Introduction
Multiple myeloma (MM), also termed myeloma, is a
plasmocyte cancer, which may be categorized as a B lymphocytic
lymphoma, as plasmocytes represent the final stage of B lymphocyte
development. Tumor cells originate from bone marrow plasmocytes.
Myeloma usually develops in multiple locations within the bone
marrow, which is why it is termed MM. Tumor development is
predominantly confined to the bone marrow and may not cause any
symptoms during the early stages of disease. However,
extramedullary spread may occur at a later stage (1). MM affects the plasma cells within the
bone marrow, which are an important component of the immune system.
Osteolytic bone lesions in MM may also affect the overall prognosis
of patients, due to an increase in bone resorption and a decrease
in bone formation.
Urokinase plasminogen activator receptor (uPAR; also
known as CD87) is a specific serine protease that connects to the
cell membrane with saccharification phosphatidylinositol, which is
able to convert plasminogen to its active form, plasmin (1). uPAR is a glycosyl-phosphatidylinositol
(GPI)-anchored plasma membrane receptor (2). The uPA system comprises uPA; its
receptor, uPAR; substrate molecules, such as plasminogen; and the
inhibitory factors, plasminogen activator inhibitor types 1 and 2.
It is the major enzyme system involved in degradation of the
extracellular matrix and in cell-mediated transfer in the body,
under physiological or pathological conditions (3). Furthermore, uPA is involved in tissue
remodeling, cell migration and tumor metastasis. Hydrolyzation of
the extracellular matrix is an important step in the process of
tumor invasion and metastasis, and requires the involvement of a
series of proteases (4). In addition
to direct degradation of extracellular matrix components, plasmin
also catalyses metalloproteinases involved in extracellular
proteolysis, and uPA, which is secreted by tumor cells, is the
activator of plasminogen. The uPAR and its ligand, uPA, constitute
the proteolytic system, which is involved in leukocyte infiltration
and tissue reconstruction.
In the circulation of cancer patients, uPAR is
commonly present in a soluble form (suPAR), which may be detected
in bodily fluids and tumor cell extract, and is released by
uPAR-positive tumor cells (5). uPA
and uPAR are predominantly expressed in blood cells, including
neutrophils, monocytes, macrophages and activated T cells, and are
hypothesized to be important for the ability of these cells to
degrade fibrin, and to extravasate and migrate during an
inflammatory response (6). A recent
study demonstrated that malignant plasma cells may express uPA and
uPAR, and the initiation of proteolytic events by this system may
contribute to the process of invasion and destruction of the bone
marrow by myeloma cells (3). This
type of interaction is an important biological process, which may
affect the degradation of marrow, stromal infiltration of plasma
cells and the patient's clinical condition.
The present study aimed to measure the level of uPAR
and its soluble form (suPAR) in patients with MM, and to analyze
the association between uPAR/suPAR and clinical characteristics,
treatment effect and patient survival time.
Patients and methods
Ethics statement
This study was conducted according to the principles
expressed in the Declaration of Helsinki and has been approved by
the Ethics Committee of Hebei Medical University (Shijiazhuang,
China). Participants provided written informed consent to
participate in this study.
Clinical materials and grouping
Forty patients with MM, treated in Hebei Cangzhou
Central Hospital (Cangzhou, China) between 2011 and 2013 were
enrolled in the present study. Patients underwent the following
routine diagnostic investigations: Complete routine blood
examination; categorization of leukocytes in the peripheral blood;
bone marrow biopsy; immune fixation electrophoresis; immunoglobulin
class determination and quantification; measurement of
β2-microglobulin, CRP and renal function; general skeletal X-ray
examination/emission computed tomography test; and chromosomal
analysis.
Extramedullary infiltration was assessed using the
hydrothorax centrifugal smear of myeloma cells, needle aspiration
puncture, or during the period of follow-up in patients with tongue
amyloidosis (one case), lung infiltration (four cases) or gingival
infiltration (two cases). Patients with primary plasma cell
leukemia were excluded.
Patients were treated as follows: 6 patients
received MP (melphalan and prednisone) therapy, while 34 patients
were treated with the M2 regimen (carmustine, cyclophosphamide,
melphalan and prednisone), with the aim of controlling plasma cell
proliferation. Seven patients were also treated with the VAD
regimen (new catharanthus, adriamycin and dexamethasone) as the M2
regimen and MP were ineffective in these individuals. Patients were
divided into two effective groups, remission (those exhibiting
partial or complete remission) or stable disease and an ineffective
group (those with progressive disease). In addition, 30 healthy
volunteers (19 males, 11 females) with a mean age of 34 years
(range, 22–53 years) were enrolled at Hebei Cangzhou Central
Hospital as the healthy control group.
