Introduction
Meningiomas are common tumors that occur within the
spinal canal. Since chronic compression to the spinal cord is the
main pathological mechanism, complete removal of the meningioma for
cord decompression is the primary treatment choice. With
development of modern neuroradiological techniques and standard
microneurosurgical procedures, surgical treatment is usually
successful with low morbidity and good outcomes (1–3). However,
delayed neurological deficit in the absence of direct cord insult
following surgical decompression often occurs in patients with
chronic compressive spinal disorders, including cervical
spondylotic myelopathy, ossification of the spinal ligament, and
spinal stenosis. Delayed neurological deficit is also a rare but
severe postoperative complication observed in a small subset of
patients with intraspinal meningiomas (4–8).
A previous clinical study reported on 284 patients
who received surgery for a spinal meningioma at Beijing Tiantan
Hospital (Beijing, China) between the years 2004 and 2010 (9). A total of 10 patients exhibited delayed
but severe neurological deterioration following complete removal of
their tumors in the absence of any direct trauma to the cord. Of
these patients, there were 5 male and 5 female patients with a mean
age of 46.8 years. The mean duration of illness from onset of
symptoms to diagnosis was 42.8 months. Seven tumors were located in
the thoracic spine and 3 in the cervical spine. The tumors
compressed the cord severely and gross total removal was achieved
in all the cases. Immediately after the surgery, all the patients
could move all their extremities but the onset of the neurologic
deterioration became apparent during the 3–8 h following surgery in
all the cases (mean 5 h). To date, the underlying pathophysiology
of these findings remains unclear and ischemia-reperfusion injury
(IRI) is considered as the potential cause in the literature
(4,6,8). Moreover,
since all 10 patients suffered severe cord compression, the degree
of compression may be a risk factor of IRI. However, to the best of
our knowledge, no experimental research has been reported to prove
this theory of etiology.
The present study investigated whether IRI occurs
following decompression surgery using an experimental rat model of
chronic compressive spinal cord injury (SCI). Lipid peroxidation
reaction resulting from oxygen-derived free radical overproduction
initiates following IRI and is one of the main pathological
mechanisms (10,11). Therefore, the levels of superoxide
dismutase (SOD) and malondialdehyde (MDA) prior to and following
decompression were biochemically measured and analyzed, both of
which are important and reliable markers of lipid peroxidation
reaction (12,13), to determine the occurrence and extent
of IRI. To the best of our knowledge, this is the first reported
experimental study that investigates the underlying causes of
atraumatic neurological deterioration following surgery for
intraspinal meningiomas.
Materials and methods
Animal and experimental groups
All the animal experiments were approved by the
ethics committee of Beijing Tiantan Hospital, Capital Medical
University, and performed in accordance with the policies of
Chinese animal research committees and guidelines from U.S.
National Institute of Health (NIH publication No. 96-23, revised
1996). Sprague-Dawley (SD) rats were provided by the Experimental
Animal Facilities of the hospital. The number of animals used and
their suffering were minimized.
Thirty male rats (280–320 g) were randomly assigned
into 6 groups. Detailed information about groups is presented in
Table I. The rats in each group were
kept in separate cages in rooms with controlled light and
temperature and were fed standard chow and water ad libitum. Room
temperature was set at 25±3°C.
 | Table I.Groups information in the study. |
Table I.
Groups information in the study.
|
| Sham | Mild
compression | Severe
compression |
|---|
|
Non-decompression | Sham group | MC group | SC group |
| Decompression | Sham-d group | MC-d group | SC-d group |
Rat model of chronic compressive
SCI
Rat model of chronic compressive SCI was established
in accordance with the model of Wang et al (14) and Kim et al (15). All animals were prevented from
drinking in the morning of surgery. Animals were anesthetized by an
intraperitoneal (i.p.) injection of trichloroacetaldehyde (300
mg/kg; Qingdao Yulong Algae Ltd., Qingdao, China; No. H37022673)
and placed on a thermistor-controlled heating pad in prostrate
position. The fur of the animals was shaved around chest and
abdomen. Following disinfection, spinous processes and laminar arcs
of T7-10 were exposed following T5-12 midline skin incision and
paravertebral muscle dissection. The yellow ligament between the
laminae was removed and the dura underneath was separated from the
laminae carefully so as to not result in a cerebrospinal fluid
leak. The expanding compression material (mild compression size:
2.5 × 2.0 × 0.4 mm3; severe compression size: 2.5 × 2.0
× 0.8 mm3) was inserted between the T8 and T9 laminae
and dura. For the sham group, the protocol was the same as for the
experimental group, except for the insertion of the compression
material.
The compression sheet was made of a water-absorbing
material, which is a penetrating polymer network hydrogel composed
of polyvinyl alcohol and polyacrylamide (1:1). The surface of the
hydrogel is crosslinked with glutaraldehyde 10 times, and the
surface has long-term water retention capabilities. After absorbing
water, the expansion of the materials is mainly reflected as an
increase of its thickness and the final volume remains stable for a
long period of time without decomposition. The expansion rate can
gradually reach a maximum of 3 times its original thickness (mild
compression size: 3.0 × 2.5 × 1.2 mm3; severe
compression size: 3.5 × 3.0 × 2.4 mm3). Prior to the
experiment, the material was implanted subcutaneously in the
abdomen of rats. After 3 months, no obvious inflammation or other
abnormal tissue reactions were observed using histological
analysis.
Surgical procedures were performed under sterile
conditions with the assistance of a surgical microscope. Bleeding
control was performed with a bipolar coagulator. Subsequently, the
muscle and skin were sutured in layers with 6-0 Vicryl (Ethicon,
Johnson & Johnson Intl, Lanneke Marelaan, Belgium). Following
the surgical procedure, the rats were placed in a warming chamber
and their body temperatures were maintained at ~37°C until they
were completely awake. Ampicillin liquid formulation (80 mg/kg;
Suzhou Two Leaves Pharmaceuticals Inc. Ltd., Suzhou, China, Batch
No. H32021320) was injected into the back exterior muscles once per
day for 3 days to prevent infection. Temperatures were strictly
maintained and all the rats were housed individually with free
access to food and water. Padding in each cage was changed every
day to keep it dry. After surgery, bladder massage was performed
twice per day to stimulate autonomic urinary reflex.
Rats were sacrificed 12 weeks following surgery. In
the decompression groups, rats underwent laminectomy and removal of
the expanded materials and the animals were kept alive for 24 h
after decompression surgery under appropriate conditions and
veterinary control, after which decapitation took place after
anesthetization using the same anesthetic agents. In the sham-d
group, rats underwent laminectomy and the protocol after surgery
was the same as the experimental group. Spinal cord samples (15 mm)
were obtained from the compressed spinal cord area and divided into
two equal parts. Cranial parts of the tissue samples were obtained
for microscopy evaluation; caudal parts were cleaned of blood with
a scalpel and immediately stored in a −20°C freezer for biochemical
analysis.
