Introduction
Parosteal osteosarcoma (POS) is a rare, slow-growing
malignant tumor that predominantly occurs on the surface of the
metaphysis of long bones. POS accounts for ~1–2 % of primary
malignant bone tumors (1), 4–6% of
all osteosarcomas (2) and 70% of
surface osteosarcomas, with a slight female predilection and a peak
incidence in the third and fourth decades of life (1). Unlike conventional osteosarcomas, POS is
typically a well differentiated low-grade lesion that has a low
tendency to metastasize and has a better prognosis (1,3,4). Chemotherapy or radiotherapy are not
recommended for treating cases of POS that do not exhibit
dedifferentiation.
The most commonly involved sites of POS include the
distal femur, proximal tibia and proximal humerus. Approximately
90% of POS cases involve the metaphysis and ~67% are confined to
the metaphysis. The posterior aspect of the distal femur accounts
for ~70% of all cases (5), and
furthermore, diaphyseal involvement is observed in <10% of all
cases (6–8).
Wide resection of POS is recommended to prevent
local recurrence and the rate of distant metastasis; however, the
optimal technique for long bone diaphyseal reconstruction following
tumor resection is undecided. There are a number of techniques
available for femoral reconstruction of diaphyseal defects
following excision of bone tumors. These include the use of
autogenous vascularized fibular grafts (9,10),
segmental allografts (11–13), autogenous extracorporeally irradiated
bone (14,15), distraction osteogenesis (16,17) and
custom-made intercalary endoprostheses (18–20).
The present case report describes the clinical,