Flow cytometry
uPAR (CD87), CD56 and CD38 molecules on platelets
from 1 ml of bone marrow plasma cells, were measured using flow
cytometry, which was performed with phycoerythrin (PE)-conjugated
monoclonal mouse anti-human CD38 (#555460), CD56 (#556647) and uPAR
(CD87-PE; #555768) antibodies, and a fluorescein isothiocyanate
(FITC)-conjugated monoclonal mouse anti-human CD38 (CD38-FITC;
#555459) antibody (all purchased from BD Biosciences, San Jose, CA,
USA; dilution, 1:15). These monoclonal antibodies were detected in
triple stainings, and all combinations of CD38, CD78 and CD56 were
included, for the specific identification of plasma cells. Cell
reactivity was analyzed using a Becton Dickinson FACSort, with
CellQuest v3.1 software (BD Biosciences). At least 10,000 events
were acquired for each monoclonal antibody combination. The
relative fluorescence intensity was calculated as the mean
fluorescence intensity (MFI) produced by a specific antibody,
divided by the background MFI value generated by the control
antibody for each patient sample. Irrelevant isotype-matched mouse
antibodies (PE-Cy™5 Mouse IgG1 κ Isotype; #555750; BD Biosciences)
were used as negative controls.
ELISA
Peripheral venous blood (2 ml, after the first 5 ml
was discarded) was drawn into blood collection tubes containing
sodium citrate (0.5 ml), gently mixed 3–5 times and centrifuged at
402 × g for 5 min. The serum was collected and stored in the fridge
at −80°C. Serum human suPAR concentrations were determined using
quantitative human colorimetric ELISA kits (Bio-Rad Model 550,
Quanti-kine HumanuPAR Immunoassay kit, USA), according to the
manufacturer's instructions.
Statistical analysis
All data were analyzed using SPSS software version
18.0 (SPSS Inc., Chicago, IL, USA). Data are presented as mean ±
standard deviation, and P<0.05 was considered to indicate a
statistically significant difference. Comparisons of continuous
variables were performed using a t-test for paired samples, while
the χ2 test was used for the comparison of categorical
variables. Patient survival was estimated using the Kaplan-Meier
method, from the date of diagnosis until death from any cause or
until survival for >2 years. Survival curves were compared
statistically using the log-rank test. Proportional hazard
regression analysis and logistic regression analysis were used to
identify the most significant independent prognostic variables
affecting patient survival.
Results
Association between suPAR expression
level and treatment efficacy
uPAR was positive in the bone marrow in all 40
patients with MM (≥20% cells expressing uPAR was defined as
positive). Typical morphological data from 1 patient are presented
in Fig. 1, and clinical and
laboratory data of all patients are presented in Table I. suPAR levels was measured in blood
samples from 40 patients with MM and 20 age-matched normal
controls. suPAR levels in patients with MM were significantly
higher than those of the controls: The mean suPAR level in the
healthy control group was 233.47±85.22 pg/ml (range, 138.3–330.9
pg/ml). Patients with MM were divided into two effective groups,
remission (those exhibiting partial or complete remission) or
stable disease and an ineffective group (those exhibiting
progressive disease). The suPAR levels in the effective groups were
257.6±32.47 and 331.0±99.80 pg/ml, respectively, which was not
significantly different compared with levels in the normal control
group (P>0.05). By contrast, the suPAR level in the invalid
group was 562.2±291.0 pg/ml, which was significantly difference
from that in the normal control group (P<0.01) and the effective
groups (P<0.05; Table II).