Evaluations of animal model
All magnetic resonance imaging (MRI) experiments
were conducted with a 3 Tesla MRI (Magnetom Trio, Siemens Medical
Solutions, Erlangen, Germany). T2-weighted images (Echedelay
time=92 ms; Repetition time=3620 ms; flip angle α=120°; slice
thickness: 2 mm; Field of view=80 mm) were obtained at the 12th
week following the operation. All rats were placed in a prone
position. Images of the thoracic spinal cord were acquired in the
axial and sagittal planes. The cross-sectional area was measured
using the Siemens NUMARIS system software, version 4 (Siemans
Healthcare, Erlangen, Germany).
Neural function was scored to evaluate the animal
model of chronic compressive SCI in an open field according to the
Basso, Beattie and Bresnahan (BBB), locomotor rating scale of 0
(complete paralysis) to 21 (normal locomotion) (16). BBB scores categorize combinations of
rat hindlimb movements, joint movement, weight support,
fore/hindlimb coordination, trunk position and stability, stepping,
paw placement, toe clearance, and tail position, representing the
sequential injury stages that rats take after SCI. Rats are
permitted to move freely and scored over 4 min by 2 independent
observers. Locomotion activity of the hindlimb was evaluated once
per week following the surgery until the time of sacrifice. The
ranking standards were established as follows: i) The activity of
hindlimb joints were scored between 0 and 7; ii) the pace and
coordination of the hindlimbs were evaluated; and iii) the fine
activities of paws during locomotion were evaluated. Each
evaluation was completed by two independent observers who were
blinded to the experiments and the values were represented as the
mean ± standard deviation.
HE staining and Nissl staining
The specimen was immersed into 4% paraformaldehyde
in 1X phosphate buffered saline (4% PFA) and stored at 4°C for
post-fixation. One week after the fixation, they were removed from
the store and placed in fresh fixative. Fixed tissue samples were
processed routinely by paraffin embedding technique following
dehydration. A total of 150 serial cross sections with thickness of
5 µm were obtained from each rat, and processed with hematoxylin
and eosin staining (HE) and Nissl staining (Boster Biotechnology,
Wuhan, China). Motor neurons were identified by the presence of
large nuclei with well-developed, densely staining Nissl bodies in
the cytoplasm (14). In addition, the
nucleus, which is typically located centrally in the cell, contains
a well-demarcated round nucleolus. To determined the density of
large-sized neurons in the gray matter and obtain a precise
stereologicount of the neurons, a slice thickness of 5 µm and a gap
interval of >8 µm were selected, based on the following
stereological considerations. The characteristic large nucleoli
have a fairly uniform diameter of ~5 µm (13,14).
Selecting a slice thickness identical to the diameter of the
spherical nucleolus means that each section will contain tangential
contours of the nucleoli with their centers located within 2.5 µm
outside of the slice. Thus, counting nucleoli of the motor neurons
that appear on a 5-µm slice yields the number of those with the
center located within 5 µm on each side from the middle of the
slice. Leaving >8 µm intervals between the sections means the
stereological count can avoid omission or redundancy in the number
of motor neurons. Stereological counting of neurones in the
preparations was performed using a light microscope (CH30; Olympus,
Tokyo, Japan). All the sections were evaluated morphologically by
the same pathologist who was blinded to each group to assess the
histopathological change.
Biochemical analysis
Tissues from each group were homogenized at a
concentration of 100 g/l after cutting the organs into small
pieces, centrifuged at 5,000 × g for 20 min at −10°C, and stored at
−20°C. Biochemical kits (Jiancheng Institute of Biology, Nanjing,
China) were used to measure levels of SOD and MDA, in accordance
with the protocol. The activity of SOD was expressed as units per
mg protein. One unit of SOD was defined as the amount of protein
that inhibited the rate of nitroblue tetrazolium reduction by 50%.
The levels of the end product of lipid peroxidation, tissue MDA,
were expressed as nmol/mg.
Statistical analysis
SPSS software, version 16.0 (SPSS, Inc., Chicago,
IL, USA) was used for statistical calculations and graphs. Data are
expressed as the mean ± standard deviation. Samples from each group
were compared using one-way analysis of variance. P<0.05 was
considered to indicate a statistically significant difference.
Results
Postoperatively, rats were in a good, healthy
condition and did not develop any infections. Depending on the
degree of compression, a graded outcome was evident from
radiography, neurological tests, and light microscopic examination
as is described below.
Neuroradiological observations
The spinal cords were progressively compressed.
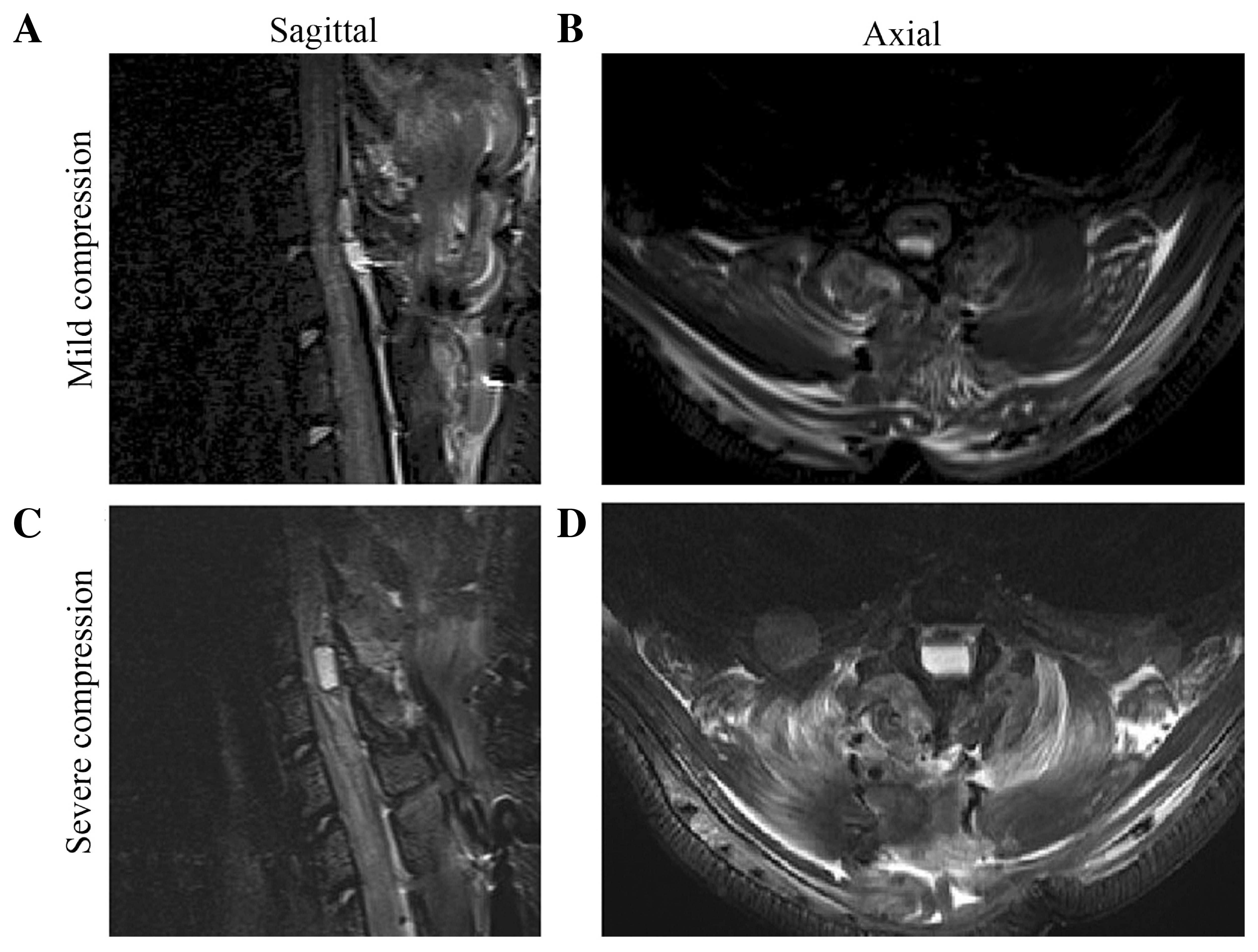
Sagittal and axial projections of the thoracic spine were obtained
to ascertain the location of the compression sheet and to evaluate
the degree of spinal cord compression (Fig. 1). The cross-sectional area in the sham
group was larger than in the compressed groups and a significant
difference was observed between the MC and SC groups (P<0.05)
(sham: 16.39±1.42 mm2; MC: 11.24±2.87 mm2;
SC: 4.31±1.59 mm2).
