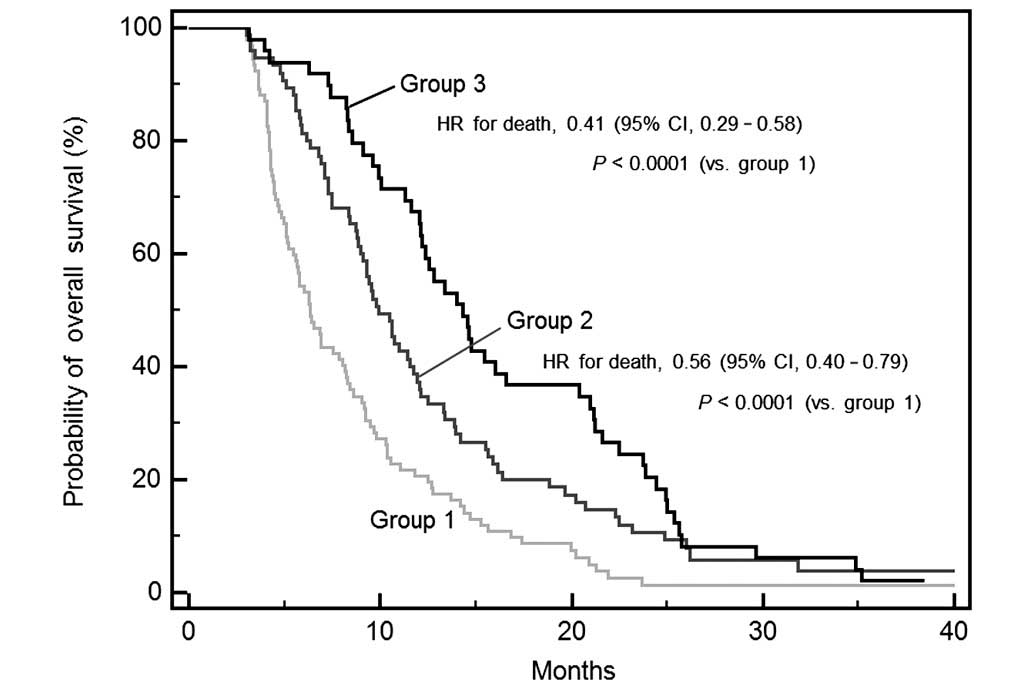
overall survival times were 6.3 months in group 1, 9.9 months in
group 2, and 14.3 months in group 3; these differences were
statistically significant (P<0.0001). The estimated HRs for
mortality were 0.56 for group 2 (95% CI, 0.40–0.79) and 0.41 for
group 3 (95% CI, 0.25–0.58; Fig.
1).
 | Table III.Univariate and multivariate analyses
of overall survival. |
Table III.
Univariate and multivariate analyses
of overall survival.
|
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|
|---|
|
Characteristics | Total, n | Median OS, months
(95% CI) | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age |
|
|
|
|
|
|
|
|
| <65
years | 126 | 9.3 (8.3–11.0) |
|
|
0.985 |
|
|
|
| ≥65
years | 90 | 9.7 (8.2–11.0) | 1.00 | 0.76–1.31 |
|
|
|
|
| Gender |
|
|
|
|
|
|
|
|
|
Male | 161 | 9.4 (8.4–10.5) |
|
|
0.648 |
|
|
|
|
Female | 55 | 9.3 (7.5–11.8) | 0.93 | 0.68–1.28 |
|
|
|
|
| ECOG performance
status |
|
|
|
|
|
|
|
|
|
0–1 | 196 | 9.9 (9.1–11.4) |
|
| <0.001 |
|
|
|
|
2–3 | 20 | 4.7 (3.9–6.9) | 3.52 | 1.57–7.86 |
| 3.25 | 1.99–5.30 | <0.0001 |
| Histological
grade |
|
|
|
|
|
|
|
|
|
Good | 72 | 10.3
(8.7–13.7) |
|
|
0.087 |
|
|
|
|
Poor | 122 | 8.4 (7.3–9.8) | 1.36 | 0.76–1.31 |
|
|
|
|
| No
data | 22 | 11.6
(9.4–20.2) | 1.03 | 0.66–1.60 |
|
|
|
|
| Timing of locally
advanced or metastatic disease |
|
|
|
|
|
|
|
|
|
Synchronous | 139 | 9.6 (8.6–10.6) |
|
|
0.551 |
|
|
|
|
Metachronous | 77 | 9.1 (6.3–11.8) | 1.09 | 0.83–1.44 |
|
|
|
|
| Peritoneal
metastases |
|
|
|
|
|
|
|
|
|
Yes | 123 | 9.5 (8.2–10.8) |
|
|
0.174 |
|
|
|
|
| No | 93 | 9.3 (8.3–11.8) | 1.21 | 0.92–1.58 |
|
|
|
|
| Number of
metastases |
|
|
|
|
|
|
|
|
| ≤1 | 98 | 9.3 (7.5–10.8) |
|
|
0.261 |
|
|
|
| 2 | 89 | 9.3 (8.3–11.0) | 1.23 | 0.92–1.66 |
|
|
|
|
| ≥3 | 29 | 9.9 (7.1–14.8) | 0.95 | 0.64–1.41 |
|
|
|
|
| Baseline
hemoglobin |
|
|
|
|
|
|
|
|
|
<10.0 g/dl | 59 | 8.9 (6.9–10.0) |
|
|
0.641 |
|
|
|
| ≥10.0
g/dl | 157 | 9.7 (8.6–11.3) | 0.93 | 0.68–1.27 |
|
|
|
|
| Baseline
albumin |