radiological and pathologic features of a 44-year old patient
diagnosed with POS localized in the diaphysis of the left femur. A
custom-made intercalary endoprosthesis was used to achieve
anatomical and functional reconstruction for a 16 cm bone defect
following en bloc tumor resection. Written informed consent was
obtained from the patient and the Ethical Review Board of Shanghai
Tenth People's Hospital, Tongji University School of Medicine
(Shanghai, China) approved the experimental procedures.
Case report
A 44-year-old female was admitted to Shanghai Tenth
People's Hospital (Shanghai, China) on December 22, 2011 for
treatment of a left femoral diaphyseal lesion. The patient had no
history of trauma or infection. Physical examination revealed a
well-defined, firm, painless mass in the patient's medial thigh,
and the patient had full active range of motion of the hip and knee
joint. There was no vascular or neurological deficit associated
with the affected limp. Routine blood investigations were not
relevant for any of the pathological findings.
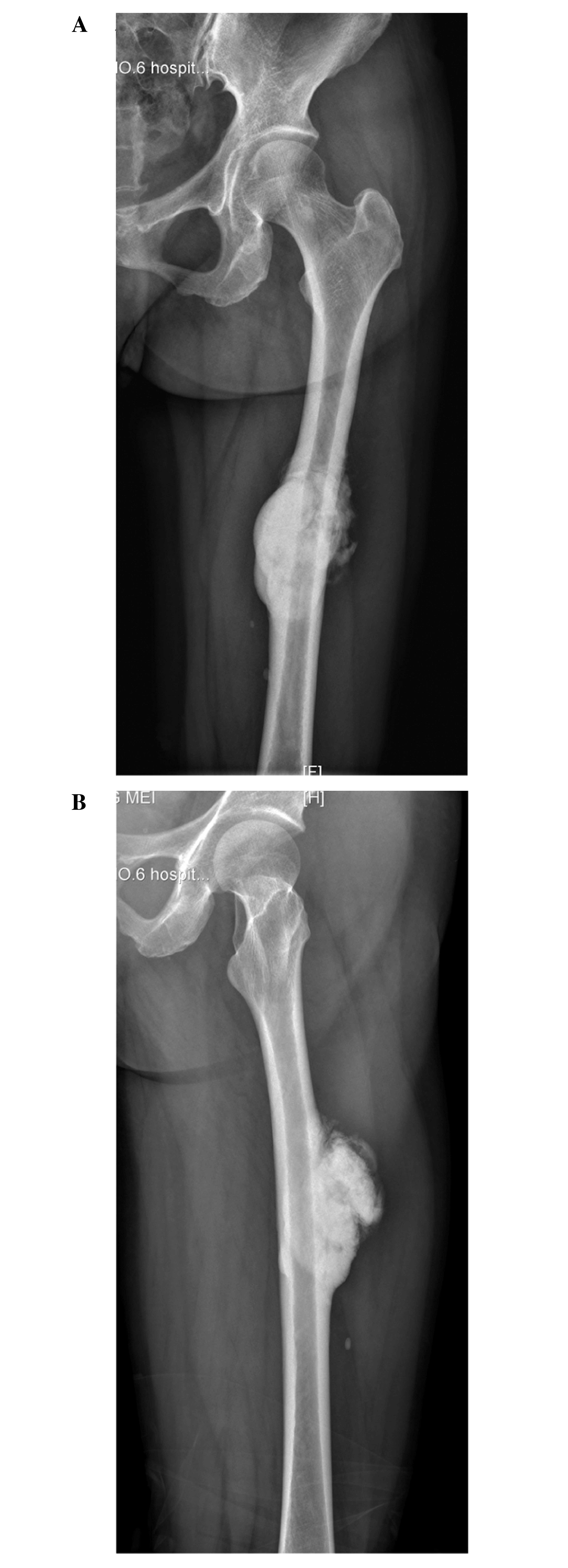
Plain radiography demonstrated a densely ossified
mass with a sessile configuration attached to the surface of the
left femur diaphysis. No radiolucent cleft separated the mass from
the host bone (Fig. 1A and B) and
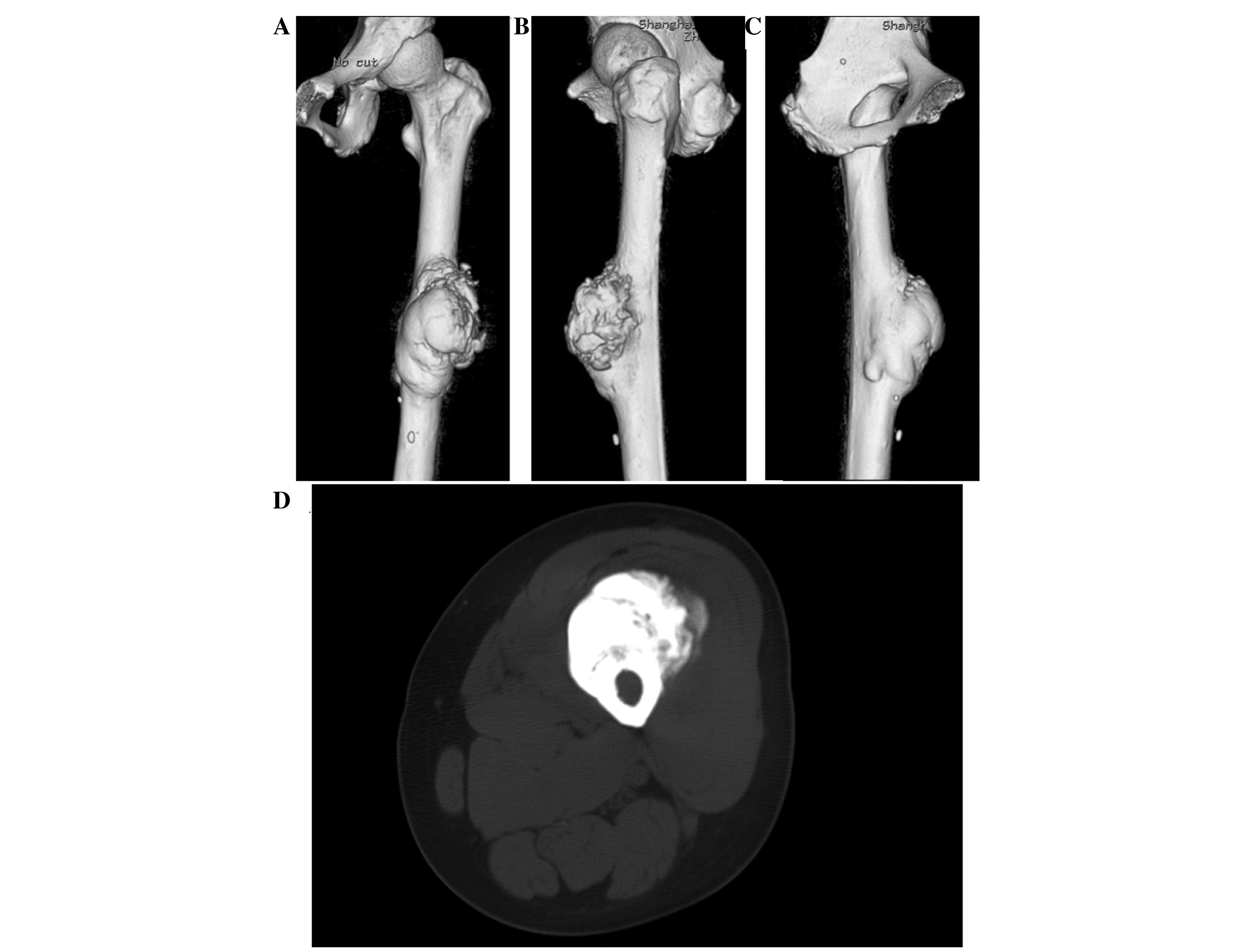
computed tomography (CT) demonstrated an intact and continuous
femoral cortex encircling the mass (Fig.
2A-C). Small unmineralized areas were observed predominantly at
the periphery of the lesion. Axial view CT scans demonstrated that
the mass had infiltrated and destroyed the cortical bone, but had
not invaded the medullary cavity (Fig.