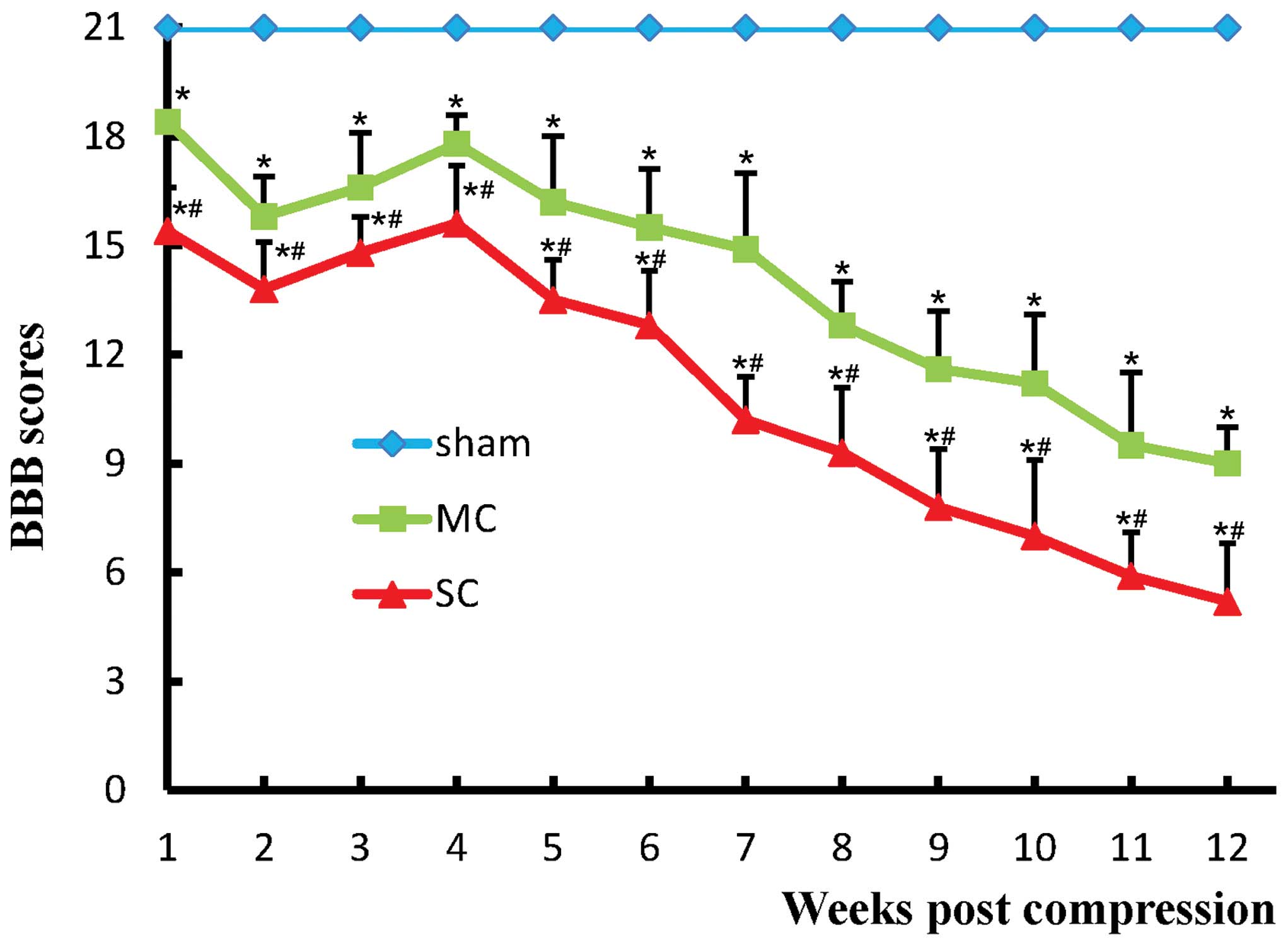
Neurobehavioral outcomes
All rats in the sham and sham-d group had a normal
postoperative neurological outcome (BBB score of 21). Rats with
compression had graded neural function injury depending on the time
and degree of compression (Fig. 2).
In the MC group, rats suffered from progressively mild motor
injury. In SC group, rats demonstrated severe motor injury and all
were observed to have paralysis, which was indicated by markedly
reduced BBB scores. A significant difference was detected between
the sham group and the compression groups (P<0.05). The BBB
scores in the MC and SC groups at all timepoints post-surgery were
statistically significant different (P<0.05).