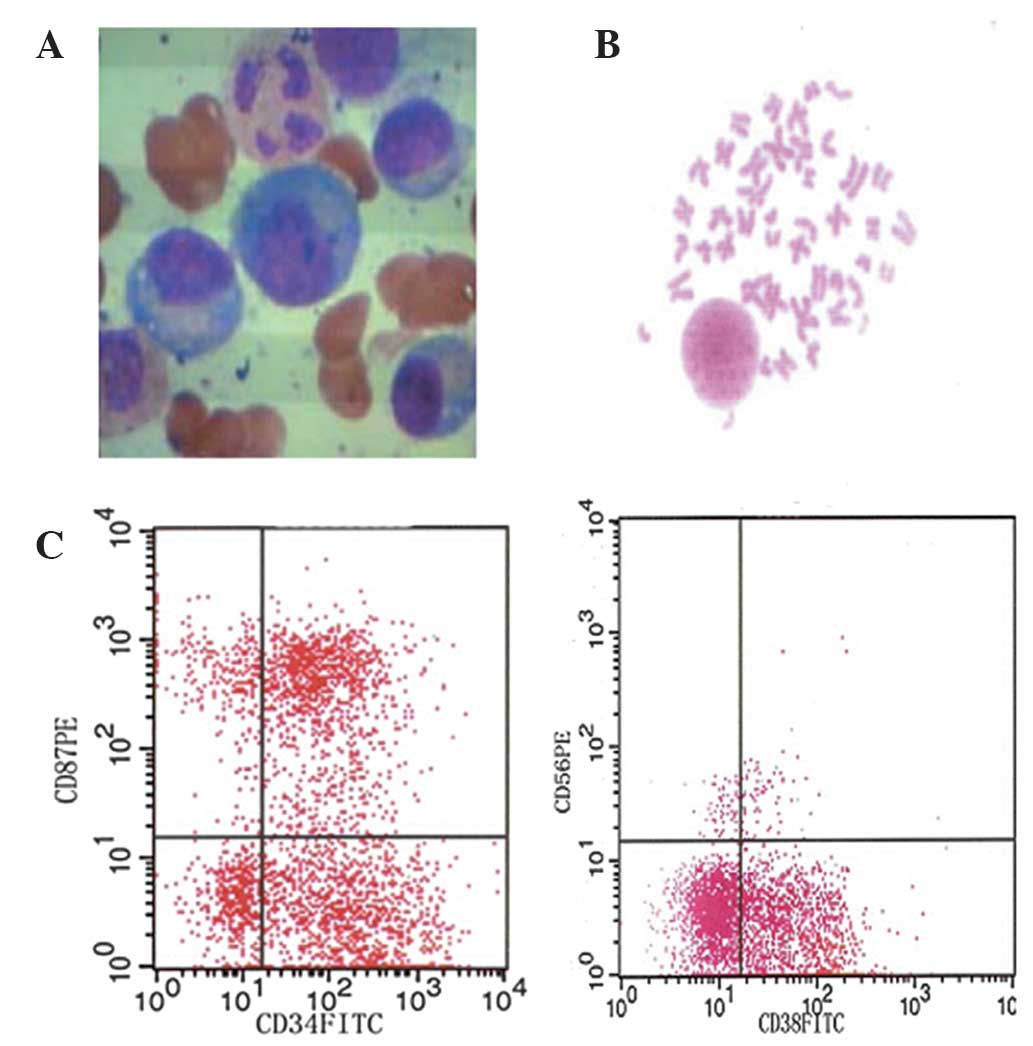 | Figure 1.(A) Bone marrow morphology, (B)
karyotype alternations and (C) immunophenotype of one MM patient
(female; age, 56 years; IgG-λ). Bone marrow aspirate smears
indicated that the percentage of plasma cells was 40.5%; Karyotype:
46, XX, del 13q21. Immunophenotype: CD87, 40.85%; CD56, (-); CD38,:
56.67%; suPAR, 970.6 pg/ml. MM, multiple myeloma; suPAR, soluble
urokinase plasminogen activator. |
 | Table I.Clinicopathological parameters of 40
patients with multiple myeloma. |
Table I.
Clinicopathological parameters of 40
patients with multiple myeloma.
| Clinicopathological
criteria | n |
|---|
| Age (years) |
|
| ≤60 | 22 |
|
>60 | 18 |
| Gender |
|
|
Female | 17 |
| Male | 23 |
| Disease stage |
|
| I–II | 11 |
| III | 29 |
| Renal
dysfunction |
|
|
Negative | 28 |
|
Positive | 12 |
| Hemoglobin
(g/dl) |
|
| ≤10 | 27 |
|
>10 | 13 |
| C-reactive protein
(mg/l) |
|
| ≤10 | 25 |
|
>10 | 15 |
| β2-microglobulin
(mg/l) |
|
| ≤4.0 | 25 |
|
>4.0 | 15 |
| Extramedullary
involvement |
|
|
| Yes | 7 |
| No | 33 |
| 13q14 |
|
|
Deleted | 6 |
|
Normal | 34 |
| Treatment |
|
| MP
(melphalan and prednisone) | 6 |
| M2
(carmustine, vincristine, cyclophosphamide, melphalan and
prednisone) | 34 |
 | Table II.Association between suPAR expression
and treatment efficacy. |
Table II.