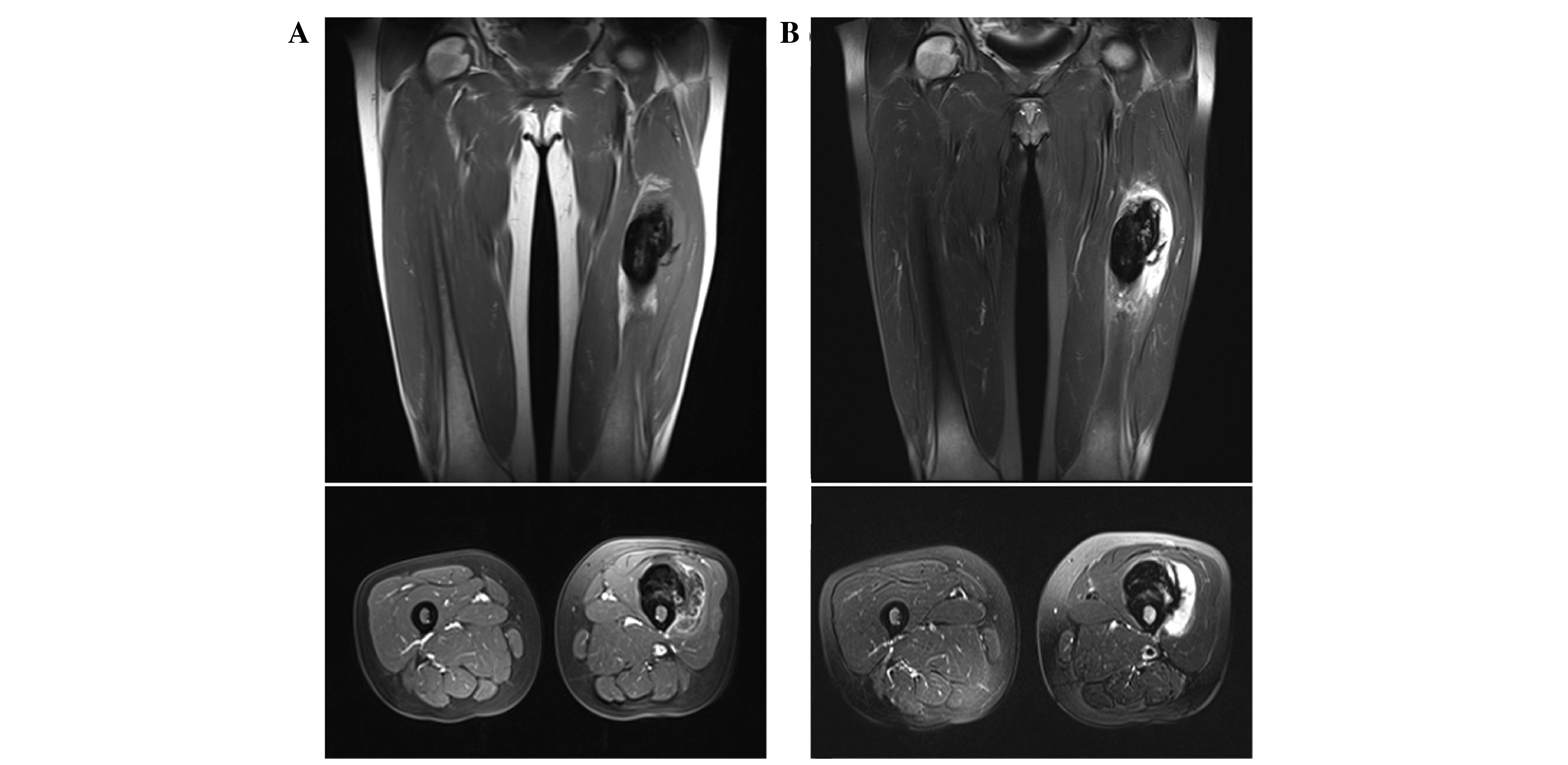
2D). Magnetic resonance imaging (MRI) revealed a tumor on the
surface of the femoral diaphysis that measured 10 × 5.5 × 4.5 cm
and displayed low-to-intermediate signal intensity (SI), when
compared to adjacent muscles on T1-weighted (T1W) images (Fig. 3A). T2-weighted images displayed a
heterogeneous lesion, containing areas of low-to-intermediate SI
mixed with peripheral hyperintense areas (Fig. 3B). Furthermore, T2W images
demonstrated no invasion of the medullar canal by the tumor, which
was initially indicated on CT scan. Subsequent CT scans of the
chest and whole-body nuclear scans revealed no metastases or other
bony abnormalities.
Following the CT and MRI scans, an incisional biopsy
was performed. The samples were fixed in 10% formaldehyde,
decalcified in EDTA (Sinopharm Chemical Reagent Co. Ltd., Shanghai,
China), dehydrated in a graded series of ethanol (70–100%) and
embedded in paraffin. Sections were cut to a thickness of 5 µm in
the longitudinal direction of the tibia, then stained with
hematoxylin and eosin (H&E) and observed using transmitted
light microscopy at a magnification of ×100 (DMI6000B; Leica
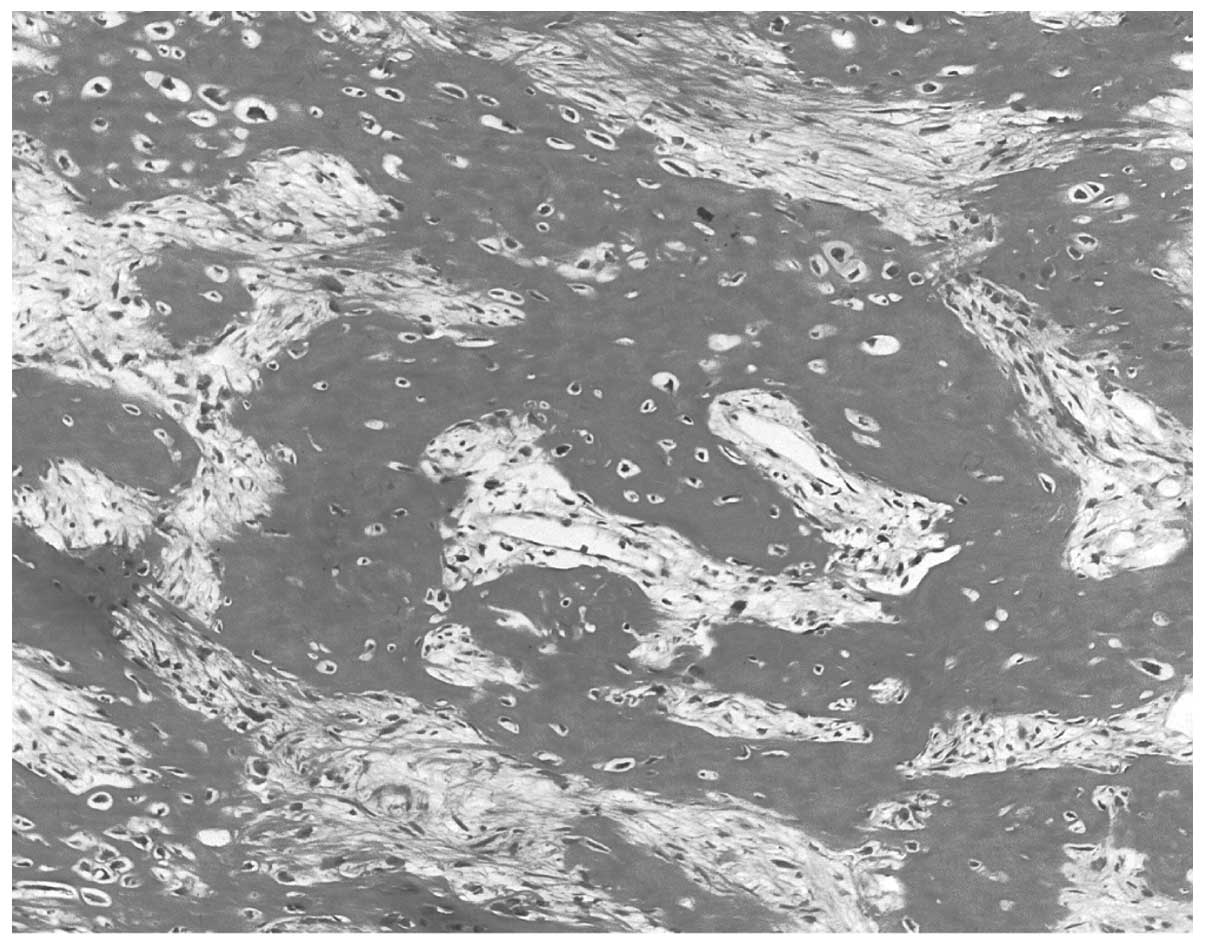
Biosystems, Wetzlar, Germany). Histologically, the lesion exhibited
moderate cellular malignant tumor proliferation, with slightly
atypical and rarely mitotic fusiform cells surrounded by osteoid