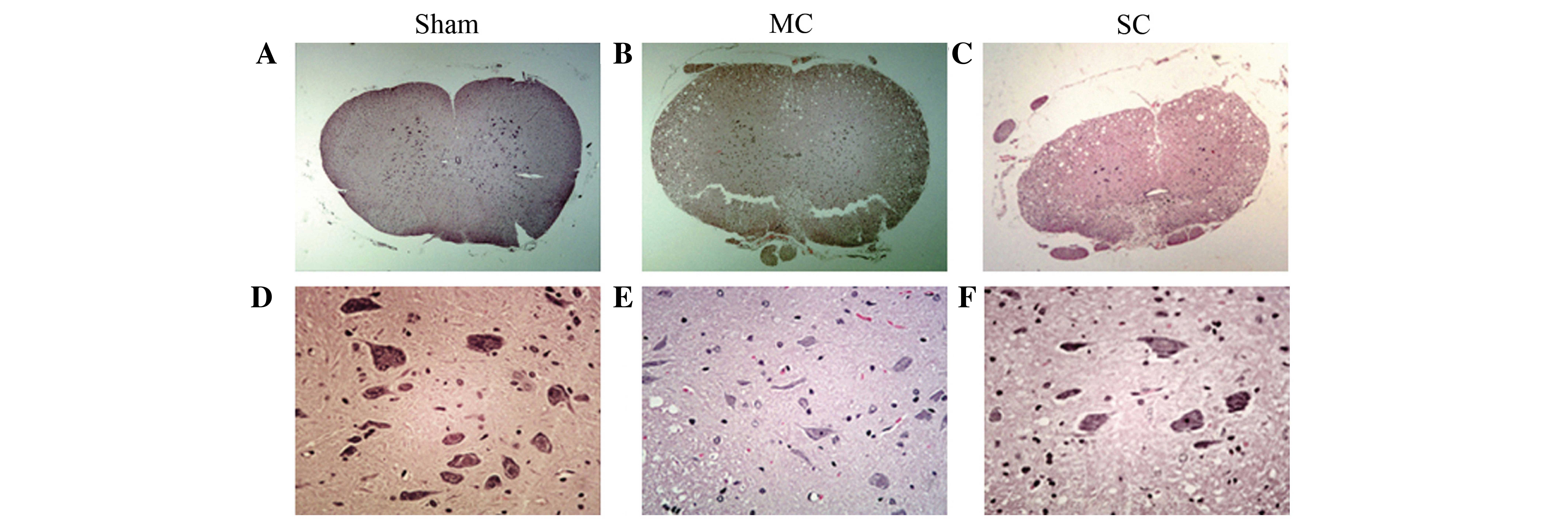
Histopathological observations
Histopathological alterations in the spinal cord
after injury were also compared. HE and Nissl staining were used to
analyze histopathological change. HE staining demonstrated that the
spinal cord in the sham group had integrated infrastructures and
clear boundary between gray and white matters, the blood vessels
and central canal also exhibited normal morphology (Fig. 3). In addition, no neuronal apoptosis
and glial proliferation were observed in the sham group (Fig. 3). Instead, in compression groups the
spinal cords were observed to have progressive pathological changes
and the extent of compression damage was proportional to the
neurological score. The number of neurons in the gray matter of
chronically compressed spinal cords reduced progressively with the
increase in degree of compression. In the MC group, a portion of
neurons were observed with condensed nucleus, darkly red stained
cytoplasm and also appearance of apoptotic bodies. However, a
proportion of neurons remained which indicated blurring structures.
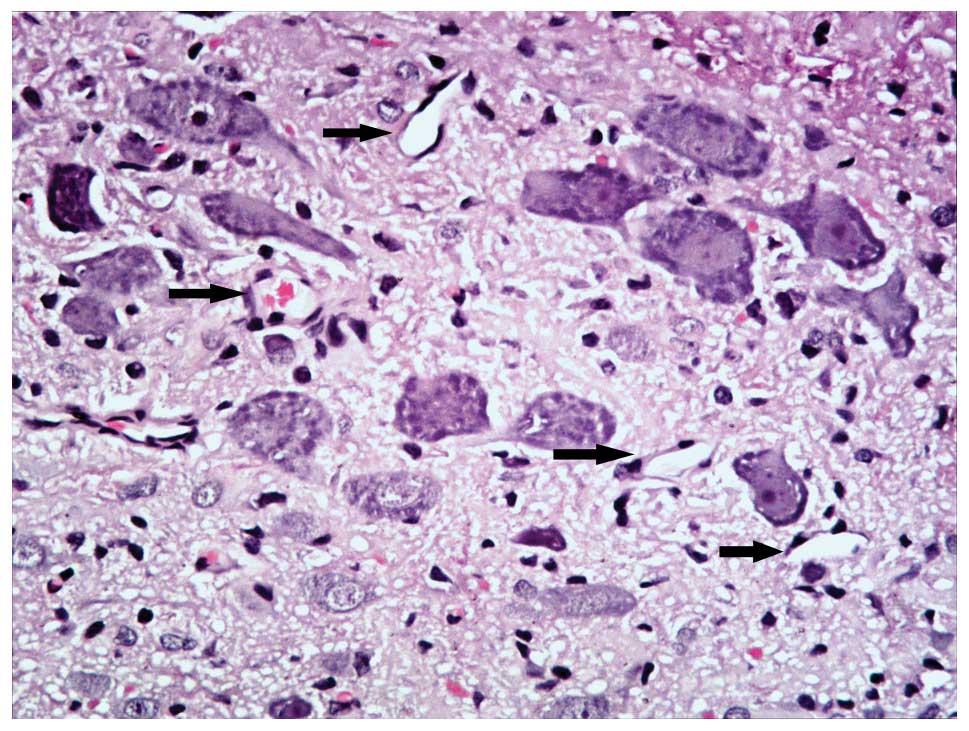
In the SC group, the spinal cord was notably flattened at the site
of compression. In the gray matter, neurons were flattened, small,
and reduced in number and patches of necrosis were seen.
Chromatolysis, both central and peripheral, was observed in the
remaining neurons. In the white matter, graded myelin damage and
loss of axons and glia were noted, as was status spongiosis. Cavity
formation and myelin ovoids were observed in the anterior, lateral,
and posterior columns. Myelin ovoids may have resulted from
phagocytosis of degenerating axons and the myelin sheath. The
spinal cord lacked clear infrastructures and cellular boundaries
compared with the MC group. Vessel stenosis and occlusion in the
gray and white matter became progressively worse with the
increasing of degree of compression, which demonstrated the
development of spinal cord ischemia.