Association between suPAR expression
and treatment efficacy.
| Effect | Patients, n | suPAR, pg/ml (mean
± SD) | P-value |
|---|
| Remission | 13 | 257.6±32.47 | NS |
| Stable disease | 19 | 331.0±99.80 | NS |
| Ineffective | 8 |
562.2±291.0b |
0.005<P<0.01a |
| Control | 30 | 233.47±85.22 |
|
Differences in suPAR expression levels
prior to and following treatment
Seventeen patients completed follow-up for
measurement of suPAR expression, from their first visit to the end
of chemotherapy. The mean suPAR level at presentation was
537.65±131.43 pg/ml. The 17 patients were treated with the M2
regimen or then VAD. Following chemotherapy, they were divided into
an effective group (10 cases; suPAR, 298.76±108.59 pg/ml) and an
ineffective group (7 cases; suPAR, 371.10±98.46 pg/ml). The mean
level of suPAR prior to treatment was higher than that following
treatment in the effective group (P<0.01) and the ineffective
group(P<0.01; Table III).
 | Table III.Contrast of suPAR expression before
and after treatment. |
Table III.
Contrast of suPAR expression before
and after treatment.
| Group | n | suPAR, pg/ml (mean
± SD) | P-value |
|---|
| Prior to
treatment | 17 |
537.65±131.43a | <0.01 |
| Following
treatment |
|
|
|
|
Effective | 10 |
298.76±108.59 | >0.05 |
|
Ineffective | 7 |
391.10±98.46a | <0.01 |
| Control | 30 | 233.47±85.22 |
|
Association between suPAR expression
level and disease severity
suPAR levels were positively correlated with disease
stage (P<0.01), renal function (P<0.05), CRP (P<0.005),
β2-microglobulin (P<0.001), extramedullary involvement
(P<0.001), chromosome 13 deletion (P<0.01) and survival <2
years (P<0.01). No correlation was observed between hemoglobin
expression and suPAR levels (Table
IV).
 | Table IV.Correlations between suPAR and other
variables. |
Table IV.
Correlations between suPAR and other
variables.
| Variable | suPAR, pg/ml (mean
± SD) | t | P-value |
|---|
| Stage |
| 1.93 | <0.05 |
|
I–II | 325.4±91.79 |
|
|
III | 465.0±231.58 |
|
| Renal
dysfunction |
| 2.45 | <0.01 |
|
Negative | 372.9±197.70 |
|
|
Positive | 551.9±242.68 |
|
| Hemoglobin
(g/dl) |
| 0.933 | >0.05 |
|
≤10 | 449.0±273.31 |
|
|
>10 | 378.0±169.79 |
|
| C-reactive protein
(mg/l) |
| 2.85 | <0.005 |
|
≤10 | 359.2±151.94 |
|
|
>10 | 566.5±287.88 |
|
| β2-microglobulin
(mg/l) |
| 3.50 | <0.001 |
|
≤4.0 | 332.34±92.90 |
|
|
>4.0 | 554.1±285.09 |
|
| Extramedullary
involvement |
| 3.84 | <0.001 |
|
Yes | 570.5±311.08 |
|
| No | 372.5±175.65 |
|
| 13q14 |
| 2.67 | <0.01 |
|
Deleted | 570.54±311.08 |
|
|
Normal | 372.53±158.97 |
|
| Survival time
(years) |
| 3.50 | <0.01 |
| ≤2 | 646.01±103.97 |
|
|
>2 | 333.02±85.37 |
|
Logistic regression was applied in order to analyze
the correlation between survival time and clinicopathological
criteria, including gender, age, disease stage, renal function,
hemoglobin, CRP, β2-microglobulin, deletion of chromosome 13,
extramedullary invasion and suPAR expression (data not shown). The
results indicated that disease stage and suPAR expression predicted
a survival time of <2 years (P<0.01).
Discussion
uPA is a specific serine protein with a molecular
weight of ~5000 Mr, which catalyzes the conversion of plasminogen
to plasmin in the extravascular space. Plasmin is a broad-spectrum
protein, which activates metalloproteinases, thereby stimulating
tissue differentiation and growth, cell adhesion and migration in
physiological and pathological conditions (7). During the catalytic process, uPA
combines with its ligand, uPAR, also termed CD87, which is a member
of the GPI-AP family. A recent study demonstrated that uPAR is a
single-chain glycoprotein with high affinity for uPA and
precursor-uPA (Kd10-9-10-12 grammole) (8). uPAR anchors to the cell membrane with
c-terminal GPI. It predominantly mediates plasminogen activation,
and is associated with cell migration, cell adhesion, tumor growth,
metastasis and chemotactic responses.