trabeculae. These observations led to a diagnosis of POS (Fig. 4).
Preoperatively, a custom-made prosthesis made of
titanium alloy (Ti6A14V), with precisely controlled architecture
for femoral diaphysis defects, was fabricated using computer
assisted design, based on plain radiographs and CT/MRI three
dimensional data files of the patient (Fig. 5). The prosthesis was designed with an
intramedullary stem at each end, these allow insertion into the
corresponding femoral canal. Both stems are screwed to provide
rotational stability, and fixation may be further enhanced with the
use of extracortical plates.
The surgical approach followed general principles of
malignant bone tumor surgery. The patient was positioned supine,
and the standard lateral approach to the femur was used, with the
incision extending from the lateral knee joint line proximally to
the greater trochanter. A 2-cm cuff of healthy tissue was left with
the tumor, and the biopsy track was left in continuity with the
specimen. The femoral vessels were dissected from proximal to
distal and small perforating branches were ligated as they enter
the femur. The sciatic nerve was located and protected. The femur
was osteotomized (proximally) 5 cm below the greater trochanter and
(distally) 15 cm above the knee. The resected tumor was removed and
specimens of the tumor margins were sent for histologic evaluation.
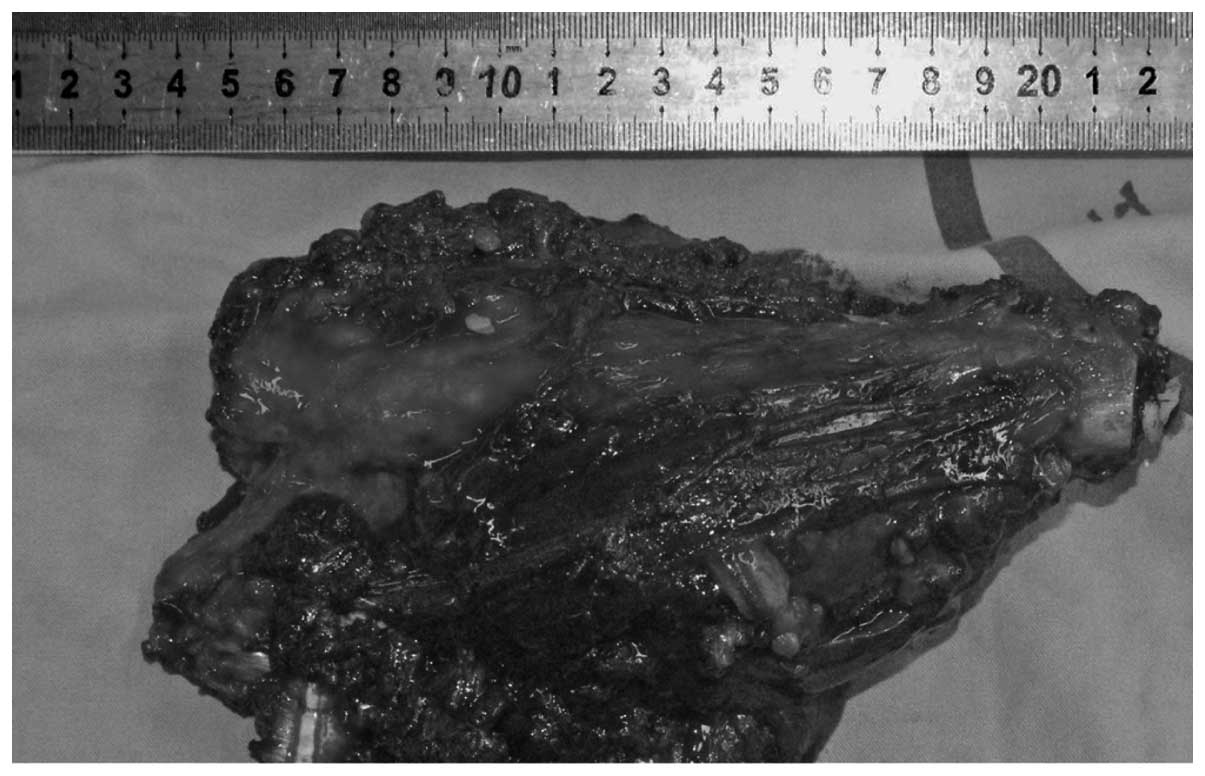
The resection length measured 16 cm (Fig.