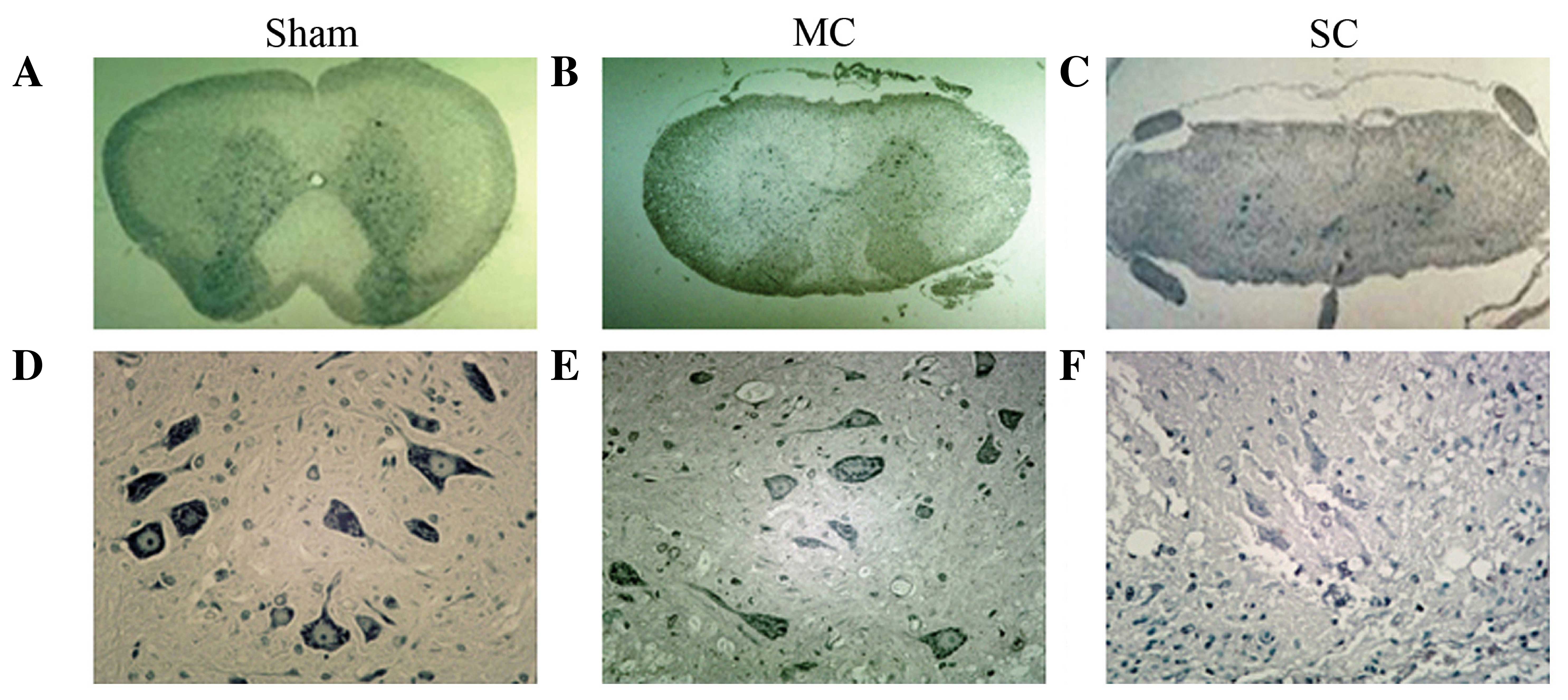
Nissl staining demonstrated that neurons in the sham
group displayed integrative and granular-like morphology (Fig. 4), and the number of motor neurons was
1050.5±128.2. The plasma was densely stained with toluidine blue,
indicating an active supply of neuronal nutrients and energy
synthesis. In the MC group, retained neurons were observed,
although the number was reduced (582.0±69.5) and tissue morphology
was relevantly maintained with lighter staining in the cytoplasm
and granular-like morphology. However, in the SC group the number
of neurons was notably reduced (274.6±92.4) and neurons appeared
irregular morphologies. Intracellular toluidine blue staining was
also significantly reduced, dimly spread out and apparent in the
periphery of the cytoplasm, which indicated chronic compressive
SCI-induced neuronal necrosis and apoptosis lead to neuronal loss.
The remaining neurons may have difficulties in energy synthesis
resulting in neural dysfunction.
No histopathological changes were observed in the
sham-d group. In the decompression groups, histopathological
manifestations exhibited no obvious changes compared with those in
the compression groups to the same degree and histopathological
improvement the in spinal cord was not apparent because it was
observed at a very early stage following decompression surgery and
the spinal cord had not had enough time to recover. However, blood
vessels in both gray and white matter were markedly dilated
(Fig. 5).
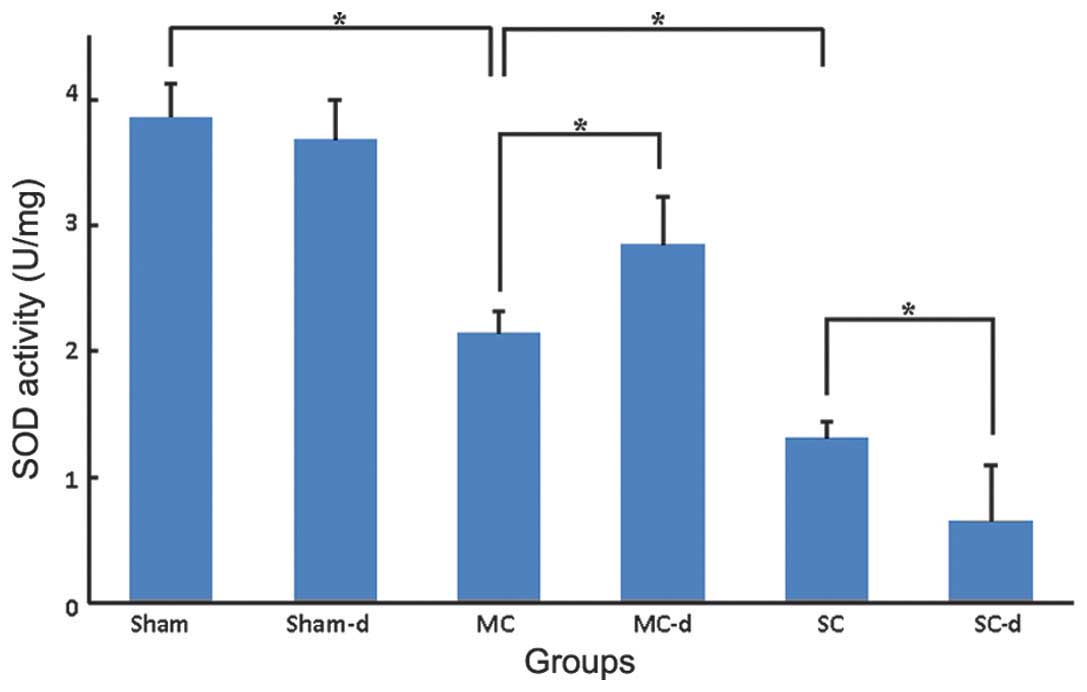
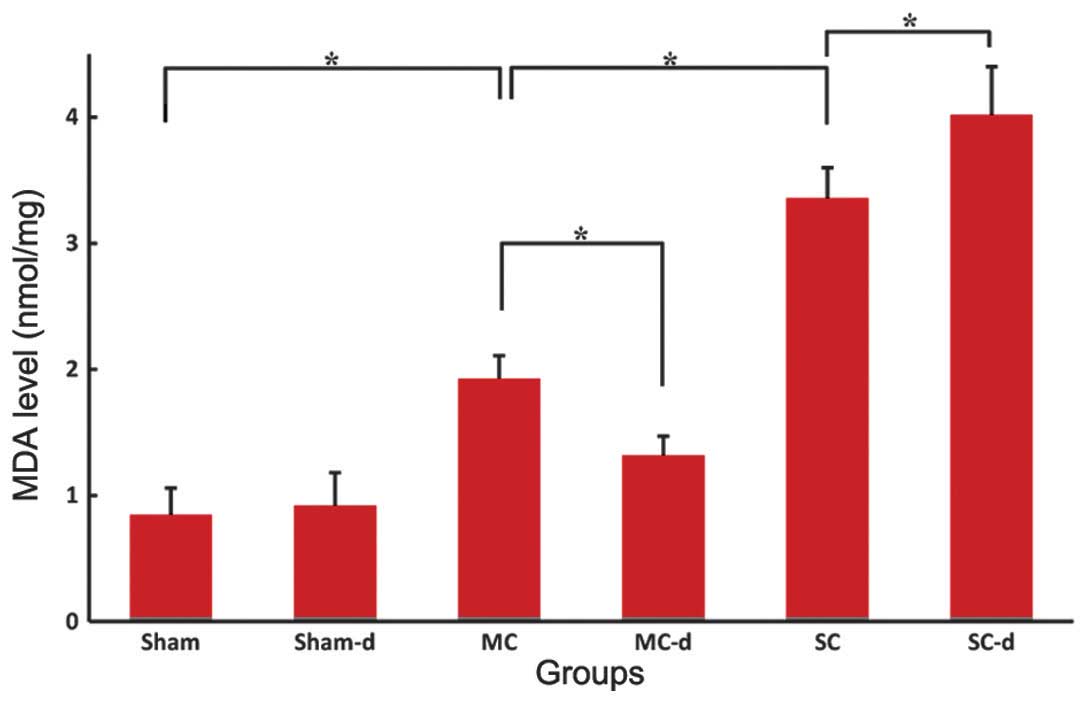
Levels of SOD and MDA
The SOD activity of the spinal cord tissues from the
sham group was 3.86±0.27 U/mg (Fig.