Under physiological conditions, uPAR is present on
the surface of a variety of types of cells, such as leukocytes,
including neutrophils, monocytes, macrophages, acidophilic
granulocyte and activated T lymphocytes; endothelial cells; and
fibroblasts. uPAR is directly involved in the chemotaxis of
neutrophils and monocytes, as a result of the change in structure
of uPAR, which consists of three parts; D1, D2 and D3 (9,10). uPA
joins to the uPAR at its n-terminal, and the c-terminal joins with
fibrinolytic enzymes on the cell membrane (11). A change in the conformation of uPAR
reveals a sequence of chemokines in the D1 and D2 sections
(7). Since uPAR may combine with the
matrix protein and mediate the cell adhesion process, uPAR and uPA
are able to regulate the adhesion of bone marrow cells and matrix
proteins (12). It was hypothesized
that uPAR may form a complex with integrins, such as CD11b, and
that it could then mediate the interaction between uPAR and the
cell scaffold, which may stimulate cell adhesion (13,14).
Furthermore, uPAR may also form a functional connection with β1, β2
or β3 integrin, or with other proteins that have tyrosine kinase
activity (15). A recent study
reported that the movement of bone marrow into fibrin, caused by
the proteolysis of plasmin, is activated by plasminogen on the
surface of MM plasma cells (16).
It has previously been shown that uPA and uPAR are
expressed on myeloma cells in patients with MM, which may lead to
the activation of the protein hydrolysis system, and that this
process may be associated with the degradation of the marrow stroma
in MM (17). Flow cytometry analysis
demonstrated that uPAR (CD87) expression was associated with the
differentiation stage of myeloma cells in MM, and that CD45+
immature plasma cells exhibited high uPAR expression, as well as
CD138 and CD56 (18). It has also
been reported that CD138 and CD56 are involved in the process of
cell adhesion (19). These studies
have shown that uPAR may participate in the regulation of plasma
cells, including CD56+ cells, and affect the proliferation of
malignant plasma cells. A separate study demonstrated that plasma
cells from patients with monoclonal γ globulin disease also
expressed uPAR. Therefore uPAR expression is not only a
characteristic of MM cells, but also an indicator of clonal plasma
cell proliferation (20).
The conventional hypothesis was that MM prognosis
was associated with disease stage, renal function, anemia, CRP,
β2-microglobulin, abnormalities of chromosome 13 and extramedullary
infiltration at presentation. Hjertner et al (21) measured uPA/uPAR expression in four
strains of myeloma cells of untreated patients with MM, using
immunocytochemistry staining and flow cytometry. The results showed
that the MM tumor cells expressed uPA/uPAR and exhibited
corresponding proteolytic activity, and that the uPA/uPAR levels
were associated with the maturity of tumor cells. The authors
hypothesized that uPAR expression may affect the invasiveness and
osseous injury of myeloma cells. Rigolin et al (20) and Luo et al (22) also proposed that uPAR expression may
be associated with extramedullary infiltration and a poor prognosis
in patients with MM. Although all patients expressed uPAR on bone
marrow cells in the present study, the expression of this molecule
was not directly correlated with disease stage, renal function,
hemoglobin, CRP, β2-microglobulin, abnormalities of chromosome 13
or extramedullary infiltration.
Therefore, a connection between uPAR expression and
clinical characteristics was required. This was hypothesized to be
suPAR. suPAR is a uPAR variant lacking a GPI anchor, and exists in
the bodily fluids or tumor tissue in soluble form. To date, it is
unclear how suPAR is formed. As mononuclear cells and neutrophils
exhibit uPAR expression, it was proposed that suPAR in healthy
individuals may be derived from cell ageing, cell death,
differentiation of the bone marrow, and the formation of
megakaryocytes and platelets (23,24).
Previous studies have also demonstrated that the elevated suPAR
levels, observed in patients with cancer, are associated with a
poor outcome (25). Although suPAR is
released by tumor cells, the speed of its secretion was not
correlated with uPAR expression and the quantity of tumor cells
(26). Other studies have
demonstrated that uPAR expression in plasma cells is not associated
with the suPAR levels in the peripheral blood (11,27). No
correlation was observed between hemoglobin and suPAR expression in
the present study.
There has been much research into the expression of
suPAR in solid tumors, including prostate cancer, ovarian cancer,
cervical cancer, liver cancer and colorectal cancer (28–30). These
studies all found that elevated suPAR expression was associated
with a poor prognosis. However, there have been few studies that
have investigated the expression of suPAR in blood cancers.