|
|
|
|
|
|
|
|
| <3.5
g/dl | 42 | 9.1 (6.9–11.6) |
|
|
0.875 |
|
|
|
| ≥3.5
g/dl | 174 | 9.4 (8.4–10.8) | 0.97 | 0.69–1.37 |
|
|
|
|
| Availability of
active cytotoxic agents |
|
|
|
|
|
|
|
|
| Group
1 | 92 | 6.3 (5.5–8.2) |
|
| <0.001 |
|
|
|
| Group
2 | 75 | 9.9 (9.0–11.8) | 0.56 | 0.40–0.79 |
| 0.58 | 0.42–0.80 | 0.0009 |
| Group
3 | 49 | 14.3
(12.1–20.4) | 0.41 | 0.29–0.58 |
| 0.40 | 0.28–0.58 | <0.0001 |
A multivariate Cox proportional hazard model
revealed that the ECOG performance status and the availability of
active cytotoxic agents were independent prognostic factors for AGC
outcome (Table III). The estimated
HRs for mortality in patients with an ECOG performance status of
2–3 compared to patients with an ECOG performance status of 0–1 was
3.25 (95% CI, 1.99–5.30; P<0.0001). The estimated HRs for
mortality in groups 2 and 3 compared to group 1 were 0.58 (95% CI,
0.42–0.80; P=0.0009) and 0.40 (95% CI, 0.28–0.58; P<0.0001),
respectively.
Discussion
The present study retrospectively analyzed data
obtained from 216 patients with AGC that had undergone palliative
chemotherapy with active cytotoxic agents, such as
fluoropyrimidines and platinums or taxanes or irinotecan. The
present analysis demonstrated that the availability of these active
cytotoxic agents in the course of treatment had a positive impact
on the survival of patients with AGC. In the present study, 92
patients (42.6%) were treated with fluoropyrimidine and platinum
only, while 75 patients (34.7%) received one more cytotoxic agent,
irinotecan or a taxane, and 49 patients (22.7%) received all four
active cytotoxic agents. Multivariate analysis revealed that the
availability of more active cytotoxic drugs was an independent
prognostic factor for survival compared with fluoropyrimidine and
platinum only. This finding suggests that it is important to use
all cytotoxic agents that have well-demonstrated clinical activity
in AGC to guarantee the maximal benefit of systemic therapy for
overall survival in patients with AGC.