6) and the MDA level was 0.85±0.21 nmol/mg (Fig. 7). In the compression groups, chronic
compressive SCI resulted in a significant graded reduction in SOD
activity (MC: 2.14±0.18 U/mg, SC: 1.31±0.13 U/mg, P<0.05) and
increase in MDA level (MC: 1.93±0.18 nmol/mg, SC: 3.36±0.24
nmol/mg, P<0.05). No significant differences were observed in
the sham-d group compared with the sham group: SOD activity of the
spinal cord tissues was 3.68±0.32 U/mg and MDA level was 0.92±0.26
nmol/mg. Compared with the compression group, decompression surgery
following removal of the expanded materials in the MC-d group
markedly rescued the SOD activity to 2.85±0.38 U/mg (P<0.05) and
significantly reduced the MDA level to 1.32±0.15 nmol/mg
(P<0.05). Instead, in the SC-d group SOD activity was
significantly reduced to 0.65±0.44 U/mg (P<0.05) and MDA level
was elevated higher to 4.02±0.38 nmol/mg (P<0.05) in comparison
with those of the SC group rats.
Discussion
A previous study reported on the outcomes of 284
patients who received surgery for intraspinal meningioma at Beijing
Tiantan Hospital and summarized the clinical features of 10
patients presented with delayed neurological deterioration
postoperatively with unknown cause. It has been proposed that
patients and surgeons should be aware of the potentially
catastrophic results after a seemingly routine tumor removal to
treat an intraspinal meningioma with chronic but severe cord
compression. It is necessary to explain the rate of neurologic
deterioration and possible complications that may arise following
surgery and to do this prior to surgical intervention. In general,
the postoperative neurological deficit is most often due to
mechanical damage of surgical procedures and intraspinal hematoma
(17–19). However, careful surgical technique and
intraoperative neuromonitoring may indicate any potential trauma to
neural tissue during tumor removal and decompression procedure. In
the absence of clear etiology, spinal cord IRI is considered to be
responsible in previous studies (8,10,20). Microcirculatory disturbance due to
reperfusion may occur in any level and any location where surgical
decompression was performed for the chronic compressive lesion
(8,21). However, no previous studies have
proven this theory in this rare postoperative complication. In the
present study, an experimental rat model of chronic compressive SCI
was established with or without decompression surgery to identify
whether spinal cord IRI is the potential etiology of patients with
unknown postoperative neurological deterioration.
There have been previous experimental animal models
of chronic spinal cord compression, including the placement of
screws and subsequent gradual tightening of the screws, the
epidural transplantation of tumor cells in rats, and the epidural
implantation of expanding materials (14,15,22–28).
Numerous investigators have used twy mice, which are a model of
spinal ligament ossification (29–32). In
the current study, a chronic spinal cord compression model was
produced using water absorbing materials to imitate the compression
process and the animal model was evaluated using neuroradiology,
neurobehavior, and histopathology.
In the compression groups, MRI performed at the
schedule time demonstrated that the spinal cord was notably
compressed and the mean spinal cord narrowing rate was
significantly different between the MC and SC groups, which
indicates different effects of graded compression in the animal
model. Assessment of neurologic function is a prevalent method for
accessing the degree of neural injury. In the present study,
compared with sham group, the BBB rating demonstrated a
progressively significant deterioration of locomotion in
compression groups at all tested time points post-surgery.
Moreover, behavioral scores were significantly different between MC
and SC groups at every timepoint. Due to the powerful spinal cord
self-repair mechanisms in rats, the behavioral function of rats
following compression gradually recovered in the early phase (3–4
weeks post-surgery) of the experiment. However, the animal models
in the present study were evaluated in the late phase (after 12
weeks of compression) and the effect of the self-repair was very
limited. Therefore, implantation of the expandable sheets in the
present study reduced the locomotive function in rats and may have
simulates the behavioral changes of chronic compressive SCI.
The spinal cord is morphologically similar to a
cylinder where gray matter is surrounded by white matter (33). Compared to white matter, gray matter
has a low density with loosely connected tissue and is full of
blood vessels. Histopathologically, when the spinal cord is
compressed from behind, more cells are dislodged and the damage is
more serious in gray matter than that in white matter (27,28). In
the present study, mild edema and ischemia, reactive gliosis and
neuronal apoptosis with condensed nuclei were observed in the MC
group. However, in the SC group a significant deterioration in the
above abnormal ultrastructures was observed, indicating that
implantation of the expandable sheets may gradually worsen the
neurological function of the animal model through progressing
tissue structure pathological changes. Furthermore, Nissl staining
was used to identify whether spinal cord neurons underwent
pathological changes. Nissl bodies are large granules observed in
neurons, which may be identified by Nissl staining and are often
used to demonstrate the neural structure of the spinal cord. Nissl
bodies are actually the rough endoplasmic reticulum (with
ribosomes) and are the site of protein synthesis, consisting of
important constituent which is associated with the nutritional
condition of neurons (34).
Therefore, a large amount of large Nissl bodies may indicate
neurons with dynamic protein synthesis and energy supply. Nissl
bodies exhibit changes under various physiological conditions and
in pathological conditions they may reduce in number, dissolve and
even disappear (13,28). In the present study, the spinal cord
tissues were observed with Nissl staining. In the rats with chronic
mild compression injury, lighter Nissl staining in the cytoplasm
and granular-like morphology in neurons was observed, indicating
neuronal function was retained. This was further demonstrated by a
restored neuronal number in the spinal cord in the animals with
mild compression injury. Whereas a significant reduction in Nissl
staining and a marked reduction in the number of neurons was
observed in the rats with severe compression injury, indicating a
loss of neurons due to the necrosis and apoptosis. The number of
surviving neurons in the MC group (582.0±69.5) was significantly
increased compared with that in the SC group (274.6±92.4;
P<0.05); however, it was significantly reduced compared with the
sham group (1050.5±128.2; P<0.05). In the decompression groups,
the blood vessels were notably dilated, which indicated that the
blood supply in the spinal cord was partially restored and the
ischemia condition of neural tissues may be relatively relieved. No
other obvious histopathological changes were observed in the spinal
cord since they were observed at the very early stages following
decompression surgery. The spinal cord of rats had limited time to
exhibit a response to decompression and therefore remained
relatively stable at cellular levels. Taken together, the present
animal model may be considered to be useful for future studies on
chronic compressive SCI.