Luo et al (22)
measured uPA and suPAR concentrations in 34 patients with MM, and
observed the uPA/suPAR expression prior to and following
chemotherapy in 6 patients with MM. The present study also
demonstrated that suPAR levels in patients with MM were
significantly higher than those of the control group. Furthermore,
the level of suPAR in patients with advanced MM was significantly
higher than the level in the control group or in the patients with
stable MM (P<0.01), while no significant difference was detected
in suPAR levels between patients with MM and the control group
(P>0.05; Table II). In the
present study, 17 patients were followed up until the end of
treatment. The mean suPAR level (537.65±131.43 pg/ml) prior to
treatment was significantly higher than that of the control group
(Table III). These 17 patients were
treated with the M2 regimen and then VAD therapy where the M2
regiment had been ineffective. Following chemotherapy, patients
were divided into an effective group (10 cases, suPAR level,
298.76±108.59 pg/ml) and an invalid group (7 cases; suPAR level,
371.10±98.46 pg/ml). There was a significant difference in suPAR
levels between the effective group and the invalid group
(P<0.05), while no significant difference was detected between
the effective group and healthy controls. Following treatment, the
suPAR level in all patients was lower than that prior to treatment,
which may have been due to the reduction in plasma cells following
chemotherapy. Therefore, suPAR expression may predict the stability
of the disease and efficacy of the treatment.
In addition, the present study analyzed the
association between suPAR expression, and disease stage, renal
function, hemoglobin, CRP, β2-microglobulin, chromosome 13
abnormalities and extramedullary infiltration. The results showed
that suPAR expression in stage III disease was higher than that in
stage I–II (P<0.05). suPAR expression in the group with abnormal
renal function was higher than that in the group with normal renal
function (P<0.01). The suPAR expression in patients with a
hemoglobin <10 g/dl group was higher than that in the group with
a hemoglobin ≥10 g/dl, although this was not statistically
significant. suPAR expression in the group with CRP >10 mg/l was
higher than that in the group with a CRP ≤10 mg/l (P<0.005). The
suPAR expression in the group with a β2-microglobulin of >4.0
mg/l was significantly higher than that in the group with
β2-microglobulin ≤4.0 mg/l (P<0.001). In addition, the suPAR
expression in those with a deletion in chromosome 13 was
significantly higher than that in those with a normal chromosome 13
or with other chromosomal abnormalities (P<0.01). Finally, there
was a significant difference in suPAR levels between those with and
without extramedullary infiltration (P<0.001). All 7 patients
with extramedullary infiltration, either had marrow infiltration at
the time of presentation or developed it <1 year following
diagnosis. This indicated that high suPAR expression may be
associated with early extramedullary infiltration. In conclusion,
disease stage, renal function, CRP, β2-microglobulin, deletion of
chromosome 13 and extramedullary infiltration were positively
correlated with suPAR expression, which further indicated that
measuring the level of suPAR may help to predict prognosis and
survival rates.
The mechanism underlying the effect of suPAR in MM
remains unclear. It has been hypothesized that the suPAR and its
components, D2 and D3, may compete with uPAR at the cell membrane,
thus influencing the uPAR utilization. High expression of suPAR is
associated with cell adhesion and extracellular matrix adhesion
(20). This process may have two
different underlying mechanisms. The first is competition with uPAR
on the plasma membrane surface to bind to integrin. The second is a
reduction in the utilization of uPAR by detachment of uPAR from the
plasma membrane. High expression of suPAR may predict a reduction
in the adhesion of plasma cells to the bone marrow stroma,
facilitating spread outside the marrow, which leads to faster
disease progress and shortened survival time (31).
The average survival time of patients with MM was 3
years in the present study. All 40 patients (17 of whom completed
post-chemotherapy suPAR assessment) were followed up until
mortality or survival at ≥2 years in order to examine the
association between the level of suPAR and patient survival. This
analysis demonstrated that suPAR expression in patients who
survived for >2 years (28 cases) was significantly lower than
that in patients who survived for <2 years (12 cases). The
results also showed that suPAR expression in patients surviving
<2 years was significantly higher than that of the normal
control group (P<0.01), which demonstrated that the high
expression of suPAR was directly correlated with survival.
Furthermore, the association between suPAR expression, and gender,
age, disease stage, renal function, hemoglobin, CRP,
β2-microglobulin, deletion of chromosome 13 and extramedullary
infiltration was analyzed, using logistic regression. The results
indicated that disease stage and suPAR were independent factors,
which predicted a survival time of <2 years.
Disease progression and marrow infiltration have
previously been considered to be the major factors affecting
prognosis in patients with MM. Early diagnosis, and early treatment
and chemotherapy were key to improving prognosis, and to prolonging
patient survival, prior to the development of rising plasma cell
counts and infiltration. Currently, clinicians rely on bone marrow
examination, CRP, β2-microglobulin and imaging in order to evaluate
the curative effect and prognosis. However, these tests may be
invasive, with poor specificity and a delay in acquiring imaging
results. Studies have also indicated that CRP and β2-microglobulin
are correlated with clinical factors and disease stage, and may
predict early mortality, although they were not found to predict
treatment response, and were unrelated to suPAR expression
(32). suPAR expression is correlated
with survival in addition to treatment efficacy. Therefore it may
be a novel predictor for use in conjunction with morphology and
imaging.