6). The frozen sections of the marrow at the site of proximal
and distal resections were reviewed to ensure the margins were
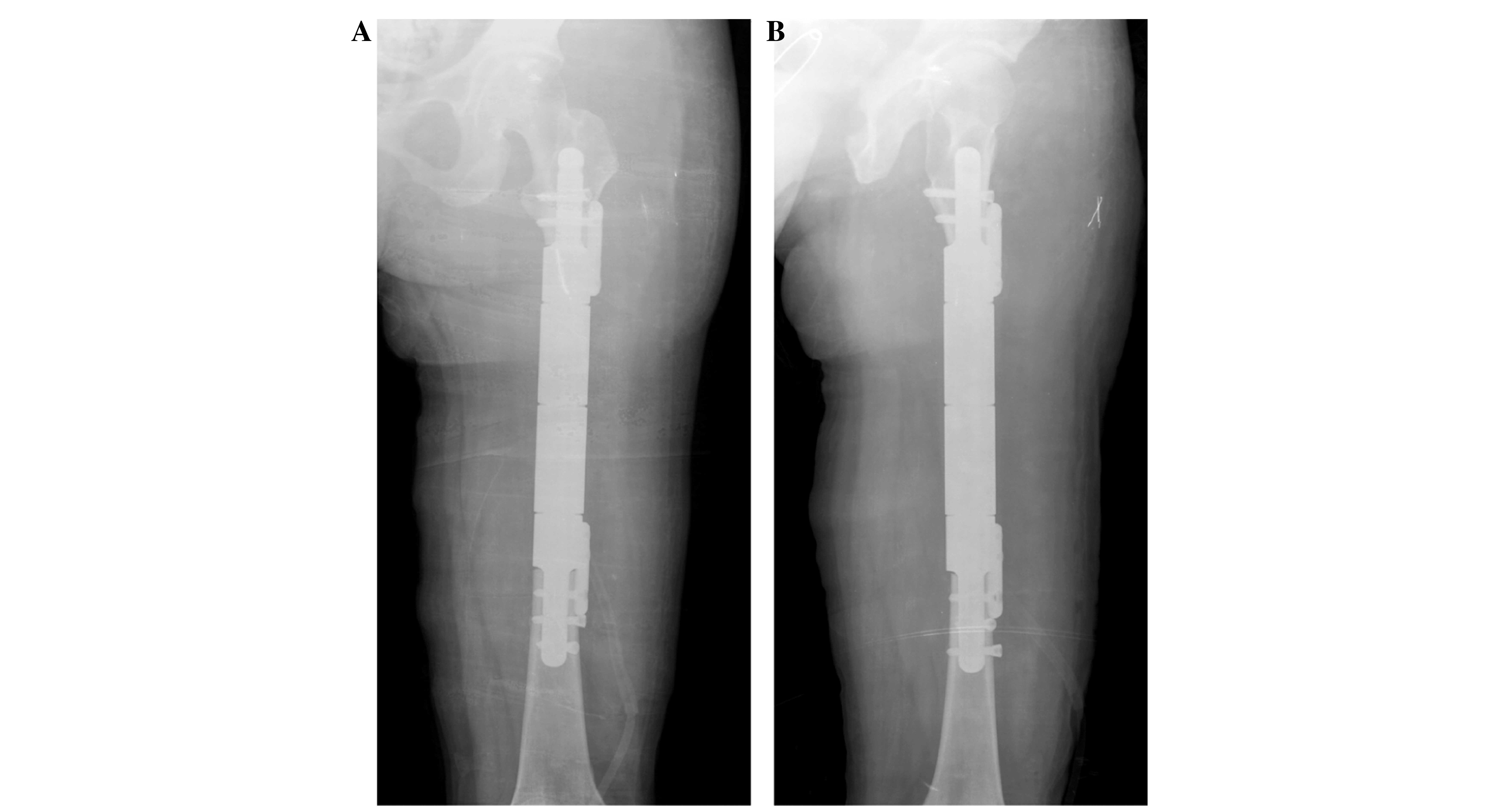
negative for tumor cells before reconstruction. The femoral
diaphysis was then reconstructed using the custom-made intercalary
endoprosthesis (Fig. 7). The patient
received prophylactic antibiotics (cefotiam, 2.0 g) twice a day for
3 days. Active physiotherapy commenced on the second day following
the operation, and the patient was able to support full weight on
the limb by the time of discharge. The patient recovered and
returned to preoperative life. No local recurrence or metastasis
was observed. The range of motion of the hip and knee was not
restricted, and the patient had a good Musculoskeletal Tumour
Society (MSTS) score (21) of 28/30
(93.3%) at the most recent 28-month follow-up.
Discussion
First described in 1951 by Geschickter and Copeland
(22), POS is a rare, slowly
progressive tumor that is typically reported in the metaphyseal of
the long bones, rarely affecting the diaphysis (7,23).
Clinically, POS presents as a hard, immobile swelling with no or
slight pain. The symptoms are often of prolonged duration (4). Pulmonary metastases occur late in the
course of the disease, typically following one or more local
relapses. Therefore, surgical intervention focuses on the local
control of the primary tumor (7).
Early recognition depends on clinical suspicion and a precise
radiological and histological evaluation.
Radiologically, the classic appearance of POS is
that of a heterogeneous, amorphous, and densely mineralized lesion,
arising from the subjacent cortical bone surface and extending into
the soft tissues (2). A
characteristic finding is a radiolucent line, separating the dense
bony mass of the tumor from the host bone, with the exception of
the site of attachment. This is called the cleavage plane, and
represents the uncalcified thickened periosteum. However,
disappearance of the cleavage plane and the preservation of a
continuous femoral cortex encircling the tumor, as observed in the
present case, are probably attributed to the gradual destruction of
the cortical bone by the circumferential growth of the lesion
(3,24). Occasionally, tumors may completely
encompass the involved bone, and the base of the lesion is
typically more ossified than the outermost portion. CT scans can
accurately define the extent of the tumor and cortical integrity
(7); however, they may not reveal
small satellite nodules beyond the main tumor (25). MRI images vary in relation to tumor
size, in addition to the presence of dense osteoid, cartilage,
hemorrhage, necrosis or areas of high-grade tumor or
dedifferentiation. Generally, POS are hypointense on both T1W and
T2W MR images, particularly when the tumors are small. Jelinek
et al (3) propose that low
signal intensity on both T1W and T2W MR images indicates a
low-grade lesion, and high signal intensity on T2-weighted images
indicates a high-grade component. MRI is optimal for identifying
the appropriate biopsy site and potential medullary invasion prior
to surgical intervention (7). MRI and
CT scans are useful in detecting characteristics of tumor
aggressiveness, such as cortical disruption and intramedullary or
soft tissue expansion. POS does not typically invade the medullary
cavity unless the lesion is of long duration or the patient has
received surgical treatment. Approximately 10–20% of patients with
POS demonstrate medullary involvement (23,25).
Histological analysis of POS typically reveals long,
narrow trabeculae or ill-defined islands of osteoid and woven bone,
separated by a fibrous stroma. The trabeculae may undergo
maturation that results in the formation of normal-appearing
lamellar bone with minimal cytological atypia and only rare mitotic
activity (1,2). There is typically minimal to no
involvement of the underlying medullary bone, and the cortex is
often thickened and deformed. These features were exhibited by the
presenting lesion of the patient in the present case, strongly
indicating the diagnosis of POS.
POS is relatively easy to diagnose according to the
imaging and the biopsy results, with the exception of advanced
cases that exhibit medullary involvement or dedifferentiation.
Radiologically, POS should be differentiated from periosteal
osteosarcoma, mature juxtacortical myositis ossificans, exostosis,
osteoma, Ewing's sarcoma, and periosteal chondrosarcoma (26,27).
Histopathologically, POS must be differentiated from various
conditions, including myositis ossificance adjacent to cortical
bone, and periosteal chondrosarcoma. A misdiagnosis with these
conditions may lead to resection with an inadequate surgical
margin.
Segmental resection of a primary bone sarcoma in the
diaphysis is a relatively low-risk method of preventing local
recurrence or distant metastasis, and preserves adjacent joints and
juxta-articular bone, as well as the epiphysis in children, making
it an attractive option for limb salvage. However, the successful
reconstruction of segmental intercalary defects following resection
of a POS in the femoral diaphysis is difficult to achieve. There
are several biological and nonbiological materials available for
the reconstruction of diaphyseal bone defects following tumor
resection. However, various factors including the ease of, and
applicability of the procedure, as well as durability,
complications, oncological safety and functional outcome need to be
considered.
Vascularized and non-vascularized autogenous fibular
grafts are associated with a significant risk of nonunion, fracture
and morbidity at the donor site, and are therefore not appropriate
for large bone defects, which are particularly common in the femur
(9,13,28). It
typically takes numerous years until hypertrophy of the graft is
sufficient to allow full weight-bearing, which can be a major
disadvantage for an individual who wishes to resume normal life,
rather than rely on crutches for a prolonged and uncertain period
of time. Previously published studies of the reconstruction of
femoral defects with autografts demonstrated that the average time
to full weight bearing was 19 months, and the incidence of
complications is closely associated with the length of the segment
replaced (29).
Large-segment allograft reconstruction is an
attractive proposal as it allows mechanical and biological
reconstruction during the same procedure. However, it is associated
with difficulty in obtaining appropriately sized allografts from
bone banks and high rates of complications, including fracture,
nonunion, transmission of disease and infection (30). To date, complete endosteal
revascularization has not been demonstrated (31,32), and
it takes 16 months for the diaphyseal allograft to unite,
therefore, the patient must remain non- or partially weight bearing
during the first few months following the operation (33). An additional study has demonstrated a
50% loss in strength of the allograft bone after 10 years (34). Loss of strength was correlated with an
increase in the prevalence of microfracture and a reduction in bone
mineral density within the cortex of the retrieved allograft.
Extracorporeally irradiated bone offers an
attractive option for reconstructing diaphyseal defects, by acting
as a close-fitting scaffold for creeping substitution and
incorporation, but it takes a long time to revascularize and
incorporate into surrounding bone (14,35).
Although the rates of nonunion and fracture are similar to those of
allograft bone, the overall rate of complication is <52%
(15,35,36).
Furthermore, the procedure is not applicable for bones that are
structurally weak and in bones with pathological fractures.