In the present study, the occurrence of IRI in the
spinal cord after decompression was examined by measuring the SOD
level and MDA concentration. SOD is an enzyme that catalyzes the
dismutation of superoxide anions. It is a major intracellular
anti-oxidative enzyme that scavenges free radicals to protect cells
from oxidative damages (35,36). The level of SOD represents the ability
of tissues and cells to evade the toxicities of free radicals.
Metabolic bursts, in which oxygen is reduced to superoxide
(O2−), hydrogen peroxide
(H2O2), and hydroxyl radical, may be elicited
by various stimuli (37). SOD
eliminates superoxides by converting them to
H2O2. H2O2 is finally
reduced to water by cytosolic antioxidants, catalase (CAT), and
glutathione peroxidase (GSH-Px) (38). MDA is the breakdown product of the
major chain reactions leading to the oxidation of polyunsaturated
fatty acids and may determine the extent of the peroxidation
reaction (35). It has previously
been established that reperfusion of neural tissue may have
deleterious clinical sequelae likely associated with the role of
reactive oxygen radical-mediated neuronal cell death (39). Animal models have demonstrated that
superoxide-mediated injury immediately occurs following reperfusion
during neuronal ischemic events (35–37).
Therefore, SOD and MDA frequently serve as important and reliable
markers of oxidative stress-mediated lipid peroxidation that
reflect the current reperfusion injury status following spinal cord
decompression.
Following chronic compressive SCI, in addition to
the direct damage at injured location, lipid peroxidation due to
free radical overproduction is the main pathological change of the
secondary injury that starts after chronic SCI. The results of the
present study demonstrated that compared with the sham group, SOD
activities in the compression groups significantly reduced, along
with a notable increase in MDA concentration. Moreover, there was a
reduced levels of SOD activity and an increased concenttration of
MDA in the SC group compared with the MC group, which indicated
progression of the secondary injury had occurred. Following
decompression, SOD activities in the MC-d group significantly
increased along with a reduction in MDA contentration compared with
the MC group, which demonstrated diminishment of lipid peroxidation
and relief of the secondary injury. These findings indicate that
decompression is effective to improve neurological recovery and may
deliver improved results for chronic mild compression of the spinal
cord. However, SOD activities in the SC-d group declined further
along with a dramatical increase in MDA content compared with the
SC group. The results reflected lipid peroxidation increased
immediately following decompression surgery which was resulted from
the reperfusion of the spinal cord. These findings indicated that
IRI may occur in the chronic severe compression of the spinal
cords. It has previously been established that neurons in the
spinal cord and subcellular components in the myelin sheath have
biological membranes which are critical in sustaining normal
physiological function and metabolism. Biological membranes contain
a large amount of unsaturated fatty acids which are vulnerable to
free radicals (37,39). Therefore, it could be proposed that
susceptible membrane are attacked by overproductive free radicals
brought by excessively reperfused blood, which leads to the
deterioration of lipid peroxidation and may result in IRI.
According to this hypothesis, potent antioxidants may be important
in the management of spinal cord IRI. In clinical practice, the
acute removal and decompression of the tumor may result in
immediate cord expansion within the open canal space, and the
long-term ischemic compressed segment of the cord is exposed to a
rush in blood supply. This sudden cord expansion and reperfusion
may have lead to disruption in the blood spinal cord barrier, and
triggered a cascade of IRI resulting in postoperative neurologic
deterioration.
It has been proposed that various pathogenic
mechanisms including mitochondria-dependant apoptosis, inflammatory
reactions, and specific phospholipid signaling cascades, may serve
important roles in IRI (15,27–29,37,38).
Chronic spinal cord ischemic injury may induce the passage of blood
borne or neurotrophic substances (specifically TNF-α) through the
blood brain barrier past its saturation point (40). It appears that decoupling of astrocyte
foot processes from endothelial cell surfaces inhibits tight
junction function in the blood brain barrier. Transport systems and
ionic buffering would then be disrupted allowing worsened
reperfusion injury upon decompression of a previously ischemic
spinal cord. However, definite specific mechanisms for
decompression-induced IRI have not yet been established and further
study is required.
The present study highlights IRI may result from a
delayed yet severe neurological deterioration in the absence of
direct insult to the spinal cord following total removal of
intraspinal meningiomas. Substantial efforts should be taken on the
mitigation of spinal cord ischemic injury in clinical practice,
including surgical techniques, pharmacological interventions, and
mechanical methods. The present study may aid to improve the
preoperative informed decision making process and further
investigation into the underlying pathophysiological mechanisms of
this finding are merited.
Acknowledgements
The authors would like to thank all of the
physicians and staff who helped this study.
References
|
1
|
Setzer M, Vatter H, Marquardt G, Seifert V
and Vrionis FD: Management of spinal meningiomas: Surgical results
and a review of the literature. Neurosurg Focus. 23:E142007.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sandalcioglu IE, Hunold A, Müller O,
Bassiouni H, Stolke D and Asgari S: Spinal meningiomas: Critical
review of 131 surgically treated patients. Eur Spine J.
17:1035–1041. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ambekar S, Sharma M, Kukreja S and Nanda
A: Complications and outcomes of surgery for spinal meningioma: A
nationwide inpatient sample analysis from 2003 to 2010. Clin Neurol
Neurosurg. 118:65–68. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Taher F, Lebl DR, Cammisa FP, Pinter DW,
Sun DY and Girardi FP: Transient neurological deficit following
midthoracic decompression for severe stenosis: A series of three
cases. Eur Spine J. 22:2057–2061. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Uematsu Y, Tokuhashi Y and Matsuzaki H:
Radiculopathy after laminoplasty of the cervical spine. Spine
(Phila Pa 1976). 23:2057–2062. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chin KR, Seale J and Cumming V: ‘White
cord syndrome’ of acute tetraplegia after anterior cervical
decompression and fusion for chronic spinal cord compression: A
case report. Case Rep Orthop. 2013:6979182013.PubMed/NCBI
|
|
7
|
Orchowski J, Bridwell KH and Lenke LG:
Neurological deficit from a purely vascular etiology after
unilateral vessel ligation during anterior thoracolumbar fusion of
the spine. Spine (Phila Pa 1976). 30:406–410. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee KS, Shim JJ, Doh JW, Yoon SM, Bae HG
and Yun IG: Transient paraparesis after laminectomy in a patient
with multi-level ossification of the spinal ligament. J Korean Med
Sci. 19:624–626. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang T, Wu L, Deng X, Yang C, Zhang Y,
Zhang D and Xu Y: Delayed neurological deterioration with an
unknown cause subsequent to surgery for intraspinal meningiomas.