In conclusion, the present study demonstrated that
uPAR was positive in the bone marrow cells in all patients with MM.
suPAR levels were positively correlated with disease stage, renal
function, CRP, β2-microglobulin, extramedullary involvement,
chromosome deletion and survival time, while they were negatively
correlated with hemoglobin concentration. The results indicated
that disease stage and suPAR were independent factors, which
predicted survival of <2 years. suPAR expression does not have a
unified reference value, due to variations in testing methods and
specimen preparation. However, a number of studies have confirmed
that its expression is significantly higher in inflammatory states
and cancer. The present study investigated the association between
suPAR expression and MM, and demonstrated that high suPAR
expression was associated with disease progression, shortened
survival and early extramedullary infiltration. However, further
investigation is required in order to fully ascertain its value in
clinical practice.
References
|
1
|
Hjertner O, Qvigstad G, HjorthHansen H,
Seidel C, Woodliff J, Epstein J, Waage A, Sundan A and Börset M:
Expression of urokinase plasminogen activator and the urokinase
plasminogen activator receptor in myeloma cells. Br J Haematol.
109:815–822. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tarui T, Mazar AP, Cines DB and Takada Y:
Urokinase-type plasminogen activator receptor (CD87) is a ligand
for integrins and mediates cell-cell interaction. J Biol Chem.
276:3983–3990. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mekkawy AH, Pourgholami MH and Morris DL:
Involvement of urokinase-type plasminogen activator system in
cancer: An overview. Med Res Rev. 34:918–956. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
DeClerck YA and Jones PA: Effect of
ascorbic acid on the resistance of the extracellular matrix to
hydrolysis by tumor cells. Cancer Res. 40:3228–3231.
1980.PubMed/NCBI
|
|
5
|
Reuning U, Sperl S, Kopitz C, et al:
Urokinase-type plasminogen activator (uPA) and its receptor (uPAR):
Development of antagonists of uPA/uPAR interaction and their
effects in vitro and in vivo. Curr Pharm Des.
9:1529–1543. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lanza F, Castoldi GL, Castagnari B, et al:
Expression and functional role of urokinase-type plasminogen
activator receptor in normal and acute leukemic cells. Br J
Haematol. 103:110–123. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wei Y, Yang X, Liu Q, et al: A role for
caveolin and the urokinase receptor in integrinmediated adhesion
and signaling. J Cell Biol. 144:1285–1294. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kindzelskii AL, Amhad I, Keller D, et al:
Pericellular proteolysis by leukocytes and tumor cells on
substrates: Focal activation and the role of urokinase-type
plasminogen activator. Histochem Cell Biol. 121:299–310. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fazioli F, Resnati M, Sidenius N, et al: A
urokinase-sensitive region of the human urokinase receptor is
responsible for its chemotactic activity. EMBO J. 16:7279–7286.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gyetko MR, Todd RF III, Wilkinson CC and
Sitrin RG: The urokinase receptor is required for monocyte
chemotaxis in vitro. J Clin Invest. 93:1380–1387. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Béné MC, Castoldi G, Knapp W, et al: CD87
(urokinase-type plasminogen activator receptor), function and
pathology in hematological disorders: A review. Leukemia.
18:394–400. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wei Y, Eble JA, Wang Z, Kreidberg JA and
Chapman HA: Urokinase receptors promote beta1 integrin function
through interactions with integrin alpha3beta1. Mol Biol Cell.
12:2975–2986. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gyetko MR, Sitrin RG, Fuller JA, Todd RF
III, Petty H and Standiford TJ: Function of the urokinase receptor
(CD87) in neutrophil chemotaxis. J Leukoc Biol. 58:533–538.
1995.PubMed/NCBI
|
|
14
|
Gyetko MR, Chen GH, McDonald RA, Goodman
R, Huffnagle GB, Wilkinson CC, Fuller JA and Toews GB: Urokinase is
required for the pulmonary inflammatory responseto Cryptococcus
neoformans: A murine transgenic model. J Clin Invest. 97:1818–1826.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wei Y, Lukashev M, Simon DI, Bodary SC,
Rosenberg S, Doyle MV and Chapman HA: Regulation of integrin
function by the urokinase receptor. Science. 273:1551–1555. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Daci E, Udagawa N, Martin TJ, Bouillon R
and Carmeliet G: The role of the plasminogen system in bone
resorption in vitro. J Bone Miner Res. 14:946–952. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hjertner O, Qvigstad G, HjorthHansen H,
Seidel C, Woodliff J, Epstein J, Waage A, Sundan A and Börset M:
Expression of urokinase plasminogen activator and the urokinase
plasminogen activator receptor in myeloma cells. Br J Haematol.
109:815–822. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kara IO, Sahin B, Paydas S and Cetiner S:
Flow cytometric evaluation of bone marrow plasma cells using CD19,
CD45, CD56, CD38, and CD138 and correlation with bone marrow
infiltration ratio in multiple myeloma patients. Saudi Med J.
25:1587–1592. 2004.PubMed/NCBI
|
|
19
|
Rawstron A, Barrans S, Blythe D, Davies F,
English A, Pratt G, Child A, Morgan G and Jack A: Distribution of
myeloma plasma cells in peripheral blood and bone marrow correlates
with CD56 expression. Br J Haematol. 104:138–143. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rigolin GM, Tieghi A, Ciccone M, Bragotti
LZ, Cavazzini F, Della Porta M, Castagnari B, Carroccia R, Guerra
G, Cuneo A, et al: Soluble urokinase-type plasminogen activator
receptor (suPAR) as an independent factor predicting worse
prognosis and extra-bone marrow involvement in multiple myeloma
patients. Br J Haematol. 120:953–959. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hjertner O, Qvigstad G, HjorthHansen H, et
al: Expression of urokinase plasminogen activator and the urokinase
plasminogen activator receptor in myeloma cells. Br J Haematol.
109:815–822. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Luo LH, Xu GB and Lu XG: Detection and
clinical significance of plasma urokinase-type plasminogen
activator and its soluble receptor in patients with multiple
myeloma. Zhejiang Da Xue Xue Bao Yi Xue Ban. 32:529–532. 2003.(In
Chinese). PubMed/NCBI
|
|
23
|
Plesner T, Behrendt N and Ploug M:
Structure, function and expression on blood and bone marrow cells
of the urokinase-type plasminogen activator receptor, uPAR. Stem
Cells. 15:398–408. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wohn KD, Kanse SM, Deutsch V, Schmidt T,
Eldor A and Preissner KT: The urokinase-receptor (CD87) is
expressed in cells of the megakaryoblastic lineage. Thromb Haemost.
77:540–547. 1997.PubMed/NCBI
|
|
25
|
Stephens RW, Nielsen HJ, Christensen IJ,
ThorlaciusUssing O, Sørensen S, Danø K and Brünner N: Plasma
urokinase receptor levels in patients with colorectal cancer:
Relationship to prognosis. J Natl Cancer Inst. 91:869–874. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Krüger A, Soeltl R, Lutz V, Wilhelm OG,
Magdolen V, Rojo EE, Hantzopoulos PA, Graeff H, Gänsbacher B and
Schmitt M: Reduction of breast carcinoma tumor growth and lung
colonization by overexpression of the soluble urokinase-type
plasminogen activator receptor (CD87). Cancer Gene Ther. 7:292–299.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mustjoki S, Sidenius N, Sier CF, Blasi F,
Elonen E, Alitalo R and Vaheri A: Soluble urokinase receptor levels
correlate with number of circulating tumor cells in acute myeloid
leukemia and decrease rapidly during chemotherapy. Cancer Res.
60:7126–7132. 2000.PubMed/NCBI
|
|
28
|
Miyake H, Hara I, Yamanaka K, Arakawa S
and Kamidono S: Elevation of urokinase-type plasminogen activator
and its receptor densities as new predictors of disease progression
and prognosis in men with prostate cancer. Int J Oncol. 14:535–541.
1999.PubMed/NCBI
|
|
29
|
Riisbro R, Stephens RW, Brünner N,
Christensen IJ, Nielsen HJ, Heilmann L and von Tempelhoff GF:
Soluble urokinase plasminogen activator receptor in preoperatively
obtained plasma from patients with gynecological cancer or benign
gynecological diseases. Gynecol Oncol. 82:523–531. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fernebro E, Madsen RR, Fernö M, Brünner N,
Bendahl P, Christensen IJ, Johnson A and Nilbert M: Prognostic
importance of the soluble plasminogen activator receptor, suPAR, in
plasma from rectal cancer patients. Eur J Cancer. 37:486–491. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Koolwijk P, Sidenius N, Peters E, Sier CF,
Hanemaaijer R, Blasi F and van Hinsbergh VW: Proteolysis of the
urokinase-type plasminogen activator receptor by
metallopoteinase-12: Implication for angiogenesis in fibrin
matrices. Blood. 97:3123–3131. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Greipp PR, Lust JA, O'Fallon WM, Katzmann
JA, Witzig TE and Kyle RA: Plasmal cell labeling index and beta
2-microglobulin predict survival independent of thymidime kinase
C-reactive protein in multiple myeloma. Blood. 81:3382–3387.
1993.PubMed/NCBI
|