Although the median survival time of patients with
AGC remained below one year, numerous cytotoxic agents have been
investigated over the previous decades, including
fluoropyrimidines, which are administered orally or intravenously,
anthracyclines, cisplatin, oxaliplatin, taxanes and irinotecan
(5–7).
Currently, various targeted agents are being tested in clinical
trials and promising data have been recently published for
trastuzumab-containing therapy, with median survival time exceeding
one year (9). Novel oral
fluoropyrimidines, including capecitabine and S-1, are not
clinically inferior to 5-FU in terms of survival, and additional
advantages of novel oral fluoropyrimidines include the convenience
of oral chemotherapy, which avoids the potential morbidity
associated with central venous access, and the opportunity to make
simple dose adjustments to the oral agent during the treatment
cycle to manage toxicity (10–13).
Cisplatin has been an integral component of AGC reference regimens
(6). However, oxaliplatin has been
extensively studied in AGC due to the specific side-effects of
cisplatin, including nephrotoxicity, emetogenicity and ototoxicity.
Due to the non-inferior efficacy, oxaliplatin may be substituted
for cisplatin in the treatment of AGC, and elderly patients may
derive a particular benefit from treatment with oxaliplatin instead
of cisplatin (10,14). Taxanes, such as docetaxel or
paclitaxel, which bind and stabilize microtubules and therefore
lead to cell-cycle arrest, have also been used as a first-line
therapy for AGC (15,16). In addition, irinotecan has been
reported to demonstrate activity in gastrointestinal cancers, and
irinotecan-based combination regimens have been studied as a
first-line alternative to platinum-based chemotherapy (17,18). The
availability of these active cytotoxic agents opened the option of
sequential salvage chemotherapy in AGC patients.
Second-line chemotherapy is currently considered to
be a standard therapy option for patients that demonstrate disease
progression during or subsequent to first-line chemotherapy.
Docetaxel and irinotecan have been evaluated extensively for
second-line therapy in patients for whom fluoropyrimidine and
platinum have failed (19). Three
randomized controlled trials have revealed the increased survival
of patients administered with either docetaxel or irinotecan
monotherapy compared with those receiving best supportive care
(20–22). As the majority of patients with AGC
are initially treated with fluoropyrimidine and platinum, it
appears more prudent to avoid these drugs in second-line regimens
for these patients. No statistically significant differences were
observed between the overall survival, progression-free survival
and response rates for patients receiving taxanes and those
receiving irinotecan (19,23). Thus, either taxanes or irinotecan may
be recommended as a treatment option for second-line chemotherapy
in patients with AGC. Although evidence is limited with regard to
the efficacy of third-line chemotherapy in AGC, this therapy may
have contributed to the prolonged overall survival time. Several
studies have demonstrated that third-line chemotherapy performs
better compared with best supportive care in patients with AGC in
terms of overall survival and quality of life (24–26). The
sequence of second and third-line regimens, including taxanes and
irinotecan, did not present any significant difference in overall
survival or time to progression subsequent to the failure of
fluoropyrimidine and platinum chemotherapy (24). The differences in toxicity profiles,
previous chemotherapy agents, and treatment schedules between the
two treatments may aid in choosing between taxanes or
irinotecan.
The current results suggest that the use of all
active cytotoxic agents improves the overall survival in patients
with AGC. However, if sequential treatment with all active
cytotoxic agents cannot be guaranteed for 100% of the patients, the
use of a triplet combination protocol may be considered as
first-line therapy. The safety and efficacy of this approach has
been assessed in previous clinical trials (15,16).
Triplet combination chemotherapy comprising an anthracycline or a
taxane in addition to fluoropyrimidine and platinum compounds has
resulted in higher response rates and a modest improvement in
overall survival compared with doublet combinations (6). In the majority of European countries,
the epirubicin, cisplatin and fluorouracil (ECF) regimen is more
commonly used, based on a phase 3 randomized trial that compared
the administration of the ECF regimen with the administration of
fluorouracil, doxorubicin and methotrexate (27). The docetaxel, cisplatin and
fluorouracil (DCF) regimen has previously been tested in the V325
phase 3 trial. In this trial, it was found that the DCF regimen not
only significantly improved the clinical benefit of chemotherapy,
but also improved the quality of life, time to progression and
overall survival compared with cisplatin and fluorouracil without
docetaxel (15). However, the high
rate of treatment-associated toxicity limits the applicability of
this regimen to all patients, particularly those that are elderly
or have a poor performance status. Therefore, several modifications
to the schedule of triplet combination chemotherapy or growth
factor support have been investigated in an attempt to minimize the
toxicity that occurs with this regimen (28,29).
Although the benefit of sequential salvage
chemotherapy is evident, the disease control rate is 30–40%. This
indicates that more than one-half of patients do not benefit from
salvage chemotherapy and suffer from toxicities. Therefore, it is
important to predict whether patients may benefit from sequential
salvage chemotherapy. Previous studies have indicated that several
factors should be considered in order to assess the response to
sequential salvage chemotherapy, such as the performance status of
the patient, extent of disease (locally advanced or metastatic),
cumulative toxicity, lack of cross-resistance of the tumor cells to
previously used drugs and progression-free survival of the patient
following previous chemotherapy (26,30).
Therefore, predictive factors for the potential survival benefit of
salvage chemotherapy with active cytotoxic agents require
additional investigation to avoid the development of toxic effects
in patients that are unlikely to benefit from the therapy.
The present study demonstrates several limitations.
Firstly, it is a retrospective analysis. However, all patients were
consecutive non-selected cases that received chemotherapy treatment
outside clinical trials and were followed by the Department of
Internal Medicine, Chungbuk National University Hospital under the
supervision of the same oncology team, which also addresses
real-life situations. Secondly, a shorter overall survival time was
demonstrated in the present study compared with previous studies.
This is due to the population in the present study possessing a
poor prognosis, with 97.7% of the patients experiencing metastatic
cancer, 56.1% possessing peritoneal carcinomatosis and 9.3%
demonstrating an ECOG performance status of 2–3.
In conclusion, the present study suggests that the
availability of active cytotoxic agents in the course of treatment
is associated with improved survival in patients with AGC.
Additional prospective studies of effective administration
schedules for patients receiving all active cytotoxic agents, in
addition to studies investigating the factors that predict the
survival benefit from salvage chemotherapy should continue in
patients with AGC.
Acknowledgements
This study was supported by a Basic Science Research
Program through the National Research Foundation of Korea, funded
by the Ministry of Education, Science, and Technology (grant no.,
2007-0054930).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jung KW, Won YJ, Kong HJ, Oh CM, Seo HG
and Lee JS: Cancer statistics in Korea: Incidence, mortality,
survival and prevalence in 2010. Cancer Res Treat. 45:1–14. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
D'Angelica M, Gonen M, Brennan MF, et al:
Patterns of initial recurrence in completely resected gastric
adenocarcinoma. Ann Surg. 240:808–816. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Macdonald JS: Gastric cancer - new
therapeutic options. N Engl J Med. 355:76–77. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wagner AD, Grothe W, Haerting J, et al:
Chemotherapy in advanced gastric cancer: A systematic review and
meta-analysis based on aggregate data. J Clin Oncol. 24:2903–2909.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wagner AD, Unverzagt S, Grothe W, et al:
Chemotherapy for advanced gastric cancer. Cochrane Database Syst
Rev. CD0040642010.PubMed/NCBI
|
|
7
|
Wesolowski R, Lee C and Kim R: Is there a
role for second-line chemotherapy in advanced gastric cancer?
Lancet Oncol. 10:903–912. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Baek SK, Kim SY, Jeong JH, Cho KS and Yoon
HJ: Second-line chemotherapy for advanced gastric cancer in Korea.
Gastric Cancer. 15:345–354. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bang YJ, Van Cutsem E, Feyereislova A, et
al: To GA Trial Investigators: Trastuzumab in combination
with chemotherapy versus chemotherapy alone for treatment of
HER2-positive advanced gastric or gastro-oesophageal junction
cancer (ToGA): A phase 3, open-label, randomised controlled trial.
Lancet. 376:687–697. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cunningham D, Starling N, Rao S, Iveson T,
Nicolson M, Coxon F, Middleton G, Daniel F, Oates J and Norman AR:
Upper Gastrointestinal Clinical Studies Group of the National
Cancer Research Institute of the United Kingdom. Capecitabine and
oxaliplatin for advanced esophagogastric cancer. N Engl J Med.
358:36–46. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kang YK, Kang WK, Shin DB, Chen J, Xiong
J, Wang J, Lichinitser M, Guan Z, Khasanov R, Zheng L, PhilcoSalas
M, et al: Capecitabine/cisplatin versus 5-fluorouracil/cisplatin as
first-line therapy in patients with advanced gastric cancer: A
randomised phase III noninferiority trial. Ann Oncol. 20:666–673.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Koizumi W, Narahara H, Hara T, Takagane A,
Akiya T, Takagi M, Miyashita K, Nishizaki T, Kobayashi O, Takiyama
W, Toh Y, et al: S-1 plus cisplatin versus S-1 alone for first-line
treatment of advanced gastric cancer (SPIRITS trial): A phase III
trial. Lancet Oncol. 9:215–221. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Boku N, Yamamoto S, Fukuda H, Shirao K,
Doi T, Sawaki A, Koizumi W, Saito H, Yamaguchi K, Takiuchi H, Nasu
J and Ohtsu A: Gastrointestinal Oncology Study Group of the Japan
Clinical Oncology Group. Fluorouracil versus combination of
irinotecan plus cisplatin versus S-1 in metastatic gastric cancer:
A randomised phase 3 study. Lancet Oncol. 10:1063–1069. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Al-Batran SE, Hartmann JT, Probst S,
Schmalenberg H, Hollerbach S, Hofheinz R, Rethwisch V, Seipelt G,
Homann N, Wilhelm G, Schuch G, et al: Arbeitsgemeinschaft
Internistische Onkologie. Phase III trial in metastatic
gastroesophageal adenocarcinoma with fluorouracil, leucovorin plus
either oxaliplatin or cisplatin: A study of the Arbeitsgemeinschaft
Internistische Onkologie. J Clin Oncol. 26:1435–1442. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
VanCutsem E, Moiseyenko VM, Tjulandin S,
Majlis A, Constenla M, Boni C, Rodrigues A, Fodor M, Chao Y, Voznyi
E, Risse ML and Ajani JA: V325 study group. Phase III study of
docetaxel and cisplatin plus fluorouracil compared with cisplatin
and fluorouracil as first-line therapy for advanced gastric cancer:
A report of the V325 study group. J Clin Oncol. 24:4991–4997. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen XL, Chen XZ, Yang C, Liao YB, Li H,
Wang L, Yang K, Li K, Hu JK, Zhang B, Chen ZX, et al: Docetaxel,
cisplatin and fluorouracil (DCF) regimen compared with
non-taxane-containing palliative chemotherapy for gastric
carcinoma: A systematic review and meta-analysis. PLoS One.
8:e603202013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dank M, Zaluski J, Barone C, Valvere V,
Yalcin S, Peschel C, Wenczl M, Goker E, Cisar L, Wang K and Bugat
R: Randomized phase III study comparing irinotecan combined with
5-fluorouracil and folinic acid to cisplatin combined with
5-fluorouracil in chemotherapy naive patients with advanced
adenocarcinoma of the stomach or esophagogastric junction. Ann
Oncol. 19:1450–1457. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Moehler M, Kanzler S, Geissler M, Raedle
J, Ebert MP, Daum S, Flieger D, Seufferlein T, Galle PR and Hoehler
T: Arbeitsgemeinschaft Internistische Onkologie, Germany. A
randomized multicenter phase II study comparing capecitabine with
irinotecan or cisplatin in metastatic adenocarcinoma of the stomach
or esophagogastric junction. Ann Oncol. 21:71–77. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim HS, Kim HJ, Kim SY, Kim TY, Lee KW,
Baek SK, Kim TY, Ryu MH, Nam BH and Zang DY: Second-line
chemotherapy versus supportive cancer treatment in advanced gastric
cancer: A meta-analysis. Ann Oncol. 24:2850–2854. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
ThussPatience PC, Kretzschmar A, Bichev D,
Deist T, Hinke A, Breithaupt K, Dogan Y, Gebauer B, Schumacher G
and Reichardt P: Survival advantage for irinotecan versus best
supportive care as second-line chemotherapy in gastric cancer - a
randomised phase III study of the Arbeitsgemeinschaft
Internistische Onkologie (AIO). Eur J Cancer. 47:2306–2314. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kang JH, Lee SI, Lim do H, Park KW, Oh SY,
Kwon HC, Hwang IG, Lee SC, Nam E, Shin DB, Lee J, et al: Salvage
chemotherapy for pretreated gastric cancer: A randomized phase III
trial comparing chemotherapy plus best supportive care with best
supportive care alone. J Clin Oncol. 30:1513–1518. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ford HE, Marshall A, Bridgewater JA,
Janowitz T, Coxon FY, Wadsley J, Mansoor W, Fyfe D, Madhusudan S,
Middleton GW, Swinson D, et al: COUGAR-02 Investigators: Docetaxel
versus active symptom control for refractory oesophagogastric
adenocarcinoma (COUGAR-02): An open-label, phase 3 randomised
controlled trial. Lancet Oncol. 15:78–86. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hironaka S, Ueda S, Yasui H, et al:
Randomized, open-label, phase III study comparing irinotecan with
paclitaxel in patients with advanced gastric cancer without severe
peritoneal metastasis after failure of prior combination
chemotherapy using fluoropyrimidine plus platinum: WJOG 4007 trial.
J Clin Oncol. 31:4438–4444. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Park JS, Lim JY, Park SK, Kim MK, Ko HS,
Yoon SO, Kim JW, Choi SH and Cho JY: Prognostic factors of second
and third line chemotherapy using 5-fu with platinum, irinotecan
and taxane for advanced gastric cancer. Cancer Res Treat.
43:236–243. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shim HJ, Yun JY, Hwang JE, Bae WK, Cho SH
and Chung IJ: Prognostic factor analysis of third-line chemotherapy
in patients with advanced gastric cancer. Gastric Cancer.
14:249–256. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lee MJ, Hwang IG, Jang JS, Choi JH, Park
BB, Chang MH, Kim ST, Park SH, Kang MH and Kang JH: Outcomes of
third-line docetaxel-based chemotherapy in advanced gastric cancer
who failed previous oxaliplatin-based and irinotecan-based
chemotherapies. Cancer Res Treat. 44:235–241. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Webb A, Cunningham D, Scarffe JH, Harper
P, Norman A, Joffe JK, Hughes M, Mansi J, Findlay M, Hill A, Oates
J, et al: Randomized trial comparing epirubicin, cisplatin, and
fluorouracil versus fluorouracil, doxorubicin and methotrexate in
advanced esophagogastric cancer. J Clin Oncol. 15:261–267. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Overman MJ, Kazmi SM, Jhamb J, Lin E, Yao
JC, Abbruzzese JL, Ho L, Ajani J and Phan A: Weekly docetaxel,
cisplatin and 5-fluorouracil as initial therapy for patients with
advanced gastric and esophageal cancer. Cancer. 116:1446–1453.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Polyzos A, Felekouras E, Karatzas T,
Griniatsos J, Dimitroulis D, Polyzos K, Kontzoglou K, Mantas D,
Karavokyros J, Nikiteas N, Tsavaris N, et al: Modified
docetaxel-cisplatin in combination with capecitabine as first-line
treatment in metastatic gastric cancer. A phase II study.
Anticancer Res. 32:4151–4156. 2012.PubMed/NCBI
|
|
30
|
Kanagavel D, Pokataev IA, Fedyanin MY,
Tryakin AA, Bazin IS, Narimanov MN, Yakovleva ES, Garin AM and
Tjulandin SA: A prognostic model in patients treated for metastatic
gastric cancer with second-line chemotherapy. Ann Oncol.
21:1779–1785. 2010. View Article : Google Scholar : PubMed/NCBI
|