Oncol Lett. 9:2325–2330. 2015.PubMed/NCBI
|
|
10
|
Wisselink W, Money SR, Crockett DE, Nguyen
JH, Becker MO, Farr GH and Hollier LH: Ischemia-reperfusion injury
of the spinal cord: Protective effect of the hydroxyl radical
scavenger dimethylthiourea. J Vasc Surg. 20:444–491. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shan LQ, Ma S, Qiu XC, Zhou Y, Zhang Y,
Zheng LH, Ren PC, Wang YC, Fan QY and Ma BA: Hydroxysafflor Yellow
A protects spinal cords from ischemia/reperfusion injury in
rabbits. BMC Neurosci. 11:982010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chan PH: Role of oxidants in ischemic
brain damage. Stroke. 27:1124–1129. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wilson JX and Gelb AW: Free radicals,
antioxidants and neurologic injury: Possible relationship to
cerebral protection by anesthetics. J Neurosurg Anesthesiol.
14:66–79. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim P, Haisa T, Kawamoto T, Kirino T and
Wakai S: Delayed myelopathy induced by chronic compression in the
rat spinal cord. Ann Neurol. 55:503–511. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang J, Rong W, Hu X, Liu X, Jiang L, Ma
Y, Dang G, Liu Z and Wei F: Hyaluronan tetrasaccharide in the
cerebrospinal fluid is associated with self-repair of rats after
chronic spinal cord compression. Neuroscience. 210:467–480. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Basso DM, Beattie MS, Bresnahan JC,
Anderson DK, Faden AI, Gruner JA, Holford TR, Hsu CY, Noble LJ,
Nockels R, et al: MASCIS evaluation of open field locomotor scores:
Effects of experience and teamwork on reliability multicenter
animal spinal cord injury study. J. Neurotrauma. 13:343–359. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kou J, Fischgrund J, Biddinger A and
Herkowitz H: Risk factors for spinal epidural hematoma after spinal
surgery. Spine (Phila Pa 1976). 27:1670–1673. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cramer DE, Maher PC, Pettigrew DB and
Kuntz C IV: Major neurologic deficit immediately after adult spinal
surgery: Incidence and etiology over 10 years at a single training
institution. J Spinal Disord Tech. 22:565–570. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ahn JS, Lee JK and Kim BK: Prognostic
factors that affect the surgical outcome of the laminoplasty in
cervical spondylotic myelopathy. Clin Orthop Surg. 2:98–104. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Young WF and Baron E: Acute neurologic
deterioration after surgical treatment for thoracic spinal
stenosis. J Clin Neurosci. 8:129–132. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sakaura H, Hosono N, Mukai Y, Ishii T and
Yoshikawa H: C5 palsy after decompression surgery for cervical
myelopathy: Review of the literature. Spine (Phila Pa 1976).
28:2447–2451. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Delattre JY, Arbit E, Thaler HT, Rosenblum
MK and Posner JB: A dose-response study of dexamethasone in a model
of spinal cord compression caused by epidural tumor. J Neurosurg.
70:920–925. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Manabe S, Tanaka H, Higo Y, Park P, Ohno T
and Tateishi A: Experimental analysis of the spinal cord compressed
by spinal metastasis. Spine (Phila Pa 1976). 14:1308–1315. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ushio Y, Posner R, Posner JB and Shapiro
WR: Experimental spinal cord compression by epidural neoplasm.
Neurology. 27:422–429. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ikeda H, Ushio Y, Hayakawa T and Mogami H:
Edema and circulatory disturbance in the spinal cord compressed by
epidural neoplasms in rabbits. J Neurosurg. 52:203–209. 1980.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Delattre JY, Arbit E, Rosenblum MK, Thaler
HT, Lau N, Galicich JH and Posner JB: High dose versus low dose
dexamethasone in experimental epidural spinal cord compression.
Neurosurgery. 22:1005–1007. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Harkey HL, al-Mefty O, Marawi I, Peeler
DF, Haines DE and Alexander LF: Experimental chronic compressive
cervical myelopathy: Effects of decompression. J Neurosurg.
83:336–341. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Schramm J, Hashizume K, Fukushima T and
Takahashi H: Experimental spinal cord injury produced by slow,
graded compression: Alterations of cortical and spinal evoked
potentials. J Neurosurg. 50:48–57. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Baba H, Maezawa Y, Uchida K, Imura S,
Kawahara N, Tomita K and Kudo M: Three-dimensional topographic
analysis of spinal accessory motoneurons under chronic mechanical
compression: An experimental study in the mouse. J Neurol.
244:222–229. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Baba H, Furusawa N, Fukuda M, Maezawa Y,
Imura S, Kawahara N, Nakahashi K and Tomita K: Potential role of
streptozotocin in enhancing ossification of the posterior
longitudinal ligament of the cervical spine in the hereditary
spinal hyperostotic mouse (twy/twy). Eur J Histochem. 41:191–202.
1997.PubMed/NCBI
|
|
31
|
Furusawa N, Baba H, Imura S and Fukuda M:
Characteristics and mechanism of the ossification of posterior
longitudinal ligament in the tip-toe walking Yoshimura (twy) mouse.
Eur J Histochem. 40:199–210. 1996.PubMed/NCBI
|
|
32
|
Okawa A, Nakamura I, Goto S, Moriya H,
Nakamura Y and Ikegawa S: Mutation in Npps in a mouse model of
ossification of the posterior longitudinal ligament of the spine.
Nat Genet. 19:271–273. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tator CH and Koyanagi L: Vascular
mechanisms in the pathophysiology of human spinal cord injury. J
Neurosurg. 86:483–492. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gupta R, Singh M and Sharma A:
Neuroprotective effect of antioxidants on ischaemia and
reperfusion-induced cerebral in jury. Pharmacol Res. 48:209–215.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ilhan A, Koltuksuz U, Ozen S, Uz E,
Ciralik H and Akyol O: The effects of caffeic acid phenethyl ester
(CAPE) on spinal cord ischemia/reperfusion injury in rabbits. Eur J
Cardiothorac Surg. 16:458–463. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Golding JD, Rigley MacDonald ST, Juurlink
BH and Rosser BW: The effect of glutamine on locomotor performance
and skeletal muscle myosins following spinal cord injury in rats. J
Appl Physiol (1985). 101:1045–1052. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cemil B, Topuz K, Demircan MN, Kurt G, Tun
K, Kutlay M, Ipcioglu O and Kucukodaci Z: Curcumin improves early
functional results after experimental spinal cord injury. Acta
Neurochir (Wien). 152:1583–1590. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ferrari R, Ceconi C, Curello S, Cargnoni
A, Alfiri O, Pardini A, Marzollo P and Visioli O: Oxygen free
radicals and myocardial damage: Protective role of thiol-containing
agents. Am J Med. 91:95S–105S. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Emmez H, Börcek AÖ, Kaymaz M, Kaymaz F,
Durdağ E, Civi S, Gülbahar O, Aykol S and Paşaoğlu A:
Neuroprotective effects of gabapentin in experimental spinal cord
injury. World Neurosurg. 73:729–734. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Christie SD, Comeau B, Myers T, Sadi D,
Purdy M and Mendez I: Duration of lipid peroxidation after acute
spinal cord injury in rats and the effect of methylprednisolone.
Neurosurg Focus. 25:E52008. View Article : Google Scholar : PubMed/NCBI
|