Distraction osteogenesis (DO) using the Ilizarov
method provides bone with adequate biomechanical strength, and it
is useful in the treatment of post-traumatic segmental bone
defects. However, this method is time-consuming and associated with
a high incidence of complications (37). Tsuchiya et al (16,17) were
the first to introduce limb-salvage surgery using DO combined with
conventional bone transport and the application of an external
fixator. In their studies, early weight bearing was allowed, and
gradual distraction started 1–2 weeks following the operation, at a
rate of 1 mm per day. Although the reported results were good, the
authors advised against using this method for segmental defects
exceeding 15 cm in length, rendering the technique inappropriate
for larger femoral tumors.
Endoprosthetic replacements of the femoral diaphysis
have demonstrated an acceptable postoperative complication rate,
long-term survival and functional outcome, following resection of
primary malignant bone tumors (19,38). The
complications encountered during this procedure include infection
(39,40), aseptic loosening, mechanical failure,
fracture either of the prosthesis or of adjacent bone (19,39–41), local
recurrence and metastatic spread (38). Compared with other reconstructive
options, the use of a custom-made intercalary endoprosthesis for
the reconstruction of the femoral diaphysis has some advantages.
These include a short operating time and hospital stay, with a low
incidence of early complications and reduced risk of disease
transmission. Furthermore, it allows early patient mobility
(without walking aids) and immediate commencement of post-operative
adjuvant therapy. Patients are able to return to a more or less
normal life much earlier (12). Hanna
et al (38) reported the mean
MSTS score for the patients retaining their diaphyseal
endoprosthesis for 10 years following surgery was 87% (range,
67–93%). In the present study, the patient returned to preoperative
life with full range of hip and knee joint motion and an MSTS score
of 28/30 (93.3%) at the 18-month post-surgery follow-up.
The femoral diaphyseal location in the present POS
case is uncommon, within an already extremely rare tumor entity. An
intercalary custom-made endoprosthesis was used to reconstruct the
damaged bone following tumor resection. No early complications,
local recurrence and distant metastasis were observed, and the MSTS
score was 28/30 (93.3%) at the 28-month post-surgery follow-up. The
present authors have described herein the clinical, radiological,
and pathological characteristics of POS and have discussed its
surgical management.
References
|
1
|
Murphey MD, Robbin MR, McRae GA, Flemming
DJ, Temple HT and Kransdorf MJ: The many faces of osteosarcoma.
Radiographics. 17:1205–1231. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Azura M, Vanel D, Alberghini M, Picci P,
Staals E and Mercuri M: Parosteal osteosarcoma dedifferentiating
into telangiectatic osteosarcoma: Importance of lytic changes and
fluid cavities at imaging. Skeletal Radiol. 38:685–690. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jelinek JS, Murphey MD, Kransdorf MJ,
Shmookler BM, Malawer MM and Hur RC: Parosteal osteosarcoma: Value
of MR imaging and CT in the prediction of histologic grade.
Radiology. 201:837–842. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Park YK and Ryu KN: Parosteal osteosarcoma
of the scapula. J Korean Med Sci. 14:586–588. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Subasi M, Kapukaya A, Buyukbayram H and
Bilici A: Unusual benign bone lesion simulating parosteal
osteosarcoma. J Orthop Sci. 11:529–532. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Partovi S, Logan PM, Janzen DL, O'Connell
JX and Connell DG: Low-grade parosteal osteosaracoma of the ulna
with dedifferentiation into high-grade osteosarcoma. Skeletal
Radiol. 25:497–500. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dönmez FY, Tüzün U, Başaran C, Tunaci M,
Bilgiç B and Acunaş G: MRI findings in parosteal osteosarcoma:
Correlation with histopathology. Diagn Interv Radiol. 14:142–152.
2008.PubMed/NCBI
|
|
8
|
Haworth JM, Watt I, Park WM and Roylance
J: Diaphyseal osteosarcoma. Br J Radiol. 54:932–938. 1981.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chang DW and Weber KL: Use of a
vascularized fibula bone flap and intercalary allograft for
diaphyseal reconstruction after resection of primary extremity bone
sarcomas. Plast Reconstr Surg. 116:1918–1925. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen CM, Disa JJ, Lee HY, et al:
Reconstruction of extremity long bone defects after sarcoma
resection with vascularized fibula flaps: a 10-year review. Plast
Reconstr Surg. 119:915–924. (discussion 925–916). 2007.PubMed/NCBI
|
|
11
|
Donati D, Di Liddo M, Zavatta M, Manfrini
M, Bacci G, Picci P, Capanna R and Mercuri M: Massive bone
allograft reconstruction in high-grade osteosarcoma. Clin Orthop
Relat Res. 377:186–194. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Muscolo DL, Ayerza MA, Aponte-Tinao L,
Ranalletta M and Abalo E: Intercalary femur and tibia segmental
allografts provide an acceptable alternative in reconstructing
tumor resections. Clin Orthop Relat Res. 426:97–102. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ogilvie CM, Crawford EA, Hosalkar HS, King
JJ and Lackman RD: Long-term results for limb salvage with
osteoarticular allograft reconstruction. Clin Orthop Relat Res.
467:2685–2690. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen TH, Chen WM and Huang CK:
Reconstruction after intercalary resection of malignant bone
tumours: Comparison between segmental allograft and
extracorporeally-irradiated autograft. J Bone Joint Surg Br.
87:704–709. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Krieg AH, Davidson AW and Stalley PD:
Intercalary femoral reconstruction with extracorporeal irradiated
autogenous bone graft in limb-salvage surgery. J Bone Joint Surg
Br. 89:366–371. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tsuchiya H, Tomita K, Minematsu K, Mori Y,
Asada N and Kitano S: Limb salvage using distraction osteogenesis.
A classification of the technique. J Bone Joint Surg Br.
79:403–411. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tsuchiya H, Wan SL, Sakayama K, Yamamoto
N, Nishida H and Tomita K: Reconstruction using an autograft
containing tumour treated by liquid nitrogen. J Bone Joint Surg Br.
87:218–225. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Abudu A, Carter SR and Grimer RJ: The
outcome and functional results of diaphyseal endoprostheses after
tumour excision. J Bone Joint Surg Br. 78:652–657. 1996.PubMed/NCBI
|
|
19
|
Aldlyami E, Abudu A, Grimer RJ, Carter SR
and Tillman RM: Endoprosthetic replacement of diaphyseal bone
defects. Long-term results. Int Orthop. 29:25–29. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ahlmann ER and Menendez LR: Intercalary
endoprosthetic reconstruction for diaphyseal bone tumours. J Bone
Joint Surg Br. 88:1487–1491. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Enneking WF, Dunham W, Gebhardt MC,
Malawar MM and Pritchard DJ: A system for the functional evaluation
of reconstructive procedures after surgical treatment of tumors of
the musculoskeletal system. Clin Orthop Relat Res. 241–246.
1993.PubMed/NCBI
|
|
22
|
Geschickter CF and Copeland MM: Parosteal
osteoma of bone: A new entity. Ann Surg. 133:790–807. 1951.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Okada K, Frassica FJ, Sim FH, Beabout JW,
Bond JR and Unni KK: Parosteal osteosarcoma. A clinicopathological
study. J Bone Joint Surg Am. 76:366–378. 1994.PubMed/NCBI
|
|
24
|
Suresh S and Saifuddin A: Radiological
appearances of appendicular osteosarcoma: A comprehensive pictorial
review. Clin Radiol. 62:314–323. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hudson TM, Springfield DS, Benjamin M,
Bertoni F and Present DA: Computed tomography of parosteal
osteosarcoma. AJR Am J Roentgenol. 144:961–965. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Uyttendaele D, De Schryver A, Claessens H,
Roels H, Berkvens P and Mondelaers W: Limb conservation in primary
bone tumours by resection, extracorporeal irradiation and
re-implantation. J Bone Joint Surg Br. 70:348–353. 1988.PubMed/NCBI
|
|
27
|
Lindell MM Jr, Shirkhoda A, Raymond AK,
Murray JA and Harle TS: Parosteal osteosarcoma:
Radiologic-pathologic correlation with emphasis on CT. AJR Am J
Roentgenol. 148:323–328. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rose PS, Shin AY, Bishop AT, Moran SL and
Sim FH: Vascularized free fibula transfer for oncologic
reconstruction of the humerus. Clin Orthop Relat Res. 438:80–84.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Han CS, Wood MB, Bishop AT and Cooney WP
III: Vascularized bone transfer. J Bone Joint Surg Am.
74:1441–1449. 1992.PubMed/NCBI
|
|
30
|
Ozaki T, Hillmann A, Bettin D, Wuisman P
and Winkelmann W: Intramedullary, antibiotic-loaded cemented,
massive allografts for skeletal reconstruction. 26 cases compared
with 19 uncemented allografts. Acta Orthop Scand. 68:387–391. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Brown KL: Limb reconstruction with
vascularized fibular grafts after bone tumor resection. Clin Orthop
Relat Res. 262:64–73. 1991.PubMed/NCBI
|
|
32
|
Kattapuram SV, Rosol MS, Rosenthal DI,
Palmer WE and Mankin HJ: Magnetic resonance imaging features of
allografts. Skeletal Radiol. 28:383–389. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Aranguren M San Julian, Leyes M, Mora G
and Cañadell J: Consolidation of massive bone allografts in
limb-preserving operations for bone tumours. Int Orthop.
19:377–382. 1995.PubMed/NCBI
|
|
34
|
Fox EJ, Hau MA, Gebhardt MC, Hornicek FJ,
Tomford WW and Mankin HJ: Long-term followup of proximal femoral
allografts. Clin Orthop Relat Res. 397:106–113. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Amitani A, Yamazaki T, Sonoda J, Tanaka M,
Hirata H, Katoh K and Uchida A: Preservation of the knee joint in
limb salvage of osteosarcoma in the proximal tibia. A case report.
Int Orthop. 22:330–334. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fuchs B, Ossendorf C, Leerapun T and Sim
FH: Intercalary segmental reconstruction after bone tumor
resection. Eur J Surg Oncol. 34:1271–1276. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hornicek FJ, Gebhardt MC, Tomford WW,
Sorger JI, Zavatta M, Menzner JP and Mankin HJ: Factors affecting
nonunion of the allograft-host junction. Clin Orthop Relat Res.
382:87–98. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hanna SA, Sewell MD, Aston WJ, Pollock RC,
Skinner JA, Cannon SR and Briggs TW: Femoral diaphyseal
endoprosthetic reconstruction after segmental resection of primary
bone tumours. J Bone Joint Surg Br. 92:867–874. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Guo W, Ji T, Yang R, Tang X and Yang Y:
Endoprosthetic replacement for primary tumours around the knee:
Experience from Peking University. J Bone Joint Surg Br.
90:1084–1089. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gosheger G, Gebert C, Ahrens H,
Streitbuerger A, Winkelmann W and Hardes J: Endoprosthetic
reconstruction in 250 patients with sarcoma. Clin Orthop Relat Res.
450:164–171. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sewell MD, Spiegelberg BG, Hanna SA, Aston
WJ, Bartlett W, Blunn GW, David LA, Cannon SR and Briggs TW: Total
femoral endoprosthetic replacement following excision of bone
tumours. J Bone Joint Surg Br. 91:1513–1520. 2009. View Article : Google Scholar : PubMed/NCBI
|