Introduction
Traumatic brain injury (TBI) is a worldwide health
problem, causing mortality and permanent disability, including
impaired attention and poor executive function as a result of
neurocognitive deficits (1). The
annual incidence and mortality rates of TBI in Europe are 235 and
15 cases per 100,000 individuals, respectively (2). However, the management for patients with
TBI has recently improved substantially (3). However, the prognosis for patients with
severe TBI remains poor, with results such as disturbance of
consciousness and motor disorders. Stem cells have been suggested
to be of potential for the repair of the damaged nervous system
(4). Mesenchymal stem cell
(MSC)-based cellular therapy has been studied in early-phase
clinical trials to improve the effects of central nervous system
(CNS) injuries (5). Human bone marrow
MSCs (BMMSCs) have been widely studied, as they are relatively easy
to access and they have potential to differentiate into the
osteogenic, adipogenic and chondrogenic lineages, and into
hepatocytes, cardiomyocytes, neurons and other types of tissues or
cells (6). Previous studies have
reported that transplanted BMMSCs accelerated neuroplasticity and
facilitated neuronal regeneration, as well as functional recovery
(7). Currently, autologous BM-derived
stem cell transplantation s is one of the most common procedures in
stem cell research. However, complications for autologous MSC
therapy, including a few serious complications, have continued to
be reported (8). As MSCs are
multipotent, the issue of safety requires further consideration.
When these cells are implanted, it may cause a low prevalence of
neoplasms (5). The present study
reports a case in which acute promyelocytic leukemia (APL)
developed following treatment with autologous BMMSC transplantation
for TBI. Written informed consent was obtained from the
patient.
Case report
A 36-year-old female was admitted to a local
hospital due to a severe traumatic brain injury caused by a traffic
accident in December 2009. Paralysis of the lower limbs and the
hands was observed following the surgery for the trauma. In early
April 2011, the patient was treated with autologous BMMSCs
transplantation by subarachnoid space injection. Following
transplantation, the patient developed skin ecchymosis that
persisted for 1 week and a fever that lasted for 3 days, and was
subsequently transferred to Zhongshan City People's
Hospital,(Zhongshan, China) on May 20, 2011. The routine blood test
was markedly abnormal, with a white blood cell count of
66.92×109/l (normal range, 4.0–10.0×109/l),
20% neutrophils (normal range, 50]70%), a hemoglobin level of 102
g/l (normal range, 110–150 g/l) and a platelet count of
61×109/l (normal range, 100–300×109/l).
Physical examination revealed scattered ecchymosis on the limbs,
bilateral cervical part lymphadenopathy and strengthened muscular
tension of limbs.
Blood chemistry on admission showed elevated lactate
dehydrogenase (1,142 U/l; normal range, 104–245 U/l), alanine (92
U/l; normal range, 10–40 U/l) and aspartate aminotransferase (96
U/l; normal range, 10–40 U/l) levels. The total protein, albumin,
serum creatinine and blood urea nitrogen levels were within normal
limits. The prothrombin time was 14 sec (normal range, 11–13 sec),
the activated partial thromboplastin time was 20.7 sec (normal
range, 31–43 sec), the thrombin time was 25.3 sec (normal range,
16–18 sec), the fibrinogen level was reduced to 0.83 g/l (normal
range, 2.0–4.0 g/l), the protamine paracoagulation test was
positive and D-dimers were increased to 2.894 mg/l (normal range,
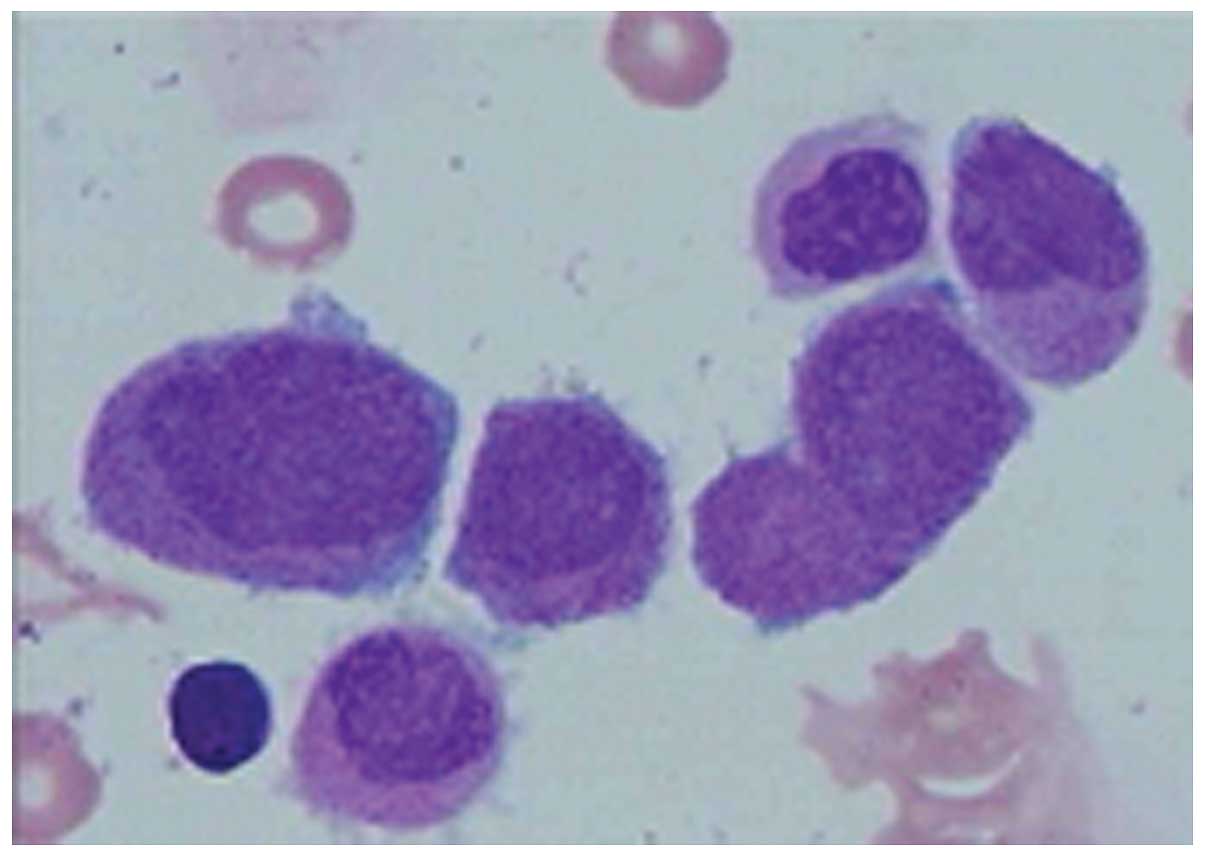
0±0.3 mg/l). BM aspiration revealed that >76% of marrow cells
were abnormal promyelocytic cells (Fig.
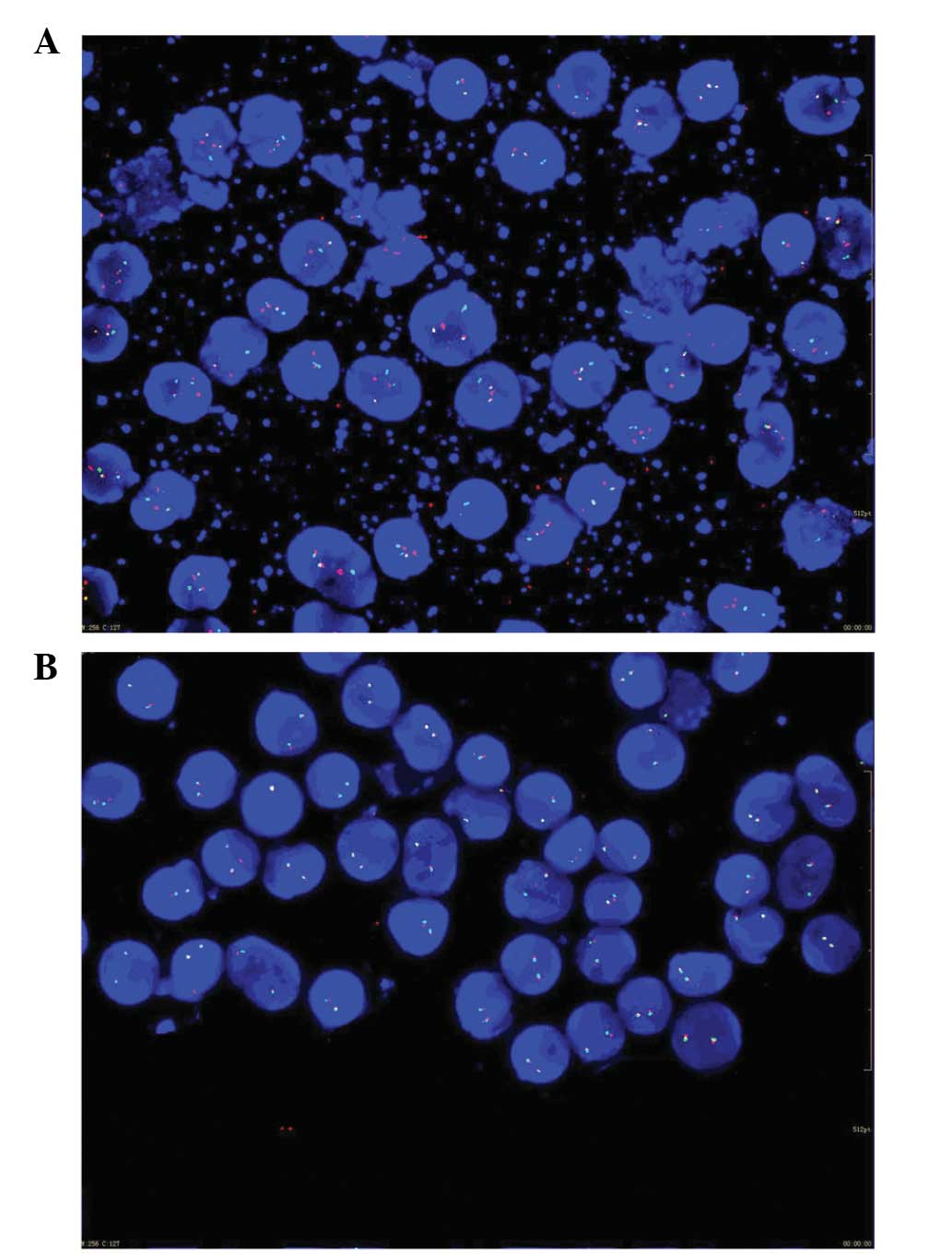
1). Translocations involving the mixed lineage leukemia (MLL)
and promyelocytic leukemia/retinoic acid receptor-α (PML/RARA)
genes were detected by fluorescence in situ hybridization
(Fig. 2). An abdominal computed
tomography scan showed slight splenomegaly. The final diagnosis was
of post-traumatic brain syndrome and APL. The patient was treated
with all-trans retinoic acid (25 mg/m2/day), and
cryoprecipitate was transfused for correction of the DIC. However,
the patient's condition deteriorated due to a severe infection of
the lung and uncontrolled gastrointestinal bleeding caused by the
DIC. Thus, the patient succumbed 10 days later.
Discussion
TBI always destroy neurons, glial cells, nerve
fibers and blood vessels directly, and the effective treatment of
neurological impairment has been a widespread problem in clinical
practice (7). Early studies suggested
that the CNS had no ability to renew or regenerate after injury.
This viewpoint has gradually been corrected through the research
progress in the fields of stem cells and neuroregeneration
(9). The compensation from uninjured
neurons, and the migration and differentiation of neural stem cells
may contribute to the neurological recovery of patients with TBI.
However, the capability of self-recovery is weak due to the limited
quantity of a patient's own stem cells. Therefore, exogenous stem
cell transplantation provides a novel method of promoting the
recovery of neurological function in patients with TBI (10).
A number of studies found that MSCs can give rise to
non-mesenchymal cell types, such as glial cells, neurons and
hepatocytes, among others (1). This
provides a promising method for replacement therapy. Transplanted
MSCs may confer beneficial effects in patients with CNS injuries by
potential mechanisms such as the migration to injured tissues,
transdifferentiation to replace neural cells that are damaged and
the induction of growth factor production (1). For example, neuronal MSC differentiation
could provide a source of cells for the replacement of neurons that
are lost due to neurodegenerative diseases. Studies have shown that
neurons differentiated from MSCs exhibit functional neuronal
properties. The major cell types used in clinical treatment are
neural stem cells, BMMSCs and umbilical cord MSCs (9). Compared with the other cell types,
autologous BMMSCs avoid ethical controversy and immune rejection,
and can be easily obtained through repeated harvests (7). Hence, BMMSCs has become an important
source of seed cells for the treatment of a wide variety of nervous
diseases.
Although the major continued investigation of MSCs
may assist in ensuring that cell based-therapy is used safely and
effectively in human disease, there is growing concern over the
clinical use of MSCs. Since MSCs are multipotent, one issue that
requires addressing further is the potential tumorigenesis
(11). Theoretically, MSCs are also
known to home in on tumors, and once set up in the tumor
microenvironment, they are able to support the growth of the tumor
and spread. Recent studies have also demonstrated that newly
injected MSCs often travel back to the BM, to inflamed
tissues/organs or to sites of growing tumors, indicating that
besides their functions in tissue repair, MSCs are important in
immunity modulation and tumor growth (11). The inflammation-cancer link has been
known for decades; MSC mobilization/activation in vivo in
response to the damage or inflammation of tissues may enable the
growth of pre-cancerous or dormant tumors (12). MSCs exhibit tumor-promoting functions
by physically providing tumor-nurturing niches when recruited to
the sites of growing tumors. Studies have also suggested that
MSC-derived soluble molecules, including fibroblast-specific
protein 1, stromal cell-derived factor-1α, chemokine (C-C motif)
ligand 5, interleukin 6 and chemokine (C-X-C motif) ligand 7, are
key to this tumor promotion, providing a mechanism by which
tumor-promoting molecules are secreted by MSCs in a paracrine
manner (12). In addition, MSCs have
been shown to favor angiogenesis, which also promotes ovarian tumor
growth. Biochemical analysis suggests that MSC-conditioned medium
induces VEGF expression in tumor cells (12). Furthermore, another critical issue
with regard to tumor promotion is the immunosuppressive prosperity
of MSCs. MSCs are known to affect the proliferation and
differentiation of dendritic cells, B and T cells,
monocytes/macrophages, natural killer cells and mast cells
(11).
Recipients receiving organ or cell transplantation
are susceptible to leukemia, which may be attributed to
donor-derived leukemic cells, the use of cytotoxic agents and long
duration immunosuppression, but the exact mechanism has yet to be
elucidated (13). Immunosuppression
is believed to be the most significant risk factor for malignancy
following transplantation. In the present case, APL occurred early
after autologous BMMSC transplantation, which is extremely rare.
Previous studies have reported that acute leukemia did not occur
between 2 months and 17 years post-organ transplantation (14,15). There
are a number of hypotheses regarding the development of leukemia in
recipients with autologous BMMSC transplantation. The majority of
reported cases of leukemia following transplantation have been
associated with a cytogenetic abnormality (16). Camós et al (15) demonstrated that the chromosomal
alterations that are typically identified in therapy-related AML,
such as monosomy or deletion of chromosomes 5 and 7, and 11q23
rearrangements, were not found in these AML cases. However, APL
with t(15,17) translocation is the most common subtype of AML, even
though few AML cases have been reported following liver
transplantation (15). AML after
solid organ transplantation is rare and, to date, only 9 cases of
this form of leukemia after liver transplantation have been
described, including 3 cases of APL and 6 with normal karyotypes.
The present patient was diagnosed with APL with PML/RARA
translocation and MLL rearrangement, also supporting this viewpoint
of leukemogenesis after cell therapy. Another possibility is
immunosuppression, where the potentially leukemogenic factor may
play a role in developing AML after transplantation. The impaired
immunosurveillance caused by the immunosuppressive effect of MSCs
may be responsible for an increased incidence of leukemia.
In conclusion, the present patient developed de
novo APL following autologous BMMSC transplantation for TBI.
Although the precise mechanism could not be identified, the
cytogenetic abnormality and the immunosuppressive effect of the
MSCs may contribute to this leukemogenesis. Despite this, MSC
transplantation is a promising treatment for TBI, although the
safety of MSC application remains a challenging issue that requires
further investigation.
References
|
1
|
Zhang R, Liu Y, Yan K, Chen L, Chen XR, Li
P, Chen FF and Jiang XD: Anti-inflammatory and immunomodulatory
mechanisms of mesenchymal stem cell transplantation in experimental
traumatic brain injury. J Neuroinflammation. 10:1062013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mauritz W, Wilbacher I, Majdan M, Leitgeb
J, Janciak I, Brazinova A and Rusnak M: Epidemiology, treatment and
outcome of patients after severe traumatic brain injury in European
regions with different economic status. Eur J Public Health.
18:575–580. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Scudday T, Brasel K, Webb T, Codner P,
Somberg L, Weigelt J, Herrmann D and Peppard W: Safety and efficacy
of prophylactic anticoagulation in patients with traumatic brain
injury. J Am Coll Surg. 213:148–153. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Parsons XH, Teng YD, Parsons JF, Snyder
EY, Smotrich DB and Moore DA: Efficient derivation of human
neuronal progenitors and neurons from pluripotent human embryonic
stem cells with small molecule induction. J Vis Exp.
28:e32732011.
|
|
5
|
Centeno CJ, Schultz JR, Cheever M, Freeman
M, Faulkner S, Robinson B and Hanson R: Safety and complications
reporting update on the re-implantation of culture-expanded
mesenchymal stem cells using autologous platelet lysate technique.
Curr Stem Cell Res Ther. 6:368–378. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dai G, Liu X, Zhang Z, Yang Z, Dai Y and
Xu R: Transplantation of autologous bone marrow mesenchymal stem
cells in the treatment of complete and chronic cervical spinal cord
injury. Brain Res. 1533:73–79. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tian C, Wang X, Wang X, Wang L, Wang X, Wu
S and Wan Z: Autologous bone marrow mesenchymal stem cell therapy
in the subacute stage of traumatic brain injury by lumbar puncture.
Exp Clin Transplant. 11:176–181. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wakitani S, Okabe T, Horibe S, Mitsuoka T,
Saito M, Koyama T, Nawata M, Tensho K, Kato H, Uematsu K, et al:
Safety of autologous bone marrow-derived mesenchymal stem cell
transplantation for cartilage repair in 41 patients with 45 joints
followed for up to 11 years and 5 months. J Tissue Eng Regen Med.
5:146–150. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lda S Meirelles and Nardi NB: Methodology,
biology and clinical applications of mesenchymal stem cells. Front
Biosci (Landmark Ed). 14:4281–4298. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang S, Cheng H, Dai G, Wang X, Hua R, Liu
X, Wang P, Chen G, Yue W and An Y: Umbilical cord mesenchymal stem
cell transplantation significantly improves neurological function
in patients with sequelae of traumatic brain injury. Brain Res.
1532:76–84. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Waterman RS, Henkle SL and Betancourt AM:
Mesenchymal stem cell 1 (MSC1)-based therapy attenuates tumor
growth whereas MSC2-treatment promotes tumor growth and metastasis.
PLoS One. 7:e455902012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhu W, Huang L, Li Y, Qian H, Shan X, Yan
Y, Mao F, Wu X and Xu WR: Mesenchymal stem cell-secreted soluble
signaling molecules potentiate tumor growth. Cell Cycle.
10:3198–3207. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu M, Liu J, Liu L, Yu L, Shi B, Ye L,
Zhang Y and Chen H: A case report of acute myeloid leukemia after
liver transplantation. Acta Haematol. 129:225–228. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jiang N, Li H, Wang GS, Zhang J, Zhang JF,
Yi SH, Yang Y, Cai CJ, Lu MQ and Chen GH: Acute leukemia, a rare
but fatal complication after liver transplantation. Leuk Res.
33:1349–1351. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Camós M, Esteve J, Rimola A, Grande L,
Rozman M, Colomer D, Villamor N, Costa D and Montserrat E:
Increased incidence of acute myeloid leukemia after liver
transplantation? Description of three new cases and review of the
literature. Transplantation. 77:311–313. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sato T, Kobayashi R, Iguchi A, Nakajima M,
Koizumi S, Furukawa H, Todoh S and Kobayashi K: Acute promyelocytic
leukemia after living donor partial orthotopic liver
transplantation in two Japanese girls. Leuk Lymphoma. 46:1057–1060.
2005. View Article : Google Scholar : PubMed/NCBI